Abstract
Aims:
The aim of this study was to assess the influence of temperature and concentration on the dynamic viscosity of sodium hypochlorite in comparison with 17% EDTA and 2% chlorhexidine gluconate.
Settings and Design:
In vitro
Materials and Methods:
Dynamic viscosity measurements of sodium hypochlorite [NaOCl (5.25%, 2.6%, 1.25%)], EDTA (17%), and chlorhexidine gluconate [CHX, 2%] were measured using a rotational digital viscometer at room temperature (25°C). The influence of temperature (45°C, 60°C) and concentration (5.25%, 2.6%, and 1.25%) on the dynamic viscosity of NaOCl was also evaluated. The measurements were performed using a circulating water bath calibrated with a thermostat, and the dynamic viscosity measurements were noted in Centipoise (Cps).
Statistical Analysis Used:
The tests used for the statistical analysis were Kolmogorov-Smirnov and Shapiro Wilk tests, one-way ANOVA, and independent sample t-test.
Results:
Viscosity statistically increased with NaOCl concentration and decreased with increasing temperature. Amongst the tested NaOCl groups, 5.25% NaOCl at room temperature was significantly the most viscous (μ =1.5300 Cps) while 1.25% NaOCl at 60°C was significantly the least viscous (μ =1.1800 Cps).
Conclusions:
5.25% NaOCl and 17% EDTA are significantly viscous at room temperature. Elevating the temperature of 1.25% NaOCl to 60°C significantly reduces the viscosity of the NaOCl.
Keywords: Chlorhexidine gluconate, concentration, dynamic viscosity, ethylenediaminetetraacetic acid, irrigation, sodium hypochlorite, temperature
INTRODUCTION
Endodontic success relies upon the complete chemo mechanical debridement of the radicular pulp space. The complex human root canal anatomy comprises of numerous irregularities, which impede optimal disinfection. These may serve as ecological niches to microbes leading to persistence of infection. Mechanical instrumentation alone does not result in complete elimination of intra-radicular microorganisms.[1] Endodontic irrigants provide access to uninstrumented intricacies when present in direct contact with the root canal system.[2]
The three most important attributes for an endodontic irrigant are its tissue dissolution ability, anti-microbicity, and ability to remove the smear layer.[3] As of now, there is no endodontic irrigant, which is able to satisfy all the desired requisites. A combination of one or more irrigants is suggested according to the infection status of the root canal. The three most commonly used endodontic irrigants are sodium hypochlorite (NaOCl), chlorhexidine gluconate (CHX), and ethylenediaminetetraacetic acid (EDTA).
NaOCl is the most recommended endodontic irrigant due to its tissue dissolution ability, which is a function of its concentration, available surface area of the involved tissue,[4,5] exposure time, variations in temperature,[6] surface tension,[7] and volume of the irrigant.[5] NaOCl has been recommended to be used in concentrations ranging from 0.5-6%.[2] The most commonly employed concentrations of NaOCl are 5.25%, 2.5%, and 1.25%.[5,8,9]
EDTA is employed as a final rinse due to its ability to remove the inorganic component of smear layer and is recommended in a concentration of 17% for a period of one minute.[10] EDTA reacts with calcium ions in dentin resulting in the formation of calcium chelates.[11] CHX has potent antimicrobial ability, especially against E. faecalis.[12] It is highly effective in reducing intra-radicular microbes in teeth with apical periodontitis[13] and is recommended in a concentration of 2% as a final rinse.
The most commonly employed irrigation protocol is with a needle and a syringe. Amongst Indian endodontic practitioners, an overwhelming majority were found to be employing conventional needle-syringe irrigation protocol.[14] However, it has been well established that conventional needle-syringe techniques result in inadequate debridement of the apical third.[4,15,16] Thus, recent focus of endodontic research has been on improving irrigation dynamics.
One of the most essential parameters related to fluid flow characteristics is its dynamic viscosity, which is the resistance exhibited by a fluid while it is being deformed by tensile or shear stresses. The lesser the viscosity, the easier is the fluid movement.[17] In any type of flow, layers of fluid move at different velocities, and the resulting dynamic viscosity arises due to the shear stress required to oppose the applied force. Temperature is one of the primary influencing variables affecting fluid viscosity.[18]
The various studies that have evaluated the effect of temperature on NaOCl have assessed it in terms of its antibacterial effectiveness and not its dynamic viscosity.[8,19] The two studies that have evaluated the dynamic viscosity of NaOCl have taken into consideration the role of its concentration at room temperature and body temperature.[20,21] The influence of different concentrations of NaOCl at varying temperatures on its dynamic viscosity has not yet been ascertained. There has also been no research so far on the influence of dynamic viscosity of 17% EDTA and 2% CHX on their irrigation dynamics.
