Abstract
Purpose
Diets rich in plant-derived polyphenols such as olive oil (OO) and/or catechins such as epigallocatechin 3-gallate (EGCG) have been shown to reduce the incidence of cardiovascular diseases, potentially by improving endothelial function, an important surrogate for atherosclerosis. The possible augmentation of endothelial function with the combined efforts of OO and EGCG is intriguing, yet unknown.
Methods
82 patients with early atherosclerosis (presence of endothelial dysfunction) were enrolled in this double-blind, randomized trial. It was the aim to compare the effect of a daily intake of 30ml simple OO, with 30ml of EGCG supplemented OO, on endothelial function as well as on inflammation and oxidative stress after a period of four months. Endothelial function was assessed non-invasively via peripheral arterial tonometry (Endo-PAT®).
Results
After 4 months, OO and EGCG supplemented OO significantly improved endothelial function (RHI, 1.59±0.25 to 1.75±0.45, p<0.05). However, there were no significant differences in results between the two olive oil groups. Interestingly, with OO supplementation there was a significant reduction in inflammatory parameters (sICAM from 196 ng/mL to 183 ng/mL, p = <0.001; white blood cells (WBCs) (6.0×109/L to 5.8×109/L, p<0.05); monocytes from 0.48×109/L to 0.44×109/L, p = 0.05 and lymphocytes from 1.85×109/L to 1.6×109/L, p = 0.01).
Conclusions
Improvement in endothelial dysfunction in patients with early atherosclerosis in association with significant reduction in leukocytes may suggest an important role of early cellular inflammatory mediators on endothelial function. The current study supports one potential mechanism for the role of olive oil, independent of EGCG, modestly supplemented to a healthy cardiovascular diet.
Keywords: Endothelial Function, Olive Oil, Inflammation, Oxidative Stress, Atherosclerosis
Introduction
Olive oil is an important source of polyphenols in the Mediterranean diet [11]. Consumption of diets rich in polyphenols has been shown to enhance nitric oxide (NO) bioavailability as well as decrease oxidative stress and inflammation [2, 14, 30]. Recent randomized trials have pointed out the important antioxidant effect of extra virgin olive oil (OO) in humans [6, 34]. The Italian Virgin Olive Oil Study [34] demonstrated a significant decrease in total antioxidative capacity (and platelet activation) in mildly dyslipidemic patients with extra virgin OO compared to highly refined olive oil, which is much lower in polyphenols. The Eurolive study [6], a randomized trial, showed a linear decrease in markers of oxidative stress with increasing polyphenolic content of the olive oils.
Epigallocatechin 3-gallate (EGCG) is a green tea derived phenol that has been described as a biologically potent faction of the polyphenol family and is thought to be the active ingredient in green tea [1, 5]. OO and EGCG might potentiate antioxidant power when combined, however the effects on cardiovascular health of dietary supplementation with either olive oil alone or in combination with EGCG are unknown.
Endothelial dysfunction is regarded as an early stage of atherosclerosis leading to subsequent cardiovascular morbidity and mortality [4]. Endothelial dysfunction is associated with most cardiovascular risk factors, and is an independent predictor of cardiovascular events [18]. Recently, studies showed conflicting results of the effect of olive oil on endothelial function, potentially because of the low polyphenol content and the brief period of OO ingestion [15, 29, 32].
Definitive evidence regarding the effect of long-term (four month) supplementation with OO on endothelial function is lacking. Additionally, there is no data regarding the effect of long-term OO and/or EGCG supplementation on endothelial function.
The aim of our study was to demonstrate the effect of OO on endothelial function over the long-term (four month), and to study whether OO with additional EGCG is more effective than OO alone in augmenting endothelial function and surrogate markers for cardiovascular disease. Thus, we utilized a double-blind, randomized-controlled study to compare the effect of four month supplementation of OO versus EGCG enriched OO (OO+EGCG) on endothelial function, as well as on oxidative stress and inflammation in patients with low-to-intermediate cardiovascular risk.
Methods
Study population
Subjects over the age of 18 years old were enrolled regardless of previous history of cardiovascular events. Patients were recruited from the Division of Cardiovascular Diseases at Mayo Clinic in Rochester, MN (ClinicalTrials.gov Identifier: NCT00865787) as well as by intra-institutional advertising seeking research participants. Participants underwent endothelial function testing with the EndoPAT® device. Those with normal EndoPAT® score (>2.0) and/or uncontrolled hypertension (blood pressure >180/100) were excluded from the study. Other exclusion criteria were history of renal or liver failure and relevant food allergies. Informed consent was obtained and signed by all participants.
