Abstract
Background
Parkinson's disease (PD) is a late-life neurodegenerative disease. Genetic and environmental factors play an etiological role. Harmane (1-methyl-9H-pyrido[3,4-b]indole) is a potent tremor-producing neurotoxin that shows structural resemblance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Objectives
In 2002 and 2007, we demonstrated elevated blood harmane concentrations [HA] in essential tremor (ET) cases. We now assessed whether blood [HA] were elevated in Parkinson's disease (PD) as well.
Methods
Blood [HA] were quantified by high performance liquid chromatography. Subjects comprised 113 PD cases and 101 controls.
Results
Mean log blood [HA] in PD cases was double that of controls (0.59 ± 0.63 g −10/ml vs. 0.27 ± 0.63 g−10/ml, p <0.001). A non-parametric test on non-transformed data (median blood [HA] = 3.31 g −10/ml in cases and 1.44 g −10/ml in controls) also showed this difference (p <0.001). In unadjusted and then adjusted logistic regression analyses, log blood [HA] was associated with PD (odds ratio [OR]unadjusted 2.31, 95% confidence interval [CI] 1.46 – 3.67, p <0.001; ORadjusted 2.54, 95% CI 1.55 – 4.16, p <0.001). In PD, log blood [HA] co-varied with family history, being lowest in PD cases with no family history (0.54 ± 0.60 g−10/ml) and highest in PD cases with a family history of both ET and PD (0.84 ± 0.68 g−10/ml)(p = 0.06).
Conclusions
Blood harmane appears to be elevated in PD. The finding needs to be reproduced in additional cohorts to assess its generalizability. The higher concentration in familial PD suggests that the mechanism may involve genetic factors.
Keywords: blood harmane concentration, Parkinson's disease
1. Introduction
β-carboline alkaloids are neurotoxins that produce tremor (Louis et al., 2013). Laboratory animals injected with high doses acutely exhibit action tremor (Fuentes and Longo, 1971; Zetler et al., 1972), and human volunteers exposed to high doses display coarse action tremor (Lewin, 1928).
Harmane (1-methyl-9H-pyrido[3,4-b]indole) is among the most potent tremor-producing β-carboline alkaloids (McKenna, 1996), is very lipid soluble (Zetler et al., 1972), and broadly distributed within the rat brain in experimental settings (Anderson et al., 2006; Matsubara et al., 1993; Moncrieff, 1989). Although harmane is produced endogenously, it is also present in the diet (particularly in meat but also in vegetables); indeed, exogenous exposure is thought to be the main source of bodily harmane (Pfau and Skog, 2004). In several studies, we demonstrated that blood harmane concentration ([HA]) was elevated in essential tremor (ET) patients compared with controls (Louis et al., 2002; Louis et al., 2008; Louis et al., 2013).
The contribution of environmental risk factors to disease etiology has been examined in epidemiological studies of ET as well as other tremor disorders, and especially Parkinson's disease (PD) or parkinsonism (Baldereschi et al., 2008; Gatto et al., 2009; Racette et al., 2001; Ritz and Yu, 2000; Rybicki et al., 1993; Semchuk et al., 1992; van der Mark et al., 2012). More specifically, identification of toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), pesticides and manganese as precipitants of PD has advanced our understanding of putative disease mechanisms; for example, the MPTP model has become the most commonly used experimental model for PD (Langston et al., 1984). Interestingly, β-carboline alkaloids, like harmane, show structural resemblance to MPTP (Kuhn et al., 1995), with the major difference being the presence of a single indole nitrogen bridge in harmane (Kuhn et al., 1996). On a functional level, β-carboline alkaloids are comparable to MPTP as inhibitors of mitochondrial respiration (Albores et al., 1990; Kuhn et al., 1995). These compounds are also similar structurally to paraquat, a pesticide linked with PD (Dick, 2006). We hypothesized that changes in harmane exposure or metabolism could be linked with PD, and in this study, measured blood [HA] in PD cases and controls.