Hence, the purpose of this study was to assess the influence of temperature (25°C, 45°C, and 60°C) and concentration (1.25%, 2.6%, and 5.25%) on the dynamic viscosity of NaOCl in comparison with 17% EDTA (25°C) and 2% CHX (25°C).
MATERIALS AND METHODS
Measurement of dynamic viscosity
The dynamic viscosity measurement was performed using a rotational digital Viscometer (Brookfield LVDV-II PRO, Middleboro, USA) with an enhanced UL adapter, which facilitates the measurement of low viscosity fluids. The measurement of viscosity was performed using a water bath maintained at constant temperature using a calibrated thermostat. The dynamic viscosity of NaOCl (Chen Chems, Chennai, India) was assessed at three concentrations of 5.25%, 2.6%, and 1.25%. The role of temperature on dynamic viscosity was further evaluated for each of the above mentioned concentrations at three temperature variants namely 25°C, 45°C, and 60°C. Saline (0.9%) was used as the control at 25°C to validate the procedure. EDTA (17%) (Chen Chems, Chennai, India) and CHX (2%) (Asep RC, Stedman Pharma) were assessed for viscosity measurements at 25°C.
An appropriate spindle corresponding to low viscosity fluids was selected, and a fluid volume of 100 mL was used at a constant speed of 100 rpm. The dynamic viscosity measurements (μ) were recorded. The procedure was then repeated ten times for each solution for each group and each concentration. Calculation of mean values and standard deviation were then performed for the ten viscosity values.
Estimation of dynamic viscosity of various irrigants at room temperature - (Groups 1-6)
Serial dilutions of 2.6% and 1.25% NaOCl were prepared from a 5.25% stock solution of NaOCl using distilled water at room temperature (25°C). The dynamic viscosity measurements were then recorded. The same procedure was repeated at room temperature using freshly prepared solutions of 17% solution of EDTA and 2% CHX. 0.9% of saline was used as the control at 25°C to ensure the validity of the experimental procedure.
Group 1-0.9% Saline (25°C), Group 2-2% CHX (25°C), Group 3-17% EDTA (25°C), Group 4-1.25% NaOCl (25°C), Group 5-2.6% NaOCl (25°C), Group 6-5.25% NaOCl (25°C).
Estimation of dynamic viscosity of NaOCl (1.25%, 2.6%, and 5.25%), at varying temperatures – (Groups 4a, 4b; 5a, 5b and 6a, 6b)
The dynamic viscosity measurement was repeated to assess the influence of temperature on the concentration of NaOCl.
Group 4a - 1.25% NaOCl (45°C), Group 4b - 1.25% NaOCl (60°C), Group 5a - 2.6% NaOCl (45°C), Group 5b - 2.6% NaOCl (60°C), Group 6a - 5.25% NaOCl (45°C), Group 6b - 5.25% NaOCl (60°C).
Statistical analysis
The tests used for the statistical analysis were Kolmogorov- Smirnov and Shapiro Wilk tests, one-way ANOVA, and independent sample T-test.
RESULTS
The normality tests Kolmogorov- Smirnov and Shapiro Wilk test results suggested that the data followed a normal distribution. Therefore, to analyze the data, parametric tests were employed.
Table 1 shows the mean and standard deviation of the viscosity of the tested groups at 25°C. One-way ANOVA analysis of the various tested irrigants at room temperature showed significant differences amongst the groups 1-6. Intergroup analysis using independent samples T test showed that all the tested samples were significantly different from each other, except between 2% CHX (Group 2) and 1.25% NaOCl (Group 4). These results indicate that at room temperature, the least viscous irrigants are 2% CHX and 1.25% NaOCl.
Table 1.
Mean and standard deviation of the viscosity of different groups at 25°C
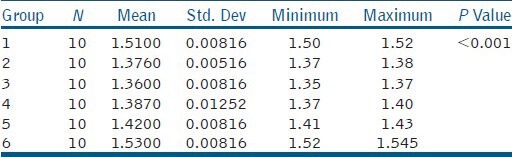
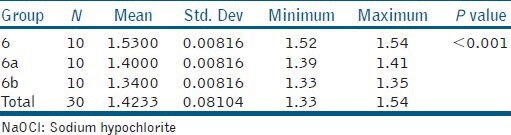
Tables 2, 3, and 4 show the one-way ANOVA analysis of 1.25% NaOCl, 2.6% NaOCl, and 5.25% NaOCl at varying temperatures (25°C, 45°C, and 60°C), respectively. The results reveal a significant influence of both concentration (P < 0.001) and temperature (P < 0.001) on viscosity. Viscosity statistically increased with NaOCl concentration and decreased with increasing temperature. Thus, amongst the tested NaOCl groups, 5.25% NaOCl at room temperature was significantly the most viscous (μ =1.5300 Cps) while 1.25% NaOCl at 60°C was significantly the least viscous (μ =1.1800 Cps).