Experimental protocol
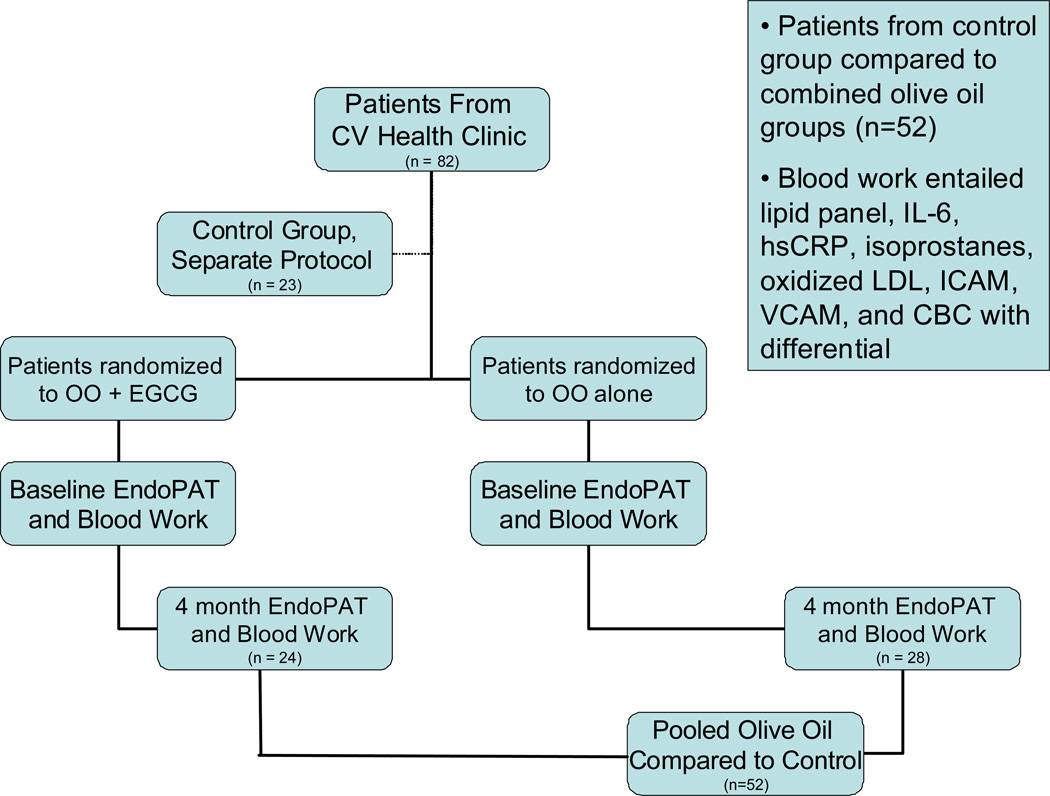
This was a controlled, randomized double-blind study conducted in accordance with the ethical standards of the responsible institutional or regional committee on human experimentation or in accordance with the Helsinki Declaration of 1975 as revised in 1983 and in accordance with the policies and procedures of the local ethics committee and Mayo Clinic Institutional Review Board. Participants were instructed to not change their diets despite olive oil supplementation, and were not given any special dietary instructions so as to have olive oil as the sole added variable in their diet. Details are in Figure 1.
Figure 1. Protocol.
Schematic representation of the patient selection and trial execution.
All participants were instructed to fast for at least four hours prior to each appointment and to abstain from caffeine and tobacco products for 24 hours. In addition, participants were instructed to hold ACE inhibitors and calcium channel blockers for 24 hours, and nitrates for six hours prior to the appointment. Examinations were performed in the morning. During a short clinical examination, blood pressure, body weight, and body height were obtained. EndoPAT® testing was performed. Participants were then randomized to receive a once daily serving of 30ml of either EGCG containing OO or OO alone for a total duration of four months. Participants were instructed to consume a single dose of the uncooked study product during one of their daily meals. Measurements were repeated after four months to assess long term effects. Participants were also contacted by phone at one and three months to assess compliance and any changes in medications or symptoms.
Endothelial function assessment
Studies were performed in a designated quiet, temperature controlled, and uniformly lit room. EndoPAT® signals were obtained using the Endo-PAT® 2000 device (Itamar Medical Inc. Ltd, Caesarea, Israel.), which has been used previously by our lab [4, 12, 17] with an high level of reproducibility [28]. Endothelial function was measured by a reactive hyperemia-peripheral arterial tonometry (RH-PAT) as previously described [3, 4]. After five minute baseline PAT recording, a blood pressure cuff was inflated on one arm to 60mmHg (or at least 200mmHg) above baseline systolic blood pressure for five minutes. Occlusion of pulsatile arterial flow was confirmed via the PAT tracing. After five minutes, the cuff was deflated and the PAT tracing recorded for another six minutes. The ratio (RH-PAT index) of the PAT signal after cuff release compared to baseline was calculated through a computer algorithm automatically normalizing for baseline RH-PAT signal, and indexed to the contra-lateral arm. Reactive hyperemia responses were recorded at baseline and after the four month treatment period.
Blood Tests
Blood measurements of lipids, inflammatory markers and endogenous oxidative stress markers were performed at baseline and at four months as previously described [20]. Inflammatory markers measured included hsCRP, IL-6, soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), fibrinogen, and a complete blood count with differential. Endogenous oxidative stress markers measured included plasma 8-isoprostane and oxidized LDL (oxLDL). Standard lipid profiles (total cholesterol, triglycerides, HDL and LDL), and lipoprotein particle subfractions by NMR [10] spectroscopy, were also assessed at baseline and four months in both OO groups.
Study olive oil and Polyphenols
OO was supplied by Olivi Agri Team Srl (Grosseto, Italy) and the enhanced/enriched sample (OO with EGCG), was prepared according to a patent (pending, Olivi Agri Team Srl). Oleuropein, EGCG, Luteolin were purchased from Extrasynthese (Geney-France), the OH-Tyr was purchased from Cayman Chemical (SPI-BIO-Europe). The enhanced samples were produced at IMET factory in Italy (Bergamasco, Italy). OO alone (340 mg/kg polyphenol content) and OO plus EGCG (600 mg/kg polyphenol content) were incorporated into participants’ diets as previously reported [24].