2. Materials and Methods
2.1 Participants
Recruitment began in 2009 and has continued to present (October 2013). PD cases were enrolled in a study of the environmental epidemiology of ET and other tremor disorders at Columbia-University Medical Center (CUMC). By design, PD cases were identified from a computerized billing database at the Center for Parkinson's Disease and Other Movement Disorders at the Neurological Institute of New York, CUMC; selection was based on date of appointment, with more recent cases being selected first. All cases had received a diagnosis of PD from their treating neurologist at the Institute and lived within two hours driving distance of CUMC. One of the authors (E.D.L.) reviewed the office records of all selected PD patients; patients with diagnoses or physical signs of dystonia, atypical parkinsonian features, or spinocerebellar ataxia were excluded, and the diagnosis of PD was confirmed using published diagnostic criteria (Hughes et al., 1992). At the time of the chart review, the following data were abstracted: most recent Unified Parkinson's Disease Rating Scale (UPDRS) score (Fahn et al., 1987), Hoehn & Yahr score (Hoehn and Yahr, 1967), and levodopa dosage (mg/day). Based on UPDRS scores, PD cases were classified into motor subtypes based on previously described methodology: tremor predominant (TP) PD or postural instability gait disorder (PIGD) PD (Jankovic et al., 1990).
Control subjects were recruited for the same study during an overlapping time period. Controls were identified using random digit telephone dialing within a defined set of telephone area codes that were represented by neurological cases (e.g., 212, 201, 203, 516, 718, 914) within the New York Metropolitan area. Controls were frequency-matched to cases based on current age (5 year intervals); the ratio of controls to cases was ~1:1.
The CUMC Internal Review Board approved of all study procedures; written informed consent was obtained upon enrollment.
2.2 Clinical Evaluation
All cases and controls were evaluated in person by a trained tester who administered clinical questionnaires. Most evaluations were performed in the late morning or early afternoon, making fasting blood [HA] impractical. Data suggest that plasma [HA] do not change significantly during the day (Rommelspacher et al., 1991). In one study (Rommelspacher et al., 1991), human subjects ingested food or ethanol, and plasma [HA] were measured hourly for eight hours. The concentration remained stable. The same investigators also demonstrated that variability in concentration was minimal over a longer (three week) period (Rommelspacher et al., 1991). Our own data indicate that log blood [HA] is not correlated with the time latency since last food consumption (Louis et al., 2011).
The tester collected demographic and clinical information (including duration of symptoms in PD cases). A structured questionnaire was used to collect family history information. Current smoking status was assessed in each subject. Medical co-morbidity was assessed with the Cumulative Illness Rating Scale, in which the severity of medical problems (0 [none] - 3 [severe]) was rated in 14 bodily systems (e.g., cardiac, hepatic) and a Cumulative Illness Rating Scale score was assigned (range = 0 – 42) to each subject (Linn et al., 1968).The names of all current medications were recorded and then collapsed into 19 classes of medications (e.g., PD medications, neuroleptic medications, cardiac medications, etc).
Weight and height were assessed and body mass index was calculated (weight in kg divided by the square of height in meters).
2.3 Blood [HA]
After phlebotomy, blood [HA] was quantified, blinded to demographic, clinical and diagnostic information, by a well-established high performance liquid chromatography method at Purdue University used in our previous studies (Louis et al., 2002; Louis et al., 2008; Zheng et al., 2000).
2.4 Statistical Analyses
Chi-square (χ2) tests were used to analyze proportions, and Student's t tests were used to examine group differences in continuous variables. Pearson's correlation coefficients were used to assess correlations between continuous variables; when variables were not normally distributed, Spearman's correlation coefficient was used.
The empirical distribution of harmane is positively skewed. Using a one-sample Kolmogorov-Smirnov test, we tested whether [HA] was normally distributed and it was not (Kolmogorov-Smirnov test, z = 6.55, p < 0.001). Therefore, [HA] were logarithmically transformed; and after log transformation were normally distributed (Kolmogorov-Smirnov test, z = 1.06, p = 0.21). Case-control differences in log blood [HA] were assessed using Student's t tests. In additional analyses, a non-parametric (Mann Whitney U) test also was performed on harmane data that were not logarithmically transformed.
