Abstract
Background:
The radiopacity of contemporary adhesive systems has been mentioned as the indication for replacement of restorations due to misinterpretation of radiographic images.
Aims:
This study aimed to evaluate the radiopacity of contemporary bonding agents and to compare their radiodensities with those of enamel and dentin.
Methods and Materials:
To measure the radiopacity, eight specimens were fabricated from Clearfil SE Bond (CF), Xeno V (XE), Adper SE Bond (ASE), Magic Bond (MB), Single Bond 2 (SB), Scotchbond Multipurpose (SM), and gutta-percha (positive control). The optical densities of enamel, dentin, the bonding agents, gutta-percha, and an aluminium (Al) step wedge were obtained from radiographic images using image analysis software.
Statistical Analysis:
The radiographic density data were analyzed statistically by analysis of variance and Tukey's test (α =0.05).
Results:
Significant differences were found between ASE and all other groups tested and between XE and CF. No statistical difference was observed between the radiodensity of 1 mm of Al and 1 mm of dentin, between 2 mm of Al and enamel, and between 5 mm of Al and gutta-percha. Five of the six adhesive resins had radiopacity values that fell below the value for dentin, whereas the radiopacity of ASE adhesive was greater than that of dentin but below that of enamel.
Conclusion:
This investigation demonstrates that only ASE presented a radiopacity within the values of dentin and enamel. CF, XE, MB, SB, and SM adhesives are all radiolucent and require alterations to their composition to facilitate their detection by means of radiographic images.
Keywords: Densitometry, dentin-bonding agents, radiography
INTRODUCTION
The phenomenon of bonding to hard dental tissues made it possible to develop new forms of cavity preparation, allowing more dental tissue conservation, a wider variety of indications of resinous materials and the ability to perform dental treatments that were impractical using conventional methods, representing great advancement in dentistry.[1] Tooth/resin bond is achieved by the application of adhesive systems, in a process known as enamel and dentin hybridization, achieved by either micromechanical means or chemical bonds, or possibly both.[2]
At present, there are an increasing variety of dental adhesive systems in the market. In addition to their biological, physical, and mechanical properties, the radiopacity of adhesives should be considered in choosing the most appropriate material for specific clinical situations.[3,4] If the adhesive is not sufficiently radiopaque, it can represent a diagnostic challenge and may result in radiolucent images suggestive of secondary caries, gaps or lack of material at the tooth/resin interface.[5,6] Because of such errors of judgment, dental restorations are frequently removed after detection of a dark halo in radiographs, resulting in the unnecessary removal of healthy dental tissue, putting at risk the pulpal health.[7,8,9] Therefore, such material must be sufficiently radiopaque to allow it to be observed radiographically and let the clinician to distinguish the material from both healthy and demineralized tissues.[6,10]
The radiopacity of contemporary adhesive systems is little known among dentists, however; this concern has been discussed in a few published studies,[3,11] and it has been mentioned as the indication for replacement due to misinterpretation of radiographic images.[5] The radiopacity of bond agents tested depend on their inorganic filler content,[3] indicating the need try out new materials introduced in market.
For that reason, it is important that all materials added to the tooth, such as the lining, base, dentinal adhesive, or restorative materials, have adequate radiopacity to allow them to be radiographically detected, and to reduce the risk of erroneous diagnoses of secondary caries and empty spaces in restorations. Therefore, this study aimed to evaluate the radiopacity of contemporary dental adhesives and compare them with healthy tooth structure.
MATERIALS AND METHODS
The study was done in accordance with the ethical principles originating in the Declaration of Helsinki and written informed consent was obtained from the donator of the tooth. Eight samples were prepared for each bonding agent tested (Clearfil™ SE Bond (CF)-Kuraray America Inc., USA; Xeno™ V (XE)- Dentsply De Trey GmbH, Germany; Adper SE plus (ASE)-3M/ESPE Dental Products, USA; Single Bond 2 (SB)-3M/ESPE Dental Products, USA; Scotchbond Multipurpose (SM)-3M/ESPE Dental Products, USA; Magic Bond DE (MB)-Vigodent Ind. Com., Brazil) using a rectangular acrylic mold with holes measuring 2 mm in diameter and 1 mm in thickness placed on a glass plate and filled with the adhesive systems. The solvent was evaporated using a gentle air flow from a dental syringe and adhesives polymerized for 40 s with a light-emitting diode source (Emiter B; Schuster Com. Equip. Odontolσgicos Ltda, Santa Maria, Brazil - 1135 mW/cm2).
