Abstract
Purpose:
The present study was aimed to evaluate and compare the color stability of two hybrid tooth-colored restorative materials, namely, resin-modified glass ionomer cement (GC Fuji II LC Capsules - GC Corporation, Tokyo, Japan) and giomer (Beautifil II - Shofu Inc, Kyoto, Japan) when subjected to immersion in various children's beverages.
Materials and Methods:
Standardized disc specimens were prepared using the test restorative materials. After preparation and rehydration of the specimens, baseline color evaluations were performed using spectrophotometer. The readings were recorded according to CIELAB color space. The experimental groups were further subdivided for immersion in orange juice, bournvita milk, and coke. Subsequent to immersion and pH cycling, new color evaluations were carried out after 1 week and 4 weeks for all the experimental groups. The mean color change values were calculated.
Results:
The obtained data was subjected to statistical analysis. The results indicated that giomer specimens exhibited less color change as compared to RMGIC specimens indicating better color stability. The maximum color changes were found with the use of coke for a period of 4 weeks.
Conclusion:
Amongst the two materials, giomer showed less color changes as compared to RMGIC indicating a better color stability.
Keywords: Beverages, color, Fuji II LC, resin composites
INTRODUCTION
The success of restorative dentistry is determined on the basis of functional results and esthetic outcomes. The constant evolution of restorative materials and techniques has made possible the achievement of optimal esthetics.[1] Currently, fluoride-releasing esthetic restorative materials have been extensively used due to their caries preventive effect. However, the color stability of these restorative materials has been a challenge to dentistry, as the oral cavity has a dynamic environment.
Due to the constant presence of oral microflora, saliva and the frequent intake of food, the color stability of an esthetic material like composite resin may be hampered. Despite its acceptance in satisfying esthetic demands, previous studies have revealed that composite resins are susceptible to various degrees of discolouration which may be attributed to intrinsic or extrinsic factors.[2,3,4,5] Intrinsic factors involve alterations or changes in the chemical structure of the composite resins under physical and chemical conditions, while extrinsic factors are mainly due to surface staining from absorption or adsorption of exogenous substances.[6] It is reported that matrix, filler composition and content, minor pigment addition, initiation components and filler coupling agents also affect color of esthetic materials.[7]
The visible color alterations in esthetic materials can also be ascribed to the proprietary differences in chemistry that may affect the polymerization, water sorption, and consequently the color stability of the material. In addition, the obvious effect of colorants in beverages and foods leads to extrinsic discoloration of composites. Thus, for suitable performance, longevity and good clinical success of esthetic restorations, the material of choice should present adequate inherent characteristics. However, the property of color stability of esthetic dental materials is often ignored over other physical and mechanical properties while making a choice. The ideal composite resin formulations that may permit an optimal esthetic outcome without compromising the mechanical properties essential for a suitable functional outcome are nevertheless under exploration. It is imperative that an ideal anterior restorative material should exhibit adequate esthetics as a function of color stability in addition to other properties such as strength and biocompatibility and at the same time aid in prevention of secondary caries formation.
The conventional glass ionomer cement (GIC) is a fluoride-containing restorative material with non-satisfactory esthetics. Thus, different hybrid restorative materials such as high-viscosity GIC, polyacid-modified resin composite, giomers, and resin-modified glass ionomer cement (RMGIC) have been developed to improve some physical properties of the materials, including the esthetics.[8] However, there is limited information regarding the color stability of these hybrid restoratives. Thus, the aim of the present study was to evaluate the color stability of two hybrid esthetic restorative materials, when subjected to immersion in various children's beverages.
MATERIALS AND METHODS
The test restorative materials used in the study were resin-modified glass ionomer cement (GC Fuji II LC Capsules-GC Corporation, Tokyo, Japan) and giomer (Beautifil II-Shofu Inc, Kyoto, Japan). For preparation of the test specimens, a custom-made Teflon ring mould was used. Thirty-six standardized specimens were prepared for each group. The capsules of RMGIC were activated and mixed in amalgamator (Gnatus Amalga mix 2) for 10 seconds according to manufacturer's instructions. The specimens were made by placing the respective materials into the mould over a glass slab sandwiched between two mylar strips using GC applier; the smoothest surfaces were obtained by curing the materials against the mylar strip. The material was light-cured for 40 seconds on each side (Henry Schein's Maxima® LED unit). Similarly, giomer specimens were prepared by incremental insertion into the mould with plastic instruments followed by sandwiching between two mylar strips and light-curing for 40 seconds on each side.