Log blood [HA] were also stratified as above the median and below the median of the controls.
To assess the null hypothesis that blood [HA] was not a predictor of diagnostic group (PD vs. control), logistic regression analysis was performed using diagnostic group as the outcome, and log blood [HA] as the primary independent variable. We considered a number of potential confounders (age in years, gender, race, years of education, body mass index, Cumulative Illness Rating scale score, current cigarette smoker, and medications [19 classes]) and included these in the adjusted logistic regression analyses if they were associated with either PD or blood [HA] in this dataset or if prior studies suggested a relationship. In some analyses, we tested for trend by treating log blood [HA] as the dependent variable in a linear regression analysis and examining the association with an ordinal independent variable (e.g., PD categories by family history). Positive family history was defined as one or more first or second degree relative with reported disease. The rationale for the trend analysis is that prior study in ET has shown that cases with a positive family history have the highest blood [HA] (Louis et al. 2008); hence, in this analysis, a family history of ET was considered of high a priori significance, and a family history of PD, of potential significance. Hence, we begin with controls, then PD cases with no family history, then PD cases with a family history of PD, PD cases with a family history of ET, and then those with a family history of both ET and PD. A priori, we expected that the latter group, with a family history of both disorders, might have the highest blood [HA].
Statistical analyses were performed in SPSS (Version 19.0).
3. Results
The 113 PD cases and 101 controls were similar in age, race, education, and other demographic and clinical factors (Table 1). The proportion of women was 43.4% in PD cases and 56.4% in controls, a difference that was marginally significant (p = 0.06). The median evaluation start time was 11:00 AM both for PD cases and controls (Mann Whitney = 1.02, p = 0.31). PD symptom duration, levodopa dosage, and Hoehn & Yahr score are shown (Table 1). Thirty-five (31.0%) PD cases were TP PD, 13 (11.5%) were PIGD PD, and the remainder (57.5%) were intermediate.
Table 1.
Demographic and Clinical Characteristics of 113 PD cases vs. 101 controls
| Characteristic | PD Cases (N = 113) | Controls (N = 101) | Significance |
|---|---|---|---|
|
| |||
| Age in years | 69.4 ± 7.8 | 71.0 ± 9.1 | t = 1.44, p = 0.15 |
|
| |||
| Female gender | 49 (43.4) | 57 (56.4) | X2 = 3.65, p = 0.06 |
|
| |||
| Non-Hispanic white race | 104 (92.0) | 88 (87.1) | X2 = 1.39, p = 0.24 |
|
| |||
| Education in years | 16.5 ± 2.9 | 16.1 ± 2.4 | t = 1.19, p = 0.24 |
|
| |||
| Body mass index kg/m2 | 25.0 ± 5.6 | 25.4 ± 5.6 | t = 0.45, p = 0.66 |
|
| |||
| Current cigarette smoker | 3 (2.7) | 4 (4.0) | X2 = 0.29, p = 0.59 |
|
| |||
| Cumulative Illness Rating Scale score | 7.0 ± 3.6 | 6.6 ± 3.9 | t = 0.75, p = 0.45 |
|
| |||
| Duration of PD symptoms in years | 7.5 ± 5.4 | NA | NA |
|
| |||
| Levodopa dosage (mg/day) | 495.4 ± 365.8 | NA | NA |
|
| |||
| Hoehn and Yahr Score a | NA | NA | |
| I | 29 (41.4) | ||
| II | 36 (51.4) | ||
| III | 3 (4.3) | ||
| IV or higher | 2 (2.9) | ||
Values are mean ± SD or numbers (percentages).
NA = not applicable.
Missing data on some PD cases.