Statistical analysis
Statistical analysis was performed by an independent statistician blinded to the randomization after completion of the studies. Results are expressed as mean± standard deviation or median with interquartile ranges if data was not normally distributed. Discrete data are presented as frequency (percentage). Differences between randomized groups were compared using Student’s two-sample t-test, the Wilcoxon rank sum test, or Pearson’s chi-squared test, as appropriate. Among all randomized patients who had a four month follow-up study, differences between four month and baseline measures were compared using a signed rank test. A two-sample t-test of the change in EndoPAT® score at four months was used to compare those with baseline scores <1.6 versus those with scores ≥1.6 to delineate. Statistical significance was accepted at p<0.05. OO groups, with and without EGCG, were analyzed separately and also subsequently pooled as part of a subgroup analysis. These were compared using a two-way ANOVA analysis between and among groups. Additionally, these patients underwent the same rigorous enrollment and intake process described above.
Results
Olive Oil composition
The main difference on the phenol composition of the two OOs (Table 1) is the higher content of secoiridoids in the OO (61%) while the enriched with EGCG arrived up only to 48%. At the same time, the lignans, another group of main constituents, have shown almost the same amount in the two oils.
Table 1.
Phenolic composition of the two OO samples.
| Compounds | OO+EGCG* (mg/L) |
OO (mg/L) |
|---|---|---|
| Hydroxy Tyrosol | 26.18 | 9.91 |
| Tyrosol | 17.45 | 13.09 |
| 3,4 Dihydroxyphenylethanol-Elenolic Acid | 23.63 | 72.72 |
| Oleocanthal | 49.51 | 68.28 |
| Other secoiridoids | 15.57 | 15.77 |
| Lignans | 110.79 | 104.96 |
| Oleuropein Aglycone | 55.85 | 41.57 |
| Luteolin | 2.03 | 0.85 |
| Total phenols (TP) | 301.00 | 327.15 |
| % Secoiridoids/TP | 48% | 61% |
| % Lignans/TP | 37% | 33% |
to this sample 280 mg of EGCG was added
Baseline characteristics
82 subjects qualified and were randomized for the study. Of these, 52 subjects were able to complete the four month protocol, and 50 participants had follow up EndoPAT® measurements. Baseline characteristics of the OO and OO + EGCG patients evaluated in the study are displayed in Table 2. No significant differences between the two randomized olive oil groups were detected regarding factors such as comorbidities, medications, gender, risk factors or other entities known to be associated with early CVD or endothelial dysfunction. Framingham risk scores were not significantly different between the two OO groups (2.0±2.9 vs. 2.2±3.8, p= 0.81). Comparisons of participant’s medications taken prior to the study were examined and found not to be significantly different.