After preparation, the specimens were kept in distilled water for 24 hours to achieve rehydration. After rehydration, the samples were rinsed and dried with filter paper, and the baseline color measurements were performed using spectrophotometer (Gretag Macbeth Color-Eye® 7000A). Color evaluations were made with color parameters based on average daylight (D65: 6504 K) and illuminating view geometry d/10. Calibration was made using a white standard. Individual specimens were placed on aperture, and readings were recorded according to Commission Internationale de l’Eclairege L*a*b* color space (CIELAB).
After baseline evaluation, the above groups were further divided into 6 experimental subgroups for immersion in 3 children's beverages, namely, orange juice (PepsiCo, India), bournvita milk (Kraft Foods, Cadbury India), and coke (The Coca-Cola Company, Atlanta, GA). For each subgroup, 12 disc specimens were used. The bournvita milk was prepared using 15 g of bournvita poured into 500 mL of unsweetened hot milk and filtered after 10 minutes.
The specimens were subjected to pH-cycling by daily immersion in demineralizing solution for 7.5 hours and in a remineralizing solution for 16 hours. The demineralizing solution was prepared using 2.2 mM CaCl2, 2.2 mM NaH2PO4, and 50 mM acetic acid adjusted to pH 4.8. The remineralizing solution was prepared using 1.5 mM CaCl2, 0.9 mM NaH2PO4, and 0.15 M KCl adjusted to pH 7.[9] These solutions served as storage medium for the specimens so as to mimic the oral environment.
The specimens were removed daily from the pH cycling solutions and immersed in respective beverages for 30 minutes. The experiment was performed for a period of 4 weeks. Subsequent to immersion and pH cycling, new color evaluations were carried out after 1 week (T1) and 4 weeks (T4) for all the experimental groups. L*a*b* values of each specimen were measured 3 times by placing each specimen on the measuring head. The values of dL* da* and db* after 3 measurements were automatically calculated by spectrophotometer and recorded. Resistance to staining effect was expressed in delta E unit and calculated from the mean dL*da* and db* values for each specimens with the following formula,[10] Equation: delta E = [(dL*)2+ (da*)2+ (db*)2]1⁄2
The data generated during the study was processed with the aid of PASW 18.0 Software (formerly known as SPSS). Descriptive statistics such as mean and standard deviation were determined. The comparative evaluation of color change (dE) as a function of different beverages was obtained using one-way analysis of variance (ANOVA). Multiple range test by Tukey-HSD procedure and paired ‘t’ test was employed to identify the significant groups at 5% level.
RESULTS
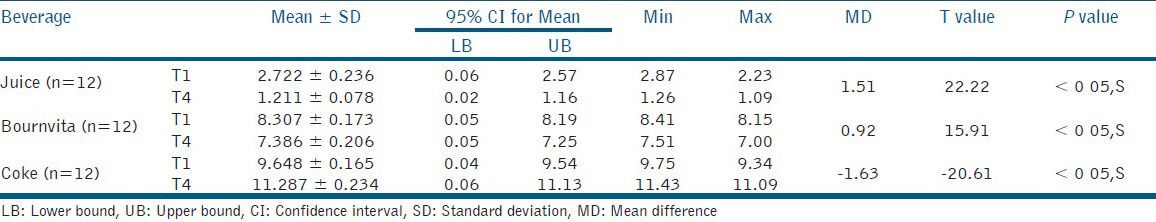
The statistical analysis indicated that the dE of bournvita group was significantly (P < 0.05) lesser than that observed with orange juice and coke groups at both time periods, i.e. T1 and T4 with RMGIC. The dE of orange juice group was significantly (P < 0.05) lesser than that observed with bournvita and coke groups at both time periods, i.e. T1 and T4 with giomer [Tables 1 and 2].
Table 1.
Mean color change (dE) recorded as a function of use of different beverages using RMGIC after 1 week (T1) and 4 weeks (T4)
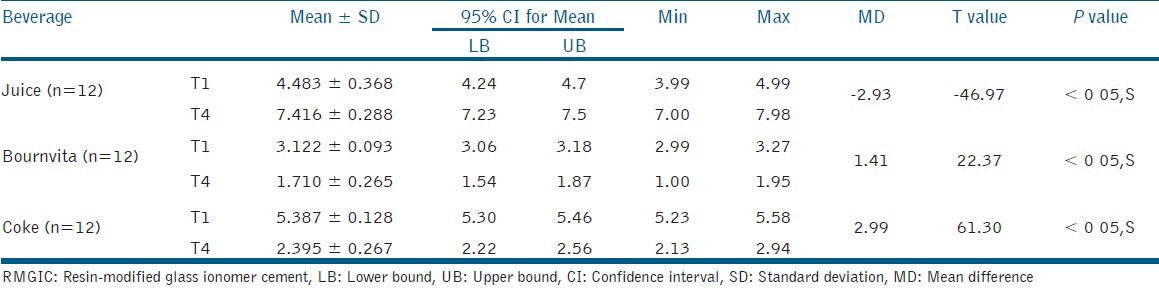
Table 2.