From CF, SM, and ASE adhesive systems, only bond resin (Bond and B liquid, respectively) was used without needs evaporation solvent phase. Enamel (E) and dentin (D) specimens were obtained from 1.0-mm-thick longitudinal sections of one freshly extracted, human, caries-free, third molar obtained from a 22-year-old male patient. The tooth was autoclaved (20 min/121°C) and then a slice was cut using a slow speed diamond disc (KG Sorensen, Barueri, Brazil) and kept in tap water until use. A 99.95% pure aluminium (Al) 8-stepwedge with 1-mm per step was used as an internal radiographic standard and as a gauge to calculate the radiopacity of each material in terms of Al thickness. Gutta-percha (G) was used as positive control.
The acrylic mold with polymerized dentin adhesives and gutta-percha (positive control) in the holes, an Al step wedge and tooth slice, [Figure 1], were captured on ultraspeed occlusal film (Eastman Kodak Co., Rochester, United States of America). To certify that the acrylic mold would not interfere with the radiographic image of the materials, an initial radiographic image was taken with an occlusal film and the step wedge only. The films were exposed for 0.20 s, with total filtration equivalent to 3.81 mm of Al at 70 kV, 7 mA, and 400-mm target film distance with a Time x 70C dental X-ray unit (Gnatus Equipamentos Médicos Odontológicos Ltd., São Paulo, Brazil) and manually processed in fresh chemicals using temperature-time table. To assure that the incidence and the object-to-focus distance were standardized an acrylic device was used. This device maintained the head of the X-ray machine in the same position.
Figure 1.
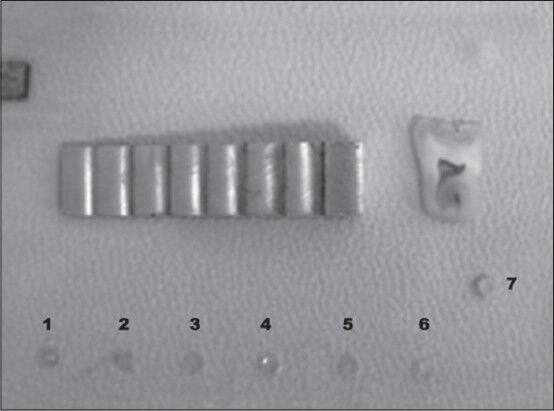
Sample discs of the bonding agents (at the bottom of the acrylic plate), from left to right: Xeno V (1), Magic Bond DE (2), Clearfil SE Bond (3), Adper SE Bond (4), Single Bond 2 (5), Scotchbond Multipurpose (6). Gutta-percha (7) in the mid-part on the right-hand side, aluminum step wedge, and tooth slice used for comparison
The radiographs [Figure 2] were digitized using a scanner with a transparent adapter and 600 dpi resolution (Astra 1220S, Umax, Taiwan, China). The images were saved as uncompressed TIFF files. All images were enlarged 2:1 in order to accurately define the areas to be measured. The digital images were analyzed using a computer graphics program (ImageJ, National Institutes of Health, Bethesda, United States of America). Three reference points at bonding agent, dentin, enamel, Al, and gutta-percha area of each sample were randomly chosen on the first radiograph and repeated for the other seven radiographs, and the optical density of each one was measured according to a scale of radiopacity also provided by the computer. The average gray scale values from 0 (black) to 255 (white) for selected regions of the image were measured using the histogram tool. The histogram provides numeric data in pixels for the optical density [OD] in the selected area, based on the 256 values of gray scale available in the system, providing the mean and standard deviation (SD). Care was taken to always measure in the same region of the radiographic images. Three OD readings were obtained from each image and the arithmetic mean calculated, corresponding to the density value of the item. One previously calibrated operator carried out all experimental procedures.
Figure 2.
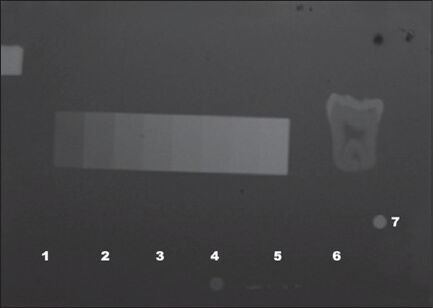
Radiographic image. Note that only one the adhesives, Adper SE Bond (4), can be clearly seen on the radiograph.
Data were statistically analyzed using one-way analysis of variance (ANOVA) and Tukey's test (SPSS version 17, SPSS Inc., Chicago, IL, USA) with level of significance set at 0.05.