Table 2.
Intergroup comparison of the mean values of 1.25% NaOCl.25°C, 45°C, and 60°C

Table 3.
Intergroup comparison of the mean values of 2.6% NaOCl.25°C, 45°C, and 60°C
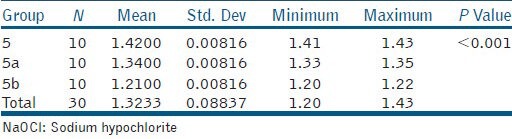
Table 4.
Intergroup comparison of the mean values of 5.25% NaOCl.25°C, 45°C, and 60°C
DISCUSSION
The objective of the current study was to evaluate the influence of temperature and concentration on the dynamic viscosity of NaOCl in comparison with 17% EDTA and 2% CHX. The effect of viscosity on fluid dynamics is related to its flow pattern, which is primarily of two types, a laminar flow when the fluid velocity is low resulting in a smooth sliding of adjacent fluid layers and a turbulent flow exhibiting erratic patterns due to the mixing of the adjacent layers. The laminar and turbulent flows have an effect on a dimensionless quantity namely the Reynolds number (Re), represented by the following formula,
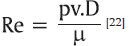
Where (ρ) is the fluid density (kg.m-3), (μ) is the fluid dynamic viscosity (Pa.s), (ν) is the average fluid velocity (m.s-1), and (D) is the characteristic Domain diameter (m).[22] When the value of the Reynolds number is less than 2,300, it indicates a laminar flow, values greater than 4,000 refer to a turbulent flow. Values in the range of 2300-4000 indicate a transient flow.[23] The Reynolds number is inversely proportional to the fluid viscosity (μ) while directly proportional to the fluid density (ρ), velocity (ν), and canal diameter (D). Viscosity is the key attribute, which reduces the Reynolds number, resulting in a laminar flow. Parameters, which decrease the viscosity, would increase the Reynolds number, thereby improving irrigant dynamics converting the flow pattern from laminar to turbulent.
The apical canal ana tomy exhibits numerous variations in the form of lateral canals, apical ramifications, deltas, and anastomoses. Residual infection may persist owing to the colonization of intra-radicular microorganisms organized as biofilms in the complex apical third of the root canal.[24] The most recommended irrigation regimen employs a combination of 5.25% NaOCl followed by a final rinse with 17% EDTA. SEM studies evaluating smear layer removal have demonstrated that a combination of NaOCl and EDTA is effective in removal of the smear layer from the coronal and middle thirds of a root canal.[25] However, smear layer removal is difficult in the apical third of root canals. This could be attributed to the narrowing of the root canal diameter, which would inhibit the placement of needle into the apical third and the resultant fluid dynamics. In our study, at room temperature, 5.25% NaOCl and 17% EDTA were found to be significantly more viscous than the other tested irrigants (2% CHX, 1.25% NaOCl, 2.6% NaOCl, and 0.9% saline). The poor efficiency of 5.25% NaOCl and 17% EDTA in removing the smear layer in the apical third of root canals could be attributed to their high viscosity. The resultant irrigant flow would most probably be less turbulent and more laminar in nature.
One of the effective ways of improving the efficiency of NaOCl is by increasing its temperature. The effect of elevating the temperature of NaOCl has been assessed only in terms of its antimicrobial efficacy[6,8,26] and tissue dissolution ability.[5,6,7] On heating, there is thermal agitation of the irrigant molecules, which enhances its flow properties. This is also associated with a reported increase in bactericidal efficacy that is almost doubled for every 5°C elevation in temperature.[27] Thus, the desired irrigant requisites can be achieved by heating low concentration solutions of NaOCl (1.25%) to 60°C for enhanced irrigation dynamics.
This improvement in flow characteristics of heated NaOCl could be attributed to the reduction in dynamic viscosity, thereby improving the flow of the irrigant into canal intricacies. In canals with complex anatomies, which rely primarily on irrigation rather than instrumentation, employing a 1.25% solution of NaOCl at 45°C and 60°C would prove to be clinically beneficial.
CONCLUSION
Within the limitations of this study, the following clinical conclusions can be made,
A concentration of 5.25% NaOCl and 17% EDTA make these irrigants significantly viscous at room temperature. This higher viscosity would be detrimental to irrigation dynamics in the crucial apical third in terms of tissue dissolution, smear layer removal, and antimicrobial ability.
Elevating the temperature of 1.25% NaOCl to 60°C significantly reduces the viscosity of the irrigant. This would clinically translate into improved irrigation dynamics.