Using controls, we examined the correlates of log blood [HA]. Log blood [HA] was not associated with age in years (r = 0.13, p = 0.21), education (r = −0.13, p = 0.20), body mass index (r = 0.08, p = 0.45) or Cumulative Illness Rating Score (r = 0.07, p = 0.48). Log blood [HA] did not differ by gender (0.27 ± 0.70 g −10/ml for men and 0.27 ± 0.57 g −10/ml for women, t = 0.00, p = 1.00). The log blood [HA] was similar in current smokers and nonsmokers (0.03 ± 0.40 g −10/ml for smokers and 0.28 ± 0.63 g −10/ml for nonsmokers, t = 0.76, p = 0.45). Log blood [HA] was not associated with white vs. non-white race (t = 0.72, p = 0.47); and it was not associated with current use of any of the 19 classes of medications (data not shown) or evaluation start time (i.e., time of day that evaluation began) (Spearman's r = 0.06, p = 0.57).
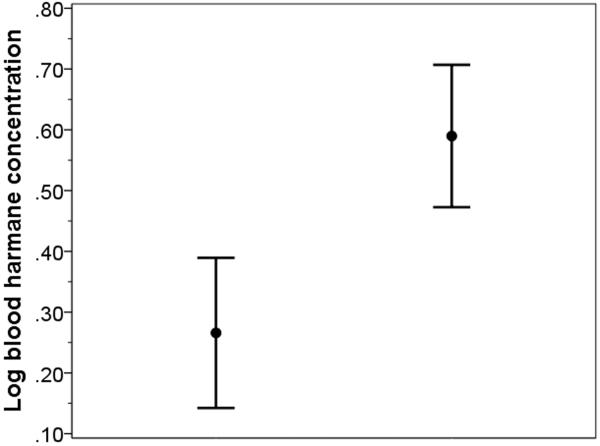
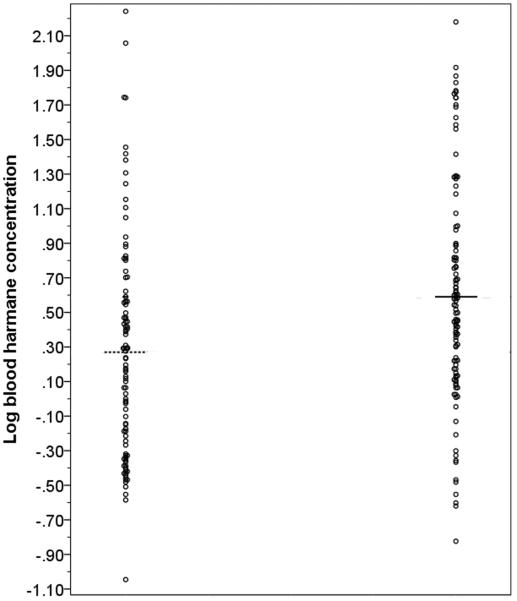
Mean log blood [HA] in PD cases was approximately double that of controls (0.59 ± 0.63 g −10/ml vs. 0.27 ± 0.63 g−10/ml, t = 3.77, p <0.001). Although the ranges overlapped, the distribution of data points for cases and controls differed (Figures 1 and 2). A non-parametric test (Mann Whitney U) on non-transformed data (median harmane = 3.31 g −10/ml in PD cases and 1.44 g −10/ml in controls) also showed this case-control difference (z = 3.90, p < 0.001). We stratified log blood [HA] based on the median value in the controls (0.24 g −10/ml); 78 (69.0%) PD cases vs. 50 (49.5%) controls had a high log blood [HA] based on this median split (odds ratio [OR] = 2.27, 95% confidence interval [CI] = 1.30 – 3.97, p = 0.004).
Figure 1.
Log blood harmane concentration in controls (left) and PD cases (right). The central point represents the mean and the bars represent the 95% confidence interval.
Figure 2.
Scatter plot of log blood harmane concentration in controls (left) and PD cases (right). The lines represent the means for the respective groups.