Mean color change (dE) recorded as a function of use of different beverages using Giomer after 1 week (T1) and 4 weeks (T4)
In RMGIC group, it was observed that there was a significant increase in mean color change after 4 weeks when compared to that observed after 1 week of immersion in orange juice. The maximum color change took place after 1 week of immersion in bournvita and coke. The color change gradually decreased over the period of 4 weeks with bournvita and coke [Table 1].
The results for giomer showed that the maximum color change took place after 1 week of immersion in orange juice and bournvita. The color change gradually decreased over the period of 4 weeks with orange juice and bournvita. There was a significant increase in mean color change after 4 weeks when compared to that observed after 1 week of immersion in coke [Table 2].
It could be concluded on the basis of statistical analysis of the data that comparatively, giomer indicates a better color stability than that exhibited with material RMGIC [Graph 1].
Graph 1.
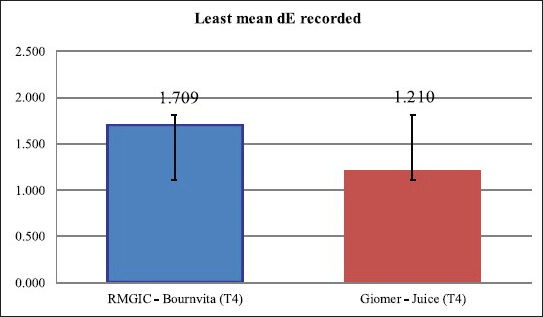
Comparative assessment of the least mean color change (dE) obtained with RMGIC and Giomer according to paired ‘t’ test
DISCUSSION
The discoloration of tooth-colored, resin-based materials may be caused by intrinsic or extrinsic factors.[11] Visual or instrumental techniques can be used to evaluate discoloration. The use of instrumental methods like spectrophotometers and colorimeters to quantify tooth color could potentially eliminate the subjective aspects of color assessment.[12] The American Dental Association recommends the use of the CIELAB color differential system in assessing chromatic differences.[13] In the present study, the color evaluations were made using a reflectance spectrophotometer using the CIELAB color system. This system is a useful mode that provides information about location of object color in a uniform 3-D color space. It quantifies the color in terms of 3 co-ordinate values L*, a*, and b*. Here, L* represents brightness or lightness (value), and a* and b* serve as numeric correlates both for hue and chroma. The a* and b* values represent position on a red/green and yellow/blue axis, respectively. The magnitude of the color difference (delta E) perceived between two objects is thus calculated. The higher the delta E, the greater the difference between the two samples being compared. A delta E value of 3.3 or less is considered to be clinically acceptable in dentistry.[14,15]
The results of the present study showed that giomer exhibited greater stain resistance as compared to RMGIC at both time periods with respect to all of the tested beverages. This difference in the color stability of the two materials tested could be attributed to the differences in various factors as discussed further. The oral environment is exposed to a variety of media on a daily basis, many of which may stain or alter the surfaces of dental restorations, potentially causing esthetic degradation. Staining of oral tissues and restorations is known to be affected by dietary factors. While many studies exist on the staining effects of beverages on tooth-colored restorations, the beverages used in most of these studies were coffee, tea, and wine, which are normally associated with adult tooth stains.[16,17,18] A few studies have evaluated the effects of common beverages ingested by children and their staining effects on restorative dental materials,[19,20] but studies on fluoride-releasing restorative materials have not been previously reported. Consumption of soft drinks is known to have increased in recent years, and is especially high among younger individuals. Other drinks that are frequently consumed by children include fruit juices and milk. The effect of cola on the color stability of restorative materials has been evaluated;[20] however, the effects of chocolate milk and orange juice have not been reported. Therefore, the present study measured the color stability of RMGIC and giomer after exposure to various children's drinks. Also, the choice of the beverages in the present study attempted to represent diverse areas of the color spectrum. There are differences in consistency also as juice is a thin, watery solution, bournvita milk is sugary, and Coca-Cola is carbonated. Additionally, the beverages show varying pH like 3.0 (cola), 3.88 (orange juice), and 6.9 (bournvita milk). In the present study, although color change was observed maximum in 1 week, it gradually increased from 1 week to 4 weeks with cola samples. This could be attributed to low pH of cola, which may lead to increased surface roughness.