Table 2. Baseline Demographics.
Baseline characteristics of the subjects involved in the study.
| OO + ECGC | OO alone | ||
|---|---|---|---|
| Variable | (N=24) | (N=28) | P-value |
| Age | 41.8 ± 14.4 | 41.3 ± 14.7 | 0.92 |
| Gender, No. (%) | 0.73 | ||
| Male | 10 (42%) | 13 (46%) | |
| Female | 14 (58%) | 15 (54%) | |
| Baseline BMI | 27.1 ± 5.7 | 28.2 ± 6.1 | 0.52 |
| Known Cardiovascular Disease, No. (%) | 2 (8%) | 1 (4%) | 0.46 |
| Has had cardiac catheterization, No. (%) | 2 (8%) | 1 (4%) | 0.46 |
| Family history of CAD, No. (%) | 7 (29%) | 9 (35%) | 0.68 |
| Hyperlipidemia, No. (%) | 12 (57%) | 16 (76%) | 0.19 |
| Smoking, No. (%) | 0 (0%) | 1 (4%) | 0.35 |
| Hypertension currently drug treated, No. | 6 (25%) | 6 (21%) | 0.76 |
| Diabetes mellitus, No. (%) | 1 (4%) | 0 (0%) | 0.28 |
The effect of OO on hemodynamic data and endothelial function
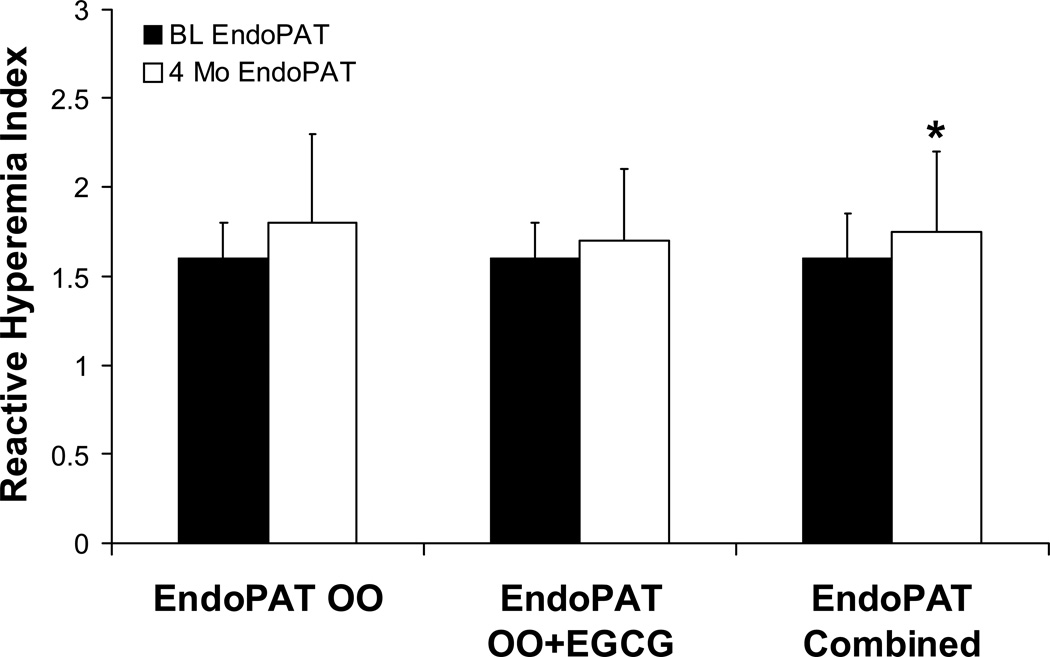
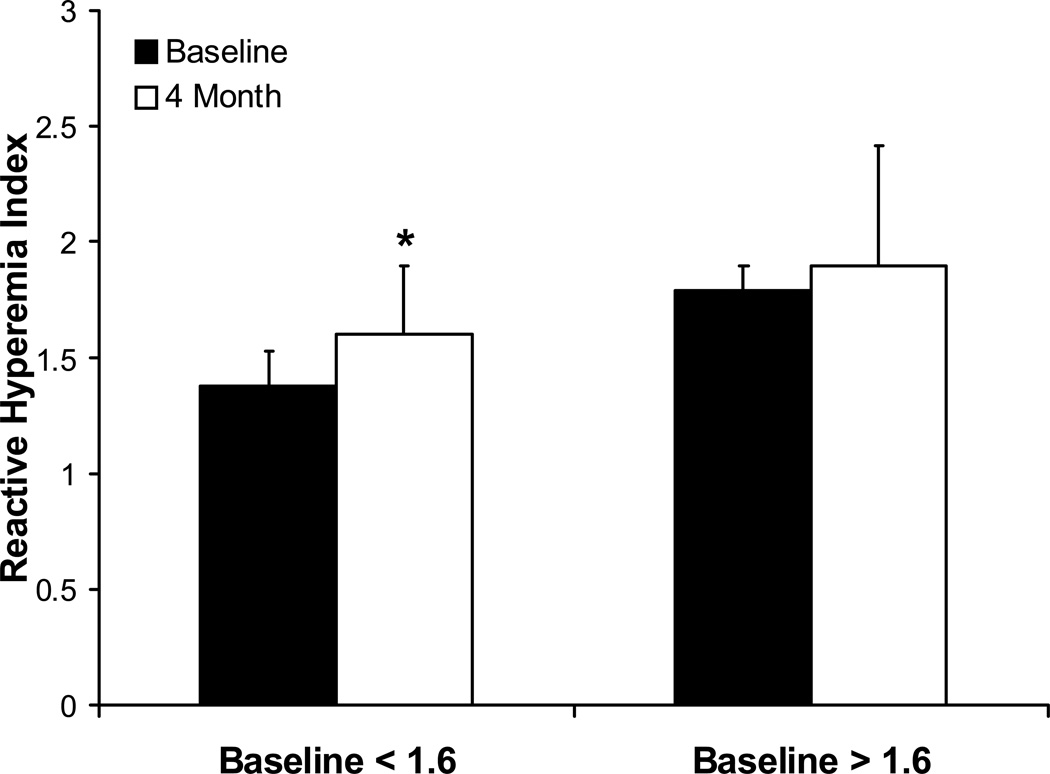
After four months of olive oil supplementation, there was no significant change in heart rate (p = 0.275), systolic (p = 0.155) or diastolic blood pressure (p = 0.726), or BMI (p = 0.64) between the two OO groups. The improvement in endothelial function as measured by EncoPAT® was similar in the OO with additional EGCG and in the OO alone group (1.6±0.2 to 1.8±0.5 and 1.6±0.2 to 1.7±0.4, respectively; p=0.85 for delta). However, when OO groups were combined, there was a significant increase in Endo-PAT® from baseline to four months (1.595 to 1.675, p = 0.03, Figure 2). In those patients who had poor endothelial function at baseline (RHI < 1.6), there was a significant increase in EndoPAT® score from 1.38±0.15 to 1.60±0.3 (Figure 3, p=0.004). Those patients who had normal baseline EndoPAT® scores did not have significant improvement in their endothelial function as measured by EndoPAT® (1.79±0.11 to 1.90±0.52, p=0.21).
Figure 2.
Comparing Reactive Hyperemia Indices (RHI, ratio) for patients at baseline and after 4 months of treatment. No statistical differences exist when treatment arms of the OO groups are separated. There is a significant difference between baseline and four month endothelial function when the groups are combined (A+B) suggesting that OO alone can improve endothelial function (* p<0.05).
Figure 3.
Graphical representation demonstrating that those with reduced endothelial function (baseline Endo-PAT® reactive hyperemia index (RHI) < 1.6) show significant improvements in endothelial function after 4 months of OO treatment (p=0.004).