In an unadjusted logistic regression analysis, log blood [HA] was associated with the outcome (diagnosis of PD vs. normal) (OR = 2.31, 95% CI = 1.46 – 3.67, p <0.001) (i.e., for every doubling of the [HA], the odds of PD increased by 131%). In logistic regression analyses that adjusted for each one of the following variables individually and then in combination, the association remained unchanged: age in years, gender, body mass index, current smoker, Cumulative Illness Rating Scale score (e.g., in a model that adjusted for each of these variables in combination, OR = 2.54, 95% CI = 1.55 – 4.16, p < 0.001). In a model that adjusted for each of the variables listed above, and which further included a covariate for evaluation start time, OR = 2.61, 95% CI = 1.58 – 4.33, p <0.001. In another model that adjusted for each of the variables listed above, and which further included a series of 19 variables for use/non-use of each of the 19 classes of medications, OR = 2.69, 95% CI = 1.60 – 4.54, p < 0.001.
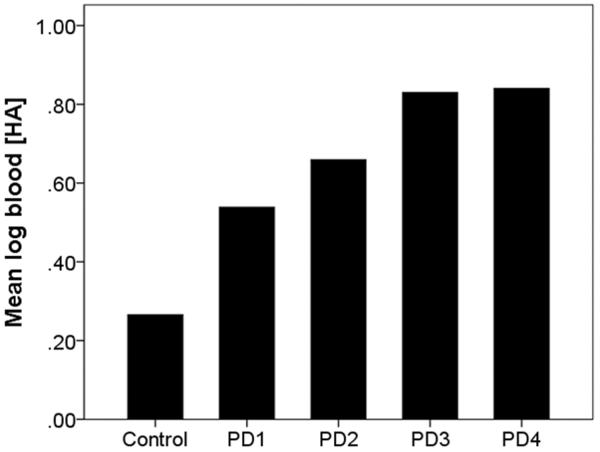
Among PD cases, there was a marginal and weak inverse correlation between symptom duration and log blood [HA] (Spearman's r = −0.18, p = 0.09), but no correlation of log blood [HA] with Hoehn & Yahr score (Spearman's r = 0.15, p = 0.22) or with daily dose of levodopa (Pearson's r = −0.12, p = 0.30). Log blood [HA] was similar in the 35 TP PD cases and 13 PIGD PD cases (0.80 ± 0.65 g−10/ml vs. 0.72 ± 0.64 g−10/ml, t = 0.36, p = 0.72). In PD, log blood [HA] covaried with family history: 0.54 ± 0.60 g−10/ml in 80 PD cases with no family history, 0.66 ± 0.71 g−10/ml in 16 PD cases with a family history of PD, 0.83 ± 0.70 g−10/ml in 6 PD cases with a family history of ET, and 0.84 ± 0.68 g−10/ml in 5 PD cases with a family history of both ET and PD (Figure 3). In a linear regression analysis with log blood [HA] as the dependent variable, there was a marginal trend, beta = 0.13, p = 0.06).
Figure 3.
Log blood harmane concentration in controls and PD cases Figure Legend:
Control = Controls (N = 101).
PD 1 = PD cases with no family history of PD or ET (N = 80).
PD 2 = PD cases with a family history of PD (N = 16).
PD 3 = PD cases with a family history of ET (N = 6).
PD 4 = PD cases with a family history of both ET and PD (N = 5).
Bars represent means.
4. Discussion
Studies in laboratory rats administered intravenous harmane indicate that harmane accumulates in several brain regions known to be of pathophysiological importance in PD (e.g., substantia nigra, striatum, basal ganglia) (Anderson et al., 2006). In humans, Kuhn et al. (1995, 1996) previously found elevated levels of plasma [HA] and cerebrospinal fluid [HA] in a small study of PD cases vs. controls. Specifically, the mean plasma [HA] was elevated in 36 PD cases vs. 36 controls: 8.7 ± 20.5 vs. 4.1 ± 9 g−12/mL (or 0.087 vs. 0.041 g−10/mL) (Kuhn et al., 1995). The study assessed plasma levels; by contrast, we assessed whole blood levels. Plasma does not contain platelets; as [HA] is particularly high in platelets, plasma levels tend to be lower than those derived from whole blood (Rommelspacher et al., 2002). Hence, the levels we report are 6 – 7 times higher than reported by Kuhn et al. (1995, 1996). In that study, the evidence for association with PD was not very strong. The p value was only marginally significant (p = 0.08), and the scatter plot showed substantial overlap in the distribution of data points for PD cases and controls (see Figure 2 in Kuhn et al., 1995). Moreover, one PD case and one control had values several-fold higher than those of others (i.e., values = 1.232 and 0.51 g−10/ml respectively); when these two individuals were excluded, the case-control difference was even less appreciable. In contrast, we now expand the number of PD cases and controls several fold, assess blood rather than plasma [HA], and examine the clinical correlates of the association. We now have data to indicate that blood [HA] is significantly elevated in our PD cases vs. controls. Kuhn et al. (1996) also reported elevated [HA] in cerebrospinal fluid of 14 PD patients vs. 14 controls: 0.151 ± 0.117 vs. 0.049 ± 0.053 g−10/mL (p = 0.01). We have not assessed cerebrospinal fluid or brain levels of this toxin in PD; however, such studies are planned.