RESULTS
The mean OD and SD of the bond agents, enamel, dentin, gutta-percha, and 1 mm of Al are shown in Table 1. Radiographically, five of the six bond agents tested presented a much lower OD than dentin, with a statistically significant difference (P < 0.05). The ANOVA showed statistically significant differences between the ASE bond agent and all the other groups tested (P = 0.000), as well as between XE and CF (P = 0.001). No statistical difference in radiodensity was observed between 1 mm of Al and 1 mm of dentin, between 2 mm of Al and 1 mm of enamel, and between 5 mm of Al and gutta-percha (P > 0.05). ASE showed the highest percentage radiodensity, higher than 1 mm of dentin (112.28%) and lower than 1 mm of enamel (85.27%). There was no statistically significant difference between XE and MB, SB and SM; and between CF and MB, SB and SM. Figure 3 summarizes the OD measurements of the tested adhesives, dentin, enamel, and gutta-percha, compared with the OD of 1 mm of Al.
Table 1.
Mean OD and standard deviation of all the groups tested
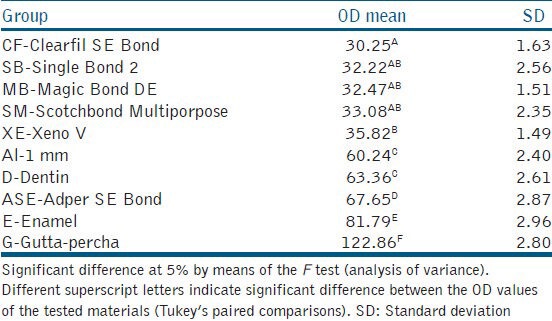
Figure 3.
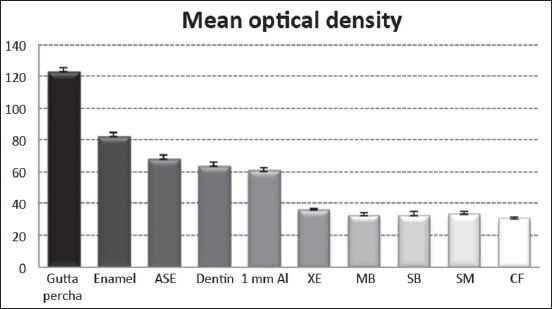
Radiopacity of the different materials tested in this study compared with 1 mm Al.
DISCUSSION
Radiopacity is one of the many desirable properties of most dental materials[9] and is an important characteristic in adhesive procedures in dentistry. There are several studies on the radiopacity of composites in the literature; however, few studies regarding comparing the radiopaque characteristics of bonding agents.[3,4,11]
In the present study, all bonding agents tested had a lower radiopacity than tooth enamel. Five bonding agents were considered radiolucent and only one (ASE) was considered radiopaque, with an OD higher than that of 1 mm of Al and 1 mm of dentin. The radiopacity of dental materials is usually expressed in terms of equivalent thickness in Al (mm). All restorative material should have a radiopacity equal to or higher than the radiographic density of dentin, which in turn has a radiopacity similar to that of Al. Although no maximum limit has been established, some authors consider that this limit must not exceed the radiopacity of enamel.[12,13]
In accordance with the International Standardization Organization,[14] the restorative material must be at least as radiopaque as the same thickness of pure Al. This means that a material specimen with same thickness would have a radiopacity roughly equal to dentin and so can it be considered adequate to be used in different clinical situations. In specification no. 27 of the American Dental Association,[15,16] it is also recommended that these materials should have a radiopacity equivalent to that of 1 mm of pure Al, which is approximately the same radiopacity as natural dentin.
The question of how the radiopacity of a restorative material should function in order to obtain the ideal diagnosis has been investigated by several authors.[12,17,18] Certain characteristics of the adhesive systems, such as application method and filler content, may affect radiographic interpretation.[5] When dental adhesives are applied in thin layers present a high risk on incomplete polymerization which might lead to a compromised restoration.[19] To the contrary, a thick adhesive layer how that is present on pooling of adhesives on the restoration corners, mainly on the gingival walls, is showed on radiography like radiolucent areas around the restorations and can lead to false replacement and non-replacement decisions.[9,20] On the other hand, a material with high radiopacity may difficult the diagnosis of incipient carious lesions by masking the image of the lesion, superimposing the image of the restorative material,[20] since the radiographic images of common use in dental practice are in two dimensions. To reach a correct diagnosis, a material with an ideal radiopacity is necessary.[9]
Bond agents, such as resins, with little or no inorganic content may have inadequate radiopacity, making it difficult to identify the limit between the cavity wall and the restorative material. This is especially important when the application of adhesive to the cavity surface has not resulted in a sufficiently thin pellicle (˜200 μm) before polymerization.[21] Even thinner pellicles (˜40 μm) can be detected radiographically[6] and if not radiopaque enough it can confused with marginal gaps, secondary caries, wear, and absence of restorative material.[6] In these cases, the interpretation of the radiographs and the decision on whether or not to remove a restoration are heavily dependent on the dentist's experience.[22]
Radiopacity of resin-based materials can be enhanced by adding the inorganic constituents, such as glass particles that contain atoms of heavy metals, such as titanium oxide (TiO2), yttrium, ytterbium, zirconium, boron silicate, barium or lithium Al silicate, zinc or strontium glass.[15,23,24,25] The great difficulty in formulating a radiopaque adhesive is the stability of the particles in the agent that will provide radiopacity, as the particles tend to agglomerate and precipitate, in addition to giving the adhesive a more opaque coloring.[23] This may make it difficult to activate the adhesive with visible light, and deeper layers of the adhesive may remain unpolymerized, compromising the bond.