The effect of OO on laboratory characteristics (inflammation and oxidative stress parameters)
Results and comparisons between OO and supplemented OO are shown in Table 2. Among the study participants there were significant reductions in white blood counts (WBCs) and sICAM-1 (Table 3) in the combined OO group after four months. Moreover, the cell types reduced by OO supplementation included lymphocytes (1.85 to 1.60×109/L, p = 0.005), monocytes (0.48 to 0.44×109/L, p = 0.047), and platelets (242 to 229×109/L, p = 0.047). Neutrophils also tended to be reduced (3.69 to 3.38×109/L, p = 0.07). However these effects did not differ between the different OO treatment groups (with and without EGCG). Inflammatory and oxidative stress markers such as hsCRP (0.10 to 0.09 mg/L, p = 0.22), IL-6 (1.3 to 1.3 pg/mL, p = 0.52), sVCAM-1 (575 to 560 ng/mL, p = 0.07) as well as oxLDL (4.29 mg/dL to 4.31 mg/dL, p = 0.78) were not affected significantly. Plasma 8-isoprostane increased with treatment of olive oil supplementation (Tables 2 and 3).
Table 3. OO vs. OO + EGCG after 4 Months.
Comparison OO vs. OO + EGCG lipid profiles, inflammatory markers, white blood cell components, and endothelial function after 4 months of supplementation. Values are reported in medians and inter-quartile ranges (parenthesis). P-values denote the comparison in differences after 4 months.
| Variable | OO (N = 24) 4 Month Change |
OO + EGCG (N = 28) 4 Month Change |
P-value |
|---|---|---|---|
| HDL Cholesterol | 0.5 (−5.0, 2.0) | −1.5 (−4.0, 4.0) | 0.78 |
| LDL Chol | 2.5 (−6.0, 12.0) | −4.5 (−16.0, 13.0) | 0.18 |
| NMR Total Chol | 2.0 (−9.0, 13.0) | −2.0 (−16.0, 18.0) | 0.36 |
| NMR Triglyceride | −1.0 (−10.0, 23.0) | 7.5 (−12.0, 27.0) | 0.70 |
| HsCRP | 0.0 (−0.0, 0.1) | −0.0 (−0.2, 0.0) | 0.12 |
| Plasma 8-isoprost | 25.1 (7.4, 152.3) | 17.3 (−41.6, 59.3) | 0.09 |
| sICAM-1 | −10.0 (−34, −3.5) | −11.5 (−36.5, −2.0) | 0.76 |
| Hemoglobin | −0.1 (−0.5, 0.3) | −0.2 (−0.6, 0.0) | 0.26 |
| Lymphocytes | −0.1 (−0.4, 0.1) | −0.1 (−0.3, 0.1) | 0.84 |
| Monocytes | −0.0 (−0.1, 0.0) | −0.0 (−0.1, 0.0) | 0.98 |
| Neutrophils | −0.0 (−0.8, 0.4) | −0.3 (−0.9, 0.0) | 0.37 |
| Platelets | −11.0 (−32.0, 18.0) | −7.0 (−27.0, 12.0) | 0.79 |
| White Blood Cells | −0.1 (−1.1, 0.4) | −0.6 (−1.0, −0.1) | 0.46 |
Serum lipoproteins
Lipid profiles did not change significantly within or between OO groups: HDL (46 mg/dL, baseline, to 51 mg/dL at four months, p = 0.79), LDL (102 mg/dL to 105 mg/dL, p = 0.56), total cholesterol (178 mg/dL to 178 mg/dL, p = 0.789) and triglycerides (106 mg/dL to 103 mg/dL, p = 0.346). Lipoprotein particles measured by NMR (nuclear magnetic resonance) spectroscopy also showed no significant changes in the OO groups.
Discussion
The current study demonstrates that longer-term supplementation of olive oil improves endothelial function in individuals with low to intermediate CV risk, an effect likely attributed to reduction in vascular inflammation. This is the first such demonstration of such a permanent endothelial benefit via long term supplementation of a macronutrient. However, further enrichment with polyphenols (EGCG) does not provide any additional benefit above those attributable to olive oil alone [19, 39]. Although the main goal of the study – demonstrating the superiority of polyphenol-enriched OO – was not achieved, the study nevertheless further supports the role, and potentially ascribes some mechanisms, of the aforementioned benefit of olive oil on cardiovascular health [6, 21, 34]. A concomitant reduction in white blood cells, particularly monocytes and neutrophils, as well as sICAM points to a potential mechanism for improved endothelial function through a reduction in vascular inflammation. Interestingly, patients with low endothelial function at baseline appear to garner the most benefit from OO. Thus, supplementation with OO seems a reasonably easy and relatively cheap dietary measure to improve endothelial function and perhaps favorably alter the progression of atherosclerotic disease, particularly in patients with already markedly impaired endothelial function.
Olive oil, a major component of the Mediterranean diet, is a rich source of a variety of polyphenols. Indeed, OO has been shown to positively and acutely impact endothelial function as soon as two hours after consumption [23], however the mechanism for this improvement and the responsible ingredient has not yet clearly been identified [27]. The current study is in accord with – and expounds upon – previously observed data, and is also the first to show a sustained effect on endothelial function after long-term OO supplementation. We also show that those with baseline endothelial dysfunction benefit the greatest from OO supplementation, perhaps offering yet another treatment modality for those patients at greatest risk for future cardiac events. Because the bioavailability of plant derived polyphenols in the blood is rather short [37], ingredients in olive oil likely have altered the expression of certain long-term endothelial modulators, such as nitric oxide synthase, to see this sustained effect. Contrary to other studies [6, 25, 34] we were not able to demonstrate a sustained antioxidant effect from OO supplementation as neither oxLDL nor the isoprostanes (markers of lipid peroxidation) were reduced by OO. The significant increase in the plasma 8-isoprostane within the EGCG group might also raise the concern of a possible pro-oxidant effect of EGCG as previous animal work has shown deleterious effects of high-dose vitamin supplementation on myocardial perfusion and coronary endothelial function [33]. Thus, it remains likely that over-supplementation with nearly any substance may not be beneficial, and may even pose greater harm to the patient. Moreover, we were unable to appreciate a significant improvement with EGCG supplementation as seen in previous studies [37]. This, again, could be due to the possible pro-oxidant effect of EGCG negating the beneficial effects of OO alone, or the fact that long-term supplementation with both substances offers no endothelial function benefit.