We examined the clinical correlates of higher blood [HA] in PD. The finding of greatest interest was that the concentration seemed to be highest in PD cases with a family history of both PD and ET, and intermediate in PD cases with a family history of PD alone; it was lowest in PD cases without a family history of PD or ET. The higher concentration in familial cases suggests that the mechanism may involve genetic factors in toxin metabolism.
The levels we report for PD are slightly higher than the levels we have previously reported for ET (e.g., mean log blood [HA] in our most recent published study of ET = 0.50+/−0.54 g−10/ml) (Louis et al., 2008). However, the ranges overlapped considerably.
Log blood [HA] was similar in the 35 TP PD cases and 13 PIGD PD cases; the small number of cases in each category makes this finding difficult to interpret. Furthermore, collapsing cases into these two categories may not be the most effective method of assessing a relationship between tremor severity and log blood [HA]. It would be of value in future studies to use total tremor score as a continuous measure of tremor severity.
The mechanism whereby PD is associated with increased blood [HA] is unclear (Langston et al., 1984). β-carboline alkaloids such as harmane are structurally very similar to MPTP (Kuhn et al., 1995), with the major difference being the presence of a single indole nitrogen bridge in harmane (Kuhn et al., 1996). On a functional level, β-carboline alkaloids like harmane are comparable to MPTP as inhibitors of mitochondrial respiration (Albores et al., 1990; Kuhn et al., 1995). These compounds are also similar to paraquat, a pesticide linked with PD (Dick, 2006). Harmane and norharmane inhibit dopamine biosynthesis by reducing tyrosine hydroxylase activity and enhance L-DOPA-induced cytotoxicity in PC12 cells (Yang et al. 2008). Bilateral intranigral administration of 2,9-dimethyl-beta-carbolinium ion has been shown to cause a significant decrease in the striatal levels of dopamine and its metabolites, which was accompanied by an enhancement of muscle tone and electromyographic activity in rats. The findings suggest that the methylated beta-carbolinium ion produces a dose-dependent degeneration of nigrostriatal neurons (Lorenc-Koci et al. 2006).The notion that ET and PD are linked in some way is not new. Both ET and PD may co-occur in the same family (Yahr et al., 2003), and clinicians have long noted that patients with ET have an increased risk of PD (Chaudhuri et al., 2005; LaRoia and Louis, 2011; Simões et al., 2012; Yahr et al., 2003). In genetic studies, there have been many attempts to establish that both disorders share the same cause (e.g., in the case of variants in LINGO1, which have been associated both with ET and PD) (Deng et al., 2012; Vilariño-Güell et al., 2010). Hence, it is conceivable that other (e.g., environmental-toxic) causes could underlie both of these tremor disorders.