Although the influence of particles on the physical and mechanical properties of dental adhesives has been studied extensively, these studies have generally used oxides with low radiopacity, such as silica, with low contrast between the dental adhesive and the teeth.[24,25] To allow marginal gaps to be distinguished from thick layers of dental adhesive, adhesive systems must contain particles with higher radiopacity than dentin.[8]
The radiopacity of adhesive systems depends on the presence of elements with a high atomic weight in the inorganic filler content.[3] According to the manufacturers, XE and SM bond agents do not contain components that provide radiodensity characteristics.
The adhesives with filler included in this study are characterized by the presence of silica, quartz, zirconium, and silicon dioxide. The use of elements with low atomic weight, such as quartz (SiO2) and silicon (Si), results in radiolucent materials.[4] This may explains radiolucent characteristic of CF, SB, and MB adhesives detected on this study.
On the contrary, barium (Ba), strontium (Sr), and zirconium (Zr) increase radiopacity due to their high atomic weight. ASE contains nanoparticles of zirconium (ZrO2), which gives it the recorded radiopacity. The scarce number of studies on the radiopacity of adhesive systems did not allow complete comparison of the results obtained; only three of the adhesives used in this research were included in previous studies.[3,4]
Pamir et al.,[5] recorded a high number of false-positives, which indicated the replacement of perfectly adapted restorations because a radiographic image suggested a lack of restorative material or secondary caries when SB and CF adhesives were used. In this study, it was found that these two adhesives were radiolucent and this is possibly due to the low percentage of load and the nature of the filler particles (silica), which led to low radiographic contrast.
Radiopacity measured by the pixel intensity of the adhesive systems in radiographic images was not capable of detecting differences between CF, SB, MB, XE, and SM adhesives. The radiopacity of these adhesive systems is insufficient to allow them to be distinguished from dentin and visible in the radiograph.
Detection of secondary caries is not an easy task. To differentiate these lesions from a lack of material under a restoration, two conditions that are radiographically perceived as radiolucent areas, is extremely difficult and may lead to restorations being replaced due to false diagnoses because the interpretation from the radiographic findings is not compatible with the real situation. The use of radiopaque adhesive systems could increase the number of correct decisions about resin composite restoration replacement.
CONCLUSION
This investigation demonstrates that of the six bond agents tested, only Adper™ SE Bond presented a radiopacity within the values of dentin and enamel. CF, XE, MB, SB, and SM bond agents are all radiolucent and require alterations to their composition to facilitate their detection by means of radiographic images.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mount GJ. A new paradigm for operative dentistry. Aust Dent J. 2007;52:264–70. doi: 10.1111/j.1834-7819.2007.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003;28:215–35. [PubMed] [Google Scholar]
- 3.Hotta M, Yamamoto K. Comparative radiopacity of bonding agents. J Adhes Dent. 2009;11:207–12. [PubMed] [Google Scholar]
- 4.Oztas B, Kursun S, Dinc G, Kamburoglu K. Radiopacity evaluation of composite restorative resins and bonding agents using digital and film x-ray systems. Eur J Dent. 2012;6:115–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Pamir T, Kaya AD, Baksi BG, Sen BH, Boyacioglu H. The influence of bonding agents on the decision to replace composite restorations. Oper Dent. 2010;35:572–8. doi: 10.2341/10-097-L. [DOI] [PubMed] [Google Scholar]
- 6.Kurşun S, Dinc G, Oztaş B, Yuksel S, Kamburoğlu K. The visibility of secondary caries under bonding agents with two different imaging modalities. Dent Mater J. 2012;31:975–9. doi: 10.4012/dmj.2012-062. [DOI] [PubMed] [Google Scholar]