We observed a simultaneous reduction in WBCs and soluble cellular adhesion molecules sICAM-1, thus pointing toward the possible role of olive oil consumption on reducing, specifically, vascular inflammation. This mirrors previous studies that have shown an inverse correlation between WBC counts and cardiovascular disease [22, 31, 36]. Moreover, increased leukocyte counts have been found in both smokers [16] and diabetics [38]; and reducing WBCs has been shown to be associated with endothelium-dependent vasodilatation [8, 35]. Our data indicate that CRP is not reduced with OO supplementation. Diets high in monounsaturated fats have been known to increase NO bioavailability, and it is possible that there could be further enhancement of NO-mediated vasodilation via reduction in inflammatory markers associated with WBCs. Myeloperoxidase has also been shown to interfere with tetrahydrobiopterin production and subsequent NO release, bioavailability, and mechanism of action [13, 26]. Therefore, cellular inflammatory mediators plausibly reduce production or bioavailability of NO, or the production of reactive oxygen species that interfere with the ability of tetrahydrobiopterin to aid in the production and synthesis of NO [26]. The reduced leukocytic inflammatory mediations would allow for increased NO-mediated vasodilation and improved endothelial function, and could underlie the vascular endothelial improvement seen with OO.
Importantly, both the OO + EGCG as well as OO alone had polyphenol concentrations that were two to five times more potent than typical commercialized olive oil [6]. This could account for the lack of difference in outcomes between the two variants of OO. The high levels of polyphenols and fats (an extra 30 g of lipids ingested daily by the patients) could also explain the high levels of isoprostanes, as the overabundance of OO could have reached levels that caused an increase in oxidative stress. Notably, however, the participants did not gain weight with the additional daily caloric intake of the olive oil. Nevertheless, there could be a target level of polyphenols in certain foods such as olive oil that provide maximum cardiovascular benefit without the side effects.
Our study did have limitations that must be taken into account. One limitation involved the lack of a control group. As OO supplementation is already known to improve endothelial function (as is green tea, of which the active ingredient is EGCG), we used OO alone as the “control” group. While this could be seen as a weakness, repeated and recent work has demonstrated that the “control” group has no change in endothelial function [7, 9]. Regardless, the addition of a “control” group would have no bearing on the fact that there was no further improvement with the addition of EGCG. Also, noted was the attrition of the subjects, albeit evenly divided, in this study mainly due to gastrointestinal discomfort and inability to incorporate the uncooked study product into their daily diet. Another limitation involves not measuring polyphenol plasma concentration, introducing the possibility that our supplementation did not achieve therapeutic concentrations. However, there were no statistical differences in fasting plasma glucose levels between the OO groups. Also of note, our sample population was of low to intermediate risk (mean Framingham 10-year risk percentage between 0.5% and 4%), thus we were examining primary prevention characteristics. Had we examined secondary prevention or a higher risk population, we might have seen further improvement in endothelial function, inflammatory markers, lipids, and WBCs.
In conclusion, OO supplementation in patients with low to intermediate risk improves endothelial function through mechanisms possibly related to improvements in inflammation. We did not observe any additive benefit of EGCG, the main component of green tea. Therefore, olive oil supplementation may be beneficial for most individuals and might theoretically reduce cardiovascular events. These benefits are consistent with the prominent role of olive oil in the Mediterranean Diet.
Table 4. The Effect of Olive Oil on Biomarkers.