This study had limitations. The PD cases were recruited from a single center, so it would be useful to generalize these results to other centers. The second issue is that we did not conduct fasting blood HA evaluations. Most evaluations were performed in the late morning or early afternoon, making fasting blood [HA]s impractical. Data suggest that plasma [HA] do not change significantly during the day (Rommelspacher et al., 1991). We did not find that log blood [HA] correlated with evaluation start time and the evaluation start time was similar in PD cases and controls; furthermore, when we adjusted for evaluation start time in a multivariate logistic regression model, the PD vs. control difference persisted. Third, we evaluated blood rather than cerebrospinal fluid or brain [HA]; brain tissue is more difficult to obtain, but it would be useful to extend these studies to include brain tissue. The study had numerous strengths, including the sample size of more than 200 subjects. All blood [HA] were performed in the same laboratory, which developed the method (Zheng et al., 2000). Measurement of blood rather than plasma concentrations allowed for the detection of higher levels of harmane. In the analyses, we were able to assess the potential confounding effects of multiple relevant covariates. This is also the first study in PD to attempt to assess the clinical correlates of elevated blood [HA], including disease duration and stage, tremor predominance, and family history.
5. Conclusions
Blood [HA] appears to be elevated in PD. The finding needs to be assessed in additional cohorts. The higher concentration in familial PD suggests that the mechanism may involve genetic factors.
HIGHLIGHTS
-
! !
The environmental correlates for Parkinson's disease (PD) are unclear.
-
! !
Harmane (1-methyl-9H-pyrido[3,4-b]indole) is a potent tremor-producing toxin.
-
! !
Blood harmane levels were quantified by high performance liquid chromatography.
-
! !
Blood harmane levels were elevated in PD cases vs. controls, and highest in familial cases.
-
! !
This study provides evidence of a possible etiological importance of this toxin in PD.
Acknowledgments
Dr. Louis drafted and revised the manuscript for content; designed the study; performed the statistical analyses; analyzed and interpreted the data; obtained funding. Monika Michalec drafted and revised the manuscript for content; acquired the data. Dr. Jiang drafted and revised the manuscript for content; acquired the data. Dr. Factor-Litvak drafted and revised the manuscript for content; designed the study, analyzed and interpreted the data; performed the statistical analyses; obtained funding. Dr. Zheng drafted and revised the manuscript for content; designed the study; analyzed and interpreted the data; contributed vital reagents, tools, and patents; obtained funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA. Mitochondrial respiratory inhibition by N-methylated beta-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc Natl Acad Sci U S A. 1990;87(23):9368–9372. doi: 10.1073/pnas.87.23.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NJ, Tyacke RJ, Husbands SM, Nutt DJ, Hudson AL, Robinson ES. In vitro and ex vivo distribution of [3H]harmane, an endogenous beta-carboline, in rat brain. Neuropharmacology. 2006;50(3):269–276. doi: 10.1016/j.neuropharm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Inzitari M, Vanni P, Di Carlo A, Inzitari D. Pesticide exposure might be a strong risk factor for Parkinson's disease. Ann Neurol. 2008;63(1):128. doi: 10.1002/ana.21049. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long duration asymmetrical postural tremor is likely to predict development of Parkinson's disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76(1):115–117. doi: 10.1136/jnnp.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Gu S, Jankovic J. LINGO1 variants in essential tremor and Parkinson's disease. Acta Neurol Scand. 2012;125(1):1–7. doi: 10.1111/j.1600-0404.2011.01516.x. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton ER. UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Macmillan; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- Fuentes JA, Longo VG. An investigation on the central effects of harmine, harmaline and related beta-carbolines. Neuropharmacology. 1971;10(1):15–23. doi: 10.1016/0028-3908(71)90004-9. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson's disease in rural California. Environ Health Perspect. 2009;117(12):1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Müller T, Grosse H, Rommelspacher H. Plasma harman and norharman in Parkinson's disease. J Neural Transm Suppl. 1995;46:291–295. [PubMed] [Google Scholar]
- Kuhn W, Müller T, Grosse H, Rommelspacher H. Elevated levels of harman and norharman in cerebrospinal fluid of parkinsonian patients. J Neural Transm. 1996;103(12):1435–1440. doi: 10.1007/BF01271257. [DOI] [PubMed] [Google Scholar]
- Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta Neurol Scand Suppl. 1984;100:49–54. [PubMed] [Google Scholar]
- LaRoia H, Louis ED. Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology. 2011;37(1):1–10. doi: 10.1159/000328866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin L. Untersuchungen Uber Banisteria caapi Sp. Arch Exp Pathol Pharmacol. 1928;129:133–149. [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Lorenc-Koci E, Rommelspacher H, Schulze G, Wernicke C, Kuter K, Smiałowska M, Wierońska J, Zieba B, Ossowska K. Parkinson's disease-like syndrome in rats induced by 2,9-dimethyl-beta-carbolinium ion, a beta-carboline occurring in the human brain. Behav Pharmacol. 2006;17(5–6):463–473. doi: 10.1097/00008877-200609000-00012. [DOI] [PubMed] [Google Scholar]
- Louis ED, Benito-León J, Moreno-García S, Vega S, Romero JP, Bermejo-Pareja F, et al. Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain. Neurotoxicology. 2013;34:264–268. doi: 10.1016/j.neuro.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Gerbin M, Jiang W, Zheng W. Blood harmane concentrations in 497 individuals relative to coffee, cigarettes, and food consumption on the morning of testing. J Toxicol. 2011:628151. doi: 10.1155/2011/628151. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Jiang W, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, et al. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008;29(2):294–300. doi: 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, et al. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59(12):1940–1944. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Collins MA, Akane A, Ikebuchi J, Neafsey EJ, Kagawa M, et al. Potential bioactivated neurotoxicants, N-methylated beta-carbolinium ions, are present in human brain. Brain Res. 1993;610(1):90–96. doi: 10.1016/0006-8993(93)91221-d. [DOI] [PubMed] [Google Scholar]
- McKenna DJ. Plant hallucinogens: springboards for psychotherapeutic drug discovery. Behav Brain Res. 1996;73(1–2):109–116. doi: 10.1016/0166-4328(96)00079-4. [DOI] [PubMed] [Google Scholar]
- Minen MT, Louis ED. Emergence of Parkinson's disease in essential tremor: a study of the clinical correlates in 53 patients. Mov Disord. 2008;23(11):1602–1605. doi: 10.1002/mds.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrieff J. Determination of pharmacological levels of harmane, harmine and harmaline in mammalian brain tissue, cerebrospinal fluid and plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr. 1989;496(2):269–278. doi: 10.1016/s0378-4347(00)82576-1. [DOI] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to beta-carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802(1):115–126. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56(1):8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Ritz B, Yu F. Parkinson's disease mortality and pesticide exposure in California 1984–1994. Int J Epidemiol. 2000;29(2):323–329. doi: 10.1093/ije/29.2.323. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Meier-Henco M, Smolka M, Kloft C. The levels of norharman are high enough after smoking to affect monoamineoxidase B in platelets. Eur J Pharmacol. 2002;441(1–2):115–125. doi: 10.1016/s0014-2999(02)01452-8. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Schmidt LG, May T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol Clin Exp Res. 1991;15(3):553–559. doi: 10.1111/j.1530-0277.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson's disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8(1):87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42(7):1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Simões RM, Constantino A, Gibadullina E, Houghton D, Louis ED, Litvan I. Examining the motor phenotype of patients with both essential tremor and Parkinson's disease. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D8CN72N0. tre-02-47-149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect. 2012;120(3):340–347. doi: 10.1289/ehp.1103881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C, Wider C, Ross OA, Jasinska-Myga B, Kachergus J, Cobb SA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010;11(4):401–408. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr MD, Orosz D, Purohit DP. Co-occurrence of essential tremor and Parkinson's disease: clinical study of a large kindred with autopsy findings. Parkinsonism Relat Disord. 2003;9(4):225–231. doi: 10.1016/s1353-8020(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Lee JJ, Jin CM, Lim SC, Lee MK. Effects of harman and norharman on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. Eur J Pharmacol. 2008;587(1–3):57–64. doi: 10.1016/j.ejphar.2008.03.050. [DOI] [PubMed] [Google Scholar]
- Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7(4):237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Barnes LF, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem. 2000;279(2):125–129. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]