- 7.Espelid I, Tveit AB, Erickson RL, Keck SC, Glasspoole EA. Radiopacity of restorations and detection of secondary caries. Dent Mater. 1991;7:114–7. doi: 10.1016/0109-5641(91)90056-5. [DOI] [PubMed] [Google Scholar]
- 8.Shulz H, Schimmoeller B, Pratsinis SE, Salz U, Bock T. Radiopaque dental adhesives: Dispersion of flame-made Ta2O5/SiO2 nanoparticles in methacrylic matrices. J Dent. 2008;36:579–87. doi: 10.1016/j.jdent.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Collares FM, Leitune VC, Ogliari FA, Piva E, Fontanella VR, Samuel SM. Influence of the composition of an experimental adhesive on conversion kinetics, flexural strength and radiodensity. Rev Odonto Ciênc. 2009;24:414–9. [Google Scholar]
- 10.Krejci I, Lutz F, Sener B, Jenss J. The x-ray opacity of tooth-coloring inlay materials and composite cements. Schweiz Monatsschr Zahnmed. 1991;101:299–304. [PubMed] [Google Scholar]
- 11.Bueno M, Moraes RR, Azevedo EC, Baldissera RA. Radiographic view of adhesive layer and relationship with marginal leakage in class II composite resin restorations. Arq Odontol. 2007;43:149–54. [Google Scholar]
- 12.Salzedas LM, Louzada MJ, de Oliveira Filho AB. Radiopacity of restorative materials using digital images. J Appl Oral Sci. 2006;14:147–52. doi: 10.1590/S1678-77572006000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devito KL, Ortega AI, Haiter-Neto F. Radiopacity of calcium hydroxide cement compared with human tooth structure. J Appl Oral Sci. 2004;12:290–3. doi: 10.1590/s1678-77572004000400007. [DOI] [PubMed] [Google Scholar]
- 14.Geneva, Switzerland: International Organization for Standardization; 2000. International Organization for Standardization ISO Standard 4049: Dentistry Resin-based Filling Materials 7.11.1-4. [Google Scholar]
- 15.The desirability of using radiopaque plastics in dentistry: A status report. Council on Dental Materials, Instruments, and Equipment. J Am Dent Assoc. 1981;102:347–9. doi: 10.14219/jada.archive.1981.0058. [DOI] [PubMed] [Google Scholar]
- 16.Phillips RW, Lutz F. Status report on posterior composites. Council on Dental Materials, Instruments, and Equipment. J Am Dent Assoc. 1983;107:74–6. [PubMed] [Google Scholar]
- 17.Ergüçü Z, Türkün LS, Onem E, Güneri P. Comparative radiopacity of six flowable resin composites. Oper Dent. 2010;35:436–40. doi: 10.2341/09-340-L. [DOI] [PubMed] [Google Scholar]
- 18.Jandt KD, Al-Jasser AM, Al-Ateeq K, Vowles RW, Allen GC. Mechanical properties and radiopacity of experimental glass-silica-metal hybrid composites. Dent Mater. 2002;18:429–35. doi: 10.1016/s0109-5641(01)00064-1. [DOI] [PubMed] [Google Scholar]
- 19.Lin NJ, Bailey LO, Becker ML, Washburn NR, Henderson LA. Macrophage response to methacrylate conversion using a gradient approach. Acta Biomater. 2007;3:163–73. doi: 10.1016/j.actbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Goshima T, Goshima Y. Radiographic detection of recurrent carious lesions associated with composite restorations. Oral Surg Oral Med Oral Pathol. 1990;70:236–9. doi: 10.1016/0030-4220(90)90126-d. [DOI] [PubMed] [Google Scholar]
- 21.Akinmade AO, Nicholson JW. Effect of adhesive layer thickness on the bond strength of a zinc polycarboxylate dental cement. Biomaterials. 1995;16:149–54. doi: 10.1016/0142-9612(95)98279-n. [DOI] [PubMed] [Google Scholar]
- 22.Hewlett ER, Atchison KA, White SC, Flack V. Radiographic secondary caries prevalence in teeth with clinically defective restorations. J Dent Res. 1993;72:1604–8. doi: 10.1177/00220345930720121301. [DOI] [PubMed] [Google Scholar]
- 23.Klapdohr S, Moszner N. New inorganic components for dental filling composites. Monatshefte Für Chemie. 2005;136:21–45. [Google Scholar]
- 24.Lee YK, Pinzon LM, O’Keefe KL, Powers JM. Effect of filler addition on the bonding parameters of dentin bonding adhesives bonded to human dentin. Am J Dent. 2006;19:23–7. [PubMed] [Google Scholar]
- 25.Tay FR, Moulding KM, Pashley DH. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999;1:103–17. [PubMed] [Google Scholar]