| Variable | N | Baseline (SD) | 4 Mo (SD) | Delta (SD) | P-value |
|---|---|---|---|---|---|
| EndoPAT score | 50 | 1.595 (1, 2) | 1.675 (1, 3.16) | 0.09 (−0.72, 1.37) | #0.029 |
| HDL Chol (md/dL) | 49 | 46 (29, 97) | 51 (29, 97) | 0 (−24, 23) | 0.786 |
| LDL Chol (mg/dL) | 49 | 102 (35, 244) | 105 (43, 222) | 1 (−40, 38) | 0.564 |
| Total Chol (mg/dL) | 49 | 178 (94, 312) | 178 (109, 302) | 0 (−63, 45) | 0.789 |
| Triglyceride (mg/dL) | 49 | 106 (25, 311) | 103 (38, 407) | 5 (−229, 219) | 0.346 |
| Oxidized LDL | 49 | 4.29 (2.86) | 4.31 (2.77) | 0.02 (0.85) | 0.79 |
| Hemoglobin | 49 | 13.7 (11.9, 16.6) | 13.6 (11.6, 16.9) | −0.2 (−1.7, 3.1) | 0.119 |
| HsCRP | 51 | 0.101 (0.01, 2.81) | 0.09 (0.01, 1.21) | 0 (−2.68, 0.639) | 0.220 |
| ICAM-1 | 53 | 196 (84, 298) | 183 (91, 239) | −11 (−168, 130) | #<.001 |
| IL-6 | 51 | 1.3 (0.3, 33) | 1.3 (0.5, 5.1) | −0.1 (−30.9, 2.7) | 0.518 |
| WBC | 49 | 6 (3.8, 11.7) | 5.8 (3.7, 10.4) | −0.4 (−5.2, 2.8) | #0.009 |
| Lymphocytes | 48 | 1.845 (0.47, 3.63) | 1.6 (0.64, 3.07) | −0.13 (−0.81, 1.12) | #0.005 |
| Monocytes | 48 | 0.48 (0.27, 0.94) | 0.44 (0.26, 0.97) | −0.03 (−0.51, 0.34) | #0.047 |
| Neutrophils | 48 | 3.69 (1.37, 8.94) | 3.38 (2.11, 6.4) | −0.15 (−5.02, 2.91) | 0.072 |
| Platelet Count | 49 | 242 (124, 367) | 229 (146, 338) | −11 (−128, 51) | #0.047 |
| VCAM-1 | 52 | 575 (368, 1054) | 560 (346, 1166) | −26 (−284, 200) | 0.068 |
| Plasma 8-isoprostanes | 52 | 130.8 (28.71, 310.6) | 164.4 (55.37, 491.6) | 22.39 (−113, 324.7) | 0.003* |
Significant reductions (#) in ICAM-1, Lymphocytes, Monocytes, Platelets, and WBCs are seen after 4 months of OO treatment. There was a trend toward reduction in neutrophils as well. No significant reductions existed with regard to lipid profiles or inflammatory markers. Plasma-8 isoprostane actually increased (*) after OO treatment.
Acknowledgments
Sources of funding
This study was partly supported by Olivi Agri Team Srl-Grosetto, Italy and the University of Florence. However, the study was investigator initiated and investigator driven. The clinical trial was conducted using Olive Oil and EGCG from Olivi Agri Company. This work was also partly supported by NIH grant #HL085307. AJF received a scholarship from the Walter and Gertrud Siegenthaler Foundation, Zurich, and is supported by a “Nachwuchsförderungskredit” of the young academics Support Committee of the University of Zurich, Switzerland and the Swiss National Science Foundation (PASMP3_132551).
Footnotes
Disclosures
Dr. Lerman serves on the advisory board of Itamar medical, the other authors report no actual or potential conflict of interest in connection with this study.
References
- 1.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 2.Bazzano L, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti P, Holmes DR, Jr, Lerman A, Barsness GW. Enhanced external counterpulsation for ischemic heart disease: what's behind the curtain? J Am Coll Cardiol. 2003;41:1918–1925. doi: 10.1016/s0735-1097(03)00428-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonetti P, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 5.Brown A, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covas M, Nyyssönen K, Poulsen HE, Kaikkonen J, Zunft HJ, Kiesewetter H, Gaddi A, de la Torre R, Mursu J, Bäumler H, Nascetti S, Salonen JT, Fitó M, Virtanen J, Marrugat J EUROLIVE Study Group. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Dohadwala M, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkind M, Sciacca RR, Boden-Albala B, Tondella ML, Feikin DR, Fields BS, Sacco RL, Di Tullio MR, Homma S. Leukocyte count is associated with reduced endothelial reactivity. Atherosclerosis. 2005;181:329–338. doi: 10.1016/j.atherosclerosis.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Flammer A, Martin EA, Gössl M, Widmer RJ, Lennon RJ, Sexton JA, Loeffler D, Khosla S, Lerman LO, Lerman A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur J Nutr. 2012 doi: 10.1007/s00394-012-0334-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford M, McConnell JP, Lavi S, Rihal CS, Prasad A, Sandhu GS, Hartman SJ, Lerman LO, Lerman A. Coronary artery endothelial dysfunction is positively correlated with low density lipoprotein and inversely correlated with high density lipoprotein subclass particles measured by nuclear magnetic resonance spectroscopy. Atherosclerosis. 2009;207:111–115. doi: 10.1016/j.atherosclerosis.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung T, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goor D, Sheffy J, Schnall RP, Arditti A, Caspi A, Bragdon EE, Sheps DS. Peripheral arterial tonometry: a diagnostic method for detection of myocardial ischemia induced during mental stress tests: a pilot study. Clin Cardiol. 2004;27:137–141. doi: 10.1002/clc.4960270307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Hu F, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA. 2002;288:2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Morales A, Ruano J, Delgado-Lista J, Fernandez JM, Camargo A, López-Segura F, Villarraso JC, Fuentes-Jiménez F, López-Miranda J, Pérez-Jiménez F. NOS3 Glu298Asp Polymorphism Interacts with Virgin Olive Oil Phenols to Determine the Postprandial Endothelial Function in Patients with the Metabolic Syndrome. J Clin Endocrinol Metab. 2011;96:1694–1702. doi: 10.1210/jc.2011-1056. [DOI] [PubMed] [Google Scholar]
- 16.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking Is Associated With Epicardial Coronary Endothelial Dysfunction and Elevated White Blood Cell Count in Patients With Chest Pain and Early Coronary Artery Disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 17.Lavie P, Shlitner A, Sheffy J, Schnall RP. Peripheral arterial tonometry: a novel and sensitive non-invasive monitor of brief arousals during sleep. Isr Med Assoc J. 2000;2:246–247. [PubMed] [Google Scholar]