In the present study, a pH cycling process has been used in order to simulate the oral environment. However, it has been previously reported that fluoride-releasing materials have a greater ion release when submitted to pH variations than when stored in artificial saliva or saline.[21] These facts could lead to lower color stability evident in the present study due to the great amount of ionic changes between the materials and the environmental solutions.
Staining susceptibility of resin-based materials might be attributed to their degree of water sorption and hydrophilicity of matrix resin, that is, if the resin composite can absorb water, it can also absorb other fluids like tea and coffee.[22] The glass filler particles will not absorb water into the bulk of the material; however, can absorb water onto the surface. In the literature, hydrophobic materials like resin composites are believed to exhibit greater stain resistance and color stability than hydrophilic materials like glass ionomer cements and compomers.[23] The results of the current study also add support to this conclusion. It is reported that most of the water sorption with resin-based materials was observed during the first week. Chan et al.[24] investigated the staining potential of coffee, tea, cola, and soy sauce on two different resin composites and reported that staining after 1 week of immersion differed significantly from all succeeding weeks, and the greatest amount of discoloration occurred during the first week, which is in agreement to the results obtained in the present study.
The color stability is also related to the resin matrix, dimensions of filler particles, depth of polymerization, and staining agents. Among the materials tested, a more noticeable color change was observed with all samples of RMGIC. It has been previously reported that glass ionomer cements lack color stability due to the polyacid content of the material and can be explained by the degradation of metal polyacrylate salts.[25] Giomer showed less color change when compared with RMGIC. This may be attributed to the amount of resin and filler particles present in the giomer. RMGIC is a hybrid material with a mean filler size range of 1.8 μm, with filler loading 76 wt.% by volume. Giomer is a bridge product; with filler loading 83.3 wt.% and a particle size of 0.8 μm.[26] The composition and size of the filler particles affect the surface smoothness and the susceptibility for extrinsic staining. Therefore, it can be expected that giomer, with a smaller particle size, will have a smoother surface and will retain less surface stains than rough surfaces. Moreover, it is reported that giomer consists of additional discrete nano-fillers (10-20 nm), which makes it possible to incorporate larger filler content of 68.6 vol%. In other words, it has a lower resin content compared to RMGIC. This may lead to a better polishability and surface finish and subsequently, more resistance to staining with giomer.
CONCLUSION
Based on the results of the present color stability evaluation, it can be stated that giomer material showed greater resistance to staining as compared to RMGIC at all time periods.
ACKNOWLEDGMENT
The authors wish to thank Spectra Colour Tech India Private Limited, Mumbai, Maharashtra for providing methodological aid and technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sikri VK. Color: Implications in dentistry. J Conserv Dent. 2010;13:249–55. doi: 10.4103/0972-0707.73381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke FJ, Watts DC, Wilson NH, Wilson MA. Current status and rationale for composite inlays and onlays. Br Dent J. 1991;170:269–73. doi: 10.1038/sj.bdj.4807506. [DOI] [PubMed] [Google Scholar]
- 3.Burke FJ, Wilson NH, Watts DC. Aesthetic inlays. Br Dent J. 1994;177:198–201. doi: 10.1038/sj.bdj.4808551. [DOI] [PubMed] [Google Scholar]
- 4.Tyas MJ. Colour stability of composite resins: a clinical comparison. Aust Dent J. 1992;37:88–90. doi: 10.1111/j.1834-7819.1992.tb03042.x. [DOI] [PubMed] [Google Scholar]
- 5.Inokoshi S, Burrow MF, Kataumi M, Yamada T, Takatsu T. Opacity and color changes of tooth-colored restorative materials. Oper Dent. 1996;21:73–80. [PubMed] [Google Scholar]
- 6.Horsted-Bindslev P, Mjor IA. Copenhagen: Munksgaard International Publishers; 1989. Modern Concepts in Operative Dentistry. [Google Scholar]