The role of viscosity is critical in irrigant dynamics, and future research should be addressed in assessing various methods that would influence this in the critical apical third of the root canal system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321–8. doi: 10.1111/j.1600-0722.1981.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Grossman LI, Meiman BW. Solution of pulp tissue by chemical agents. J Am Dent Assoc. 1941;28:223–5. [Google Scholar]
- 4.Rosenfeld EF, James GA, Burch BS. Vital pulp tissue response to sodium hypochlorite. J Endod. 1978;4:140–6. doi: 10.1016/S0099-2399(78)80129-0. [DOI] [PubMed] [Google Scholar]
- 5.Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15:187–96. doi: 10.1111/j.1365-2591.1982.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 6.Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31:669–71. doi: 10.1097/01.don.0000153846.62144.d2. [DOI] [PubMed] [Google Scholar]
- 7.Stojicic S, Zivkovic S, Qian W, Zhang H, Haapasalo M. Tissue dissolution by sodium hypochlorite: Effect of concentration, temperature, agitation, and surfactant. J Endod. 2010;36:1558–62. doi: 10.1016/j.joen.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham WT, Balekjian AY. Effect of temperature on collagen-dissolving ability of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980;49:175–7. doi: 10.1016/0030-4220(80)90313-8. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233–9. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28:17–9. doi: 10.1097/00004770-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kandaswamy D, Venkateshbabu N. Root canal irrigants. J Conserv Dent. 2010;13:256–64. doi: 10.4103/0972-0707.73378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onçağ O, Hoşgör M, Hilmioğlu S, Zekioğlu O, Eronat C, Burhanoğlu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 13.Siqueira JF, Jr, Guimarães-Pinto T, Rôças IN. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication with calcium hydroxide on cultivable bacteria in infected root canals. J Endod. 2007;33:800–5. doi: 10.1016/j.joen.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Gopikrishna V, Pare S, Pradeep Kumar A, Lakshmi Narayanan L. Irrigation protocol among endodontic faculty and postgraduate students in Indian dental colleges: A survey. J Conserv Dent. 2013;16:394–8. doi: 10.4103/0972-0707.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ram Z. Effectiveness of root canal irrigation. Oral Surg Oral Med Oral Pathol. 1977;44:306–12. doi: 10.1016/0030-4220(77)90285-7. [DOI] [PubMed] [Google Scholar]
- 16.Druttman AC, Stock CJ. An in vitro comparison of ultrasonic and conventional methods of irrigant replacement. Int Endod J. 1989;22:174–8. doi: 10.1111/j.1365-2591.1989.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 17.Symon KR. Mechanics. 3rd Ed. Massachusetts: Addison-Wesley; 1971. Introduction to the mechanics of continuous media; pp. 345–352. [Google Scholar]
- 18.Viswanath DS, Ghosh TK, Prasad DHL, Dutt NVK, Rani KY. 1st Ed. Netherlands: Springer; 2007. Introduction. Viscosity of Liquids Theory, Estimation, Experiment, and Data; pp. 1–8. [Google Scholar]
- 19.Radcliffe CE, Potouridou L, Qureshi R, Habahbeh N, Qualtrough A, Worthington H, et al. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and enterococcus faecalis. Int Endod J. 2004;37:438–46. doi: 10.1111/j.1365-2591.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 20.Guerisoli DM, Silva RS, Pecora JD. Evaluation of some physico-chemical properties of different concentrations of sodium hypochlorite solutions. Braz Endod J. 1998;3:21–3. [Google Scholar]
- 21.Bukiet F, Soler T, Guivarch M, Camps J, Tassery H, Cuisinier F, et al. Factors affecting the viscosity of sodium hypochlorite and their effect on irrigant flow. Int Endod J. 2013;46:954–61. doi: 10.1111/iej.12086. [DOI] [PubMed] [Google Scholar]
- 22.Boutsioukis C, Lambrianidis T, Kastrinakis E. Irrigant flow within a prepared root canal using various flow rates: A computational fluid dynamics study. Int Endod J. 2009;42:144–55. doi: 10.1111/j.1365-2591.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- 23.Holman JP. New York: McGraw Hill; 2002. Heat Transfer. [Google Scholar]
- 24.Nair PN. On the causes of persistent apical periodontitis: A review. Int Endod J. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 25.Mozayeni MA, Javaheri GH, Poorroosta P, Ashari MA, Javaheri HH. Effect of 17% EDTA and MTAD on intracanal smear layer removal: A scanning electron microscopic study. Aust Endod J. 2009;35:13–7. doi: 10.1111/j.1747-4477.2007.00111.x. [DOI] [PubMed] [Google Scholar]
- 26.Luddin N, Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dychdala GR. Chlorine and chlorine compounds. In: Block SS, editor. Disinfection, sterilization and preservation. 4th Ed. Philadelphia: Lea and Febiger; 1991. pp. 131–51. [Google Scholar]