- 18.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz M, Wessler S, Follmann E, Michaelis W, Düsterhöft T, Baumann G, Stangl K, Stangl V. A Constituent of Green Tea, Epigallocatechin-3-gallate, Activates Endothelial Nitric Oxide Synthase by a Phosphatidylinositol-3-OH-kinase-, cAMP-dependent Protein Kinase-, and Akt-dependent Pathway and Leads to Endothelial-dependent Vasorelaxation. Journal of Biological Chemistry. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 20.Meuwissen M, van der Wal AC, Niessen HWM, Koch KT, de Winter RJ, van der Loos CM, Rittersma SZH, Chamuleau SAJ, Tijssen JGP, Becker AE, Piek JJ. Colocalisation of intraplaque C reactive protein, complement, oxidised low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J Clin Pathol. 2006;59:196–201. doi: 10.1136/jcp.2005.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Jimenez F, et al. International conference on the healthy effect of virgin olive oil. Eur J Clin Invest. 2005;35:421–421. doi: 10.1111/j.1365-2362.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 22.Prentice R, Szatrowski TP, Fujikura T, Kato H, Mason M, Hamilton HH. Leukocyte Counts and Coronary Heart Disease in a Japanese Cohort. Am J Epidemiol. 1982;116:496–509. doi: 10.1093/oxfordjournals.aje.a113434. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;35:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 24.Romani A, Pinelli P, Mulinacci N, Galardi C, Vincieri FF, Liberatore L, Cichelli A. HPLC and HRGC analyses of polyphenols and secoiridoids in olive oil. Chromatographia. 2001;53:279–284. [Google Scholar]
- 25.Ruano J, Lopez-Miranda J, Fuentes F, Moreno JA, Bellido C, Perez-Martinez P, Lozano A, Gómez P, Jiménez Y, Pérez Jiménez F. Phenolic Content of Virgin Olive Oil Improves Ischemic Reactive Hyperemia in Hypercholesterolemic Patients. Journal of the American College of Cardiology. 2005;46:1864–1868. doi: 10.1016/j.jacc.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt K, Werner ER, Mayer B, Wachter H, Kukovetz WR. Tetrahydrobiopterin-dependent formation of endothelium-derived relaxing factor (nitric oxide) in aortic endothelial cells. Biochem J. 1992;281:297–300. doi: 10.1042/bj2810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt C, Dirsch VM. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide. 2009;21:77–91. doi: 10.1016/j.niox.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Selamet-Tierney E, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, Ferranti SD. Endothelial Pulse Amplitude Testing: Feasibility and Reproducibility in Adolescents. J Pediatr. 2009;154 doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira J, Smiley D, Newton C, Le NA, Gosmanov AR, Spiegelman R, Peng L, Osteen SJ, Jones DP, Quyyumi AA, Ziegler TR, Umpierrez GE. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: comparison of vascular, metabolic, and inflammatory effects. J Clin Endocrinol Metab. 2011;96:3207–3216. doi: 10.1210/jc.2011-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stocker R. Dietary and pharmacological antioxidants in atherosclerosis. Curr Opin Lipidol. 1999;10:589–597. doi: 10.1097/00041433-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Sweetnam P, Thomas HF, Yarnell JW, Baker IA, Elwood PC. Total and Differential Leukocyte Counts as Predictors of Ischemic Heart Disease: The Caerphilly and Speedwell Studies. American Journal of Epidemiology. 1997;145:416–421. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 32.Tousoulis D, Papageorgiou N, Antoniades C, Giolis A, Bouras G, Gounari P, Stefanadi E, Miliou A, Psaltopoulou T, Stefanadis C. Acute effects of different types of oil consumption on endothelial function, oxidative stress status and vascular inflammation in healthy volunteers. Br J Nutr. 2010;103:43–49. doi: 10.1017/S0007114509991346. [DOI] [PubMed] [Google Scholar]
- 33.Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, Napoli C, Lerman LO, Lerman A. Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension. 2006;47:475–481. doi: 10.1161/01.HYP.0000201445.77125.26. [DOI] [PubMed] [Google Scholar]
- 34.Visioli F, Caruso D, Grande S, Bosisio R, Villa M, Galli G, Sirtori C, Galli C. Virgin Olive Oil Study (VOLOS): vasoprotective potential of extra virgin olive oil in mildly dyslipidemic patients. Eur J Nutr. 2005;44:121–127. doi: 10.1007/s00394-004-0504-0. [DOI] [PubMed] [Google Scholar]
- 35.Walker AE, Seibert SM, Donato AJ, Pierce GL, Seals DR. Vascular Endothelial Function Is Related to White Blood Cell Count and Myeloperoxidase Among Healthy Middle-Aged and Older Adults. Hypertension. 2010;55:363–369. doi: 10.1161/HYPERTENSIONAHA.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler J, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30 374 individuals. European Heart Journal. 2004;25:1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Widlansky M, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, Keaney JF, Vita JA. Acute EGCG Supplementation Reverses Endothelial Dysfunction in Patients with Coronary Artery Disease. J Am Coll Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodman R, Watts GF, Puddey IB, Burke V, Mori TA, Hodgson JM, Beilin LJ. Leukocyte count and vascular function in Type 2 diabetic subjects with treated hypertension. Atherosclerosis. 2002;163:175–181. doi: 10.1016/s0021-9150(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 39.Yokozawa T, Nakagawa T, Kitani K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J Agric Food Chem. 2002;50:3549–3552. doi: 10.1021/jf020029h. [DOI] [PubMed] [Google Scholar]