- 7.Ergücü Z, Türkün LS, Aladag A. Color stability of nano-composites polished with one-step system. Oper Dent. 2008;33:413–20. doi: 10.2341/07-107. [DOI] [PubMed] [Google Scholar]
- 8.Arora V, Kundabala M, Parolia A, Thomas MS, Pai V. Comparison of the shear bond strength of RMGIC to a resin composite using different adhesive systems: An in vitro study. J Conserv Dent. 2010;13:80–3. doi: 10.4103/0972-0707.66716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imparato JC, Garcia A, Bonifacio CC, Scheidt L, Raggio DP, Mendes FM, et al. Color stability of esthetic ion-releasing restorative materials subjected to pH variations. J Dent Child (Chic) 2007;74:189–93. [PubMed] [Google Scholar]
- 10.Gupta G, Gupta T. Evaluation of the effect of various beverages and food material on the color stability of provisional materials - An in vitro study. J Conserv Dent. 2011;14:287–92. doi: 10.4103/0972-0707.85818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satou N, Khan AM, Matsumae I, Satou J, Shintani H. In vitro color change of composite- based resins. Dent Mater. 1989;5:384–7. doi: 10.1016/0109-5641(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 12.Seghi RR, Hewlett ER, Kim J. Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J Dent Res. 1989;68:1760–4. doi: 10.1177/00220345890680120801. [DOI] [PubMed] [Google Scholar]
- 13.Council on dental materials and devices. Revised American Dental Association specification No 12 for denture base polymers. J Am Dent Assoc. 1975;90:451–8. doi: 10.14219/jada.archive.1975.0069. [DOI] [PubMed] [Google Scholar]
- 14.Ruyter IE, Nilner K, Moller B. Color stability of dental composite resin materials for crown and bridge veneers. Dent Mater. 1987;3:246–51. doi: 10.1016/S0109-5641(87)80081-7. [DOI] [PubMed] [Google Scholar]
- 15.Vichi A, Ferrari M, Davidson CL. Color and opacity variations in three different resin-based composite products after water aging. Dent Mater. 2004;20:530–4. doi: 10.1016/j.dental.2002.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Stober T, Gilde H, Lenz P. Color stability of highly filled composite resin materials for facings. Dent Mater. 2001;17:87–94. doi: 10.1016/s0109-5641(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 17.Guler AU, Yilmaz F, Kulunk T, Guler E, Kurt S. Effects of different drinks on stainability of resin composite provisional restorative materials. J Prosthet Dent. 2005;94:118–24. doi: 10.1016/j.prosdent.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Fujita M, Kawakami S, Noda M, Sano H. Color change of newly developed esthetic restorative material immersed in food-simulating solutions. Dent Mater J. 2006;25:352–9. doi: 10.4012/dmj.25.352. [DOI] [PubMed] [Google Scholar]
- 19.Curtin JA, Lu H, Milledge JT, Hong L, Peterson J. In Vitro Staining of Resin Composites by Liquids Ingested by Children. Pediatr Dent. 2008;30:317–22. [PubMed] [Google Scholar]
- 20.Tunc SE, Bayrak S, Guler UA, Tulogu N. The effects of children's drinks on the color stability of various restorative materials. J Clin Pediatr Dent. 2009;34:147–50. doi: 10.17796/jcpd.34.2.953q255621436788. [DOI] [PubMed] [Google Scholar]
- 21.Ren YF, Feng L, Serban D, Malmstrom HS. Effects of common beverage colorants on color stability of dental composite resins: The utility of a thermocycling stain challenge model in vitro. J Dent. 2012;40(Suppl 1):e48–56. doi: 10.1016/j.jdent.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri R, Burrow MF, Tyas M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J Dent. 2005;33:389–98. doi: 10.1016/j.jdent.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Mutlu-Sagesen L, Ergün G, Ozkan Y, Semiz M. Color stability of a dental composite after immersion in various media. Dent Mater J. 2005;24:382–90. doi: 10.4012/dmj.24.382. [DOI] [PubMed] [Google Scholar]
- 24.Chan KC, Fuller JL, Hormati AA. The ability of foods to stain two composite resins. J Prosthet Dent. 1980;43:542–5. doi: 10.1016/0022-3913(80)90328-5. [DOI] [PubMed] [Google Scholar]
- 25.Hse KM, Leung SK, Wei SH. Resin-ionomer restorative materials for children: A review. Aust Dent J. 1999;44:1–11. doi: 10.1111/j.1834-7819.1999.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 26.Toledano M, Osorio R, Osorio E, Fuentes V, Prati C, Garcia-Godoy F. Sorption and solubility of resin-based restorative dental materials. J Dent. 2003;31:43–50. doi: 10.1016/s0300-5712(02)00083-0. [DOI] [PubMed] [Google Scholar]


