Abstract
Objectives
Bone mineral density decreases with antiretroviral therapy (ART)-initiation, although the pathogenesis, including the role of tenofovir (TDF), is unclear. This study assessed changes in bone-turnover markers, osteoprotegerin (OPG), soluble receptor activator for nuclear factor kappa β ligand (sRANKL), and inflammation in subjects initiating TDF- vs. non-TDF-containing regimens, and determined the relationship between bone turnover, OPG/sRANKL, and inflammation.
Methods
This was a longitudinal, observational study comparing levels of bone-turnover markers (C-terminal telopeptide of type I collagen, CTX; osteocalcin (OC)), OPG, sRANKL, and inflammatory cytokines (soluble tumor necrosis factor-α receptor-I, -II (sTNFR-I,-II), interleukin-6) prior to ART and 6–12 months after ART-initiation with a TDF- vs. non-TDF-containing regimen in HIV-infected subjects 18–50 years old.
Results
87 subjects were enrolled (TDF=44; non-TDF=43). Groups were similar except subjects on TDF had a lower CD4 nadir (P<0.01) and were more likely to receive a protease inhibitor (PI) (P=0.03). At pre-ART, 35% and 1% of subjects had CTX and OC above normal range, respectively. Both increased with ART initiation, whereas OPG, sRANKL, and inflammatory markers significantly decreased. In multivariate models, increases in OC were associated with TDF-use, PI-use, and pre-ART levels of sTNFR-I, while increases in CTX were associated with CD4 nadir <50 cell/mm3. Increases in bone markers were unrelated to pre-ART levels of OPG/sRANKL and changes in OPG/sRANKL after ART-initiation.
Conclusions
TDF-use, PI-use, TNF-α activity, and advanced HIV disease are associated with changes in bone-turnover markers, underscoring the complicated interaction between ART, bone turnover, inflammation, and immune status, which extend beyond the OPG/RANKL system.
Keywords: HIV, osteoporosis, antiretroviral therapy, bone-turnover, inflammation
INTRODUCTION
The importance of aging-related co-morbidities among HIV-infected persons, such as osteoporosis, has increased. Even among younger HIV-infected patients, osteoporosis is 3–4 times more prevalent compared to HIV-uninfected controls [1], and it likely accounts for their increased fracture risk [2, 3]. Alterations in bone metabolism have been described since early in the combination antiretroviral (ART) era [4]; however, the pathophysiologic mechanisms underlying these bone changes are poorly understood.
HIV infection and the host immune response contribute to lower bone mineral density (BMD) [4–6]. For example, HIV viral proteins, Vpr and gp120, have been shown to stimulate osteoclast activity and induce osteoblast dysfunction and apoptosis in vitro [7, 8], underlying the clinical observation of increased bone resorption markers and relative suppression of bone formation markers in untreated HIV-infected patients [4]. These effects may be mediated or exacerbated by inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which appear to contribute to bone loss in other inflammatory conditions [9, 10].
With ART-initiation, inflammation decreases markedly and bone turnover increases [4]. Initially, this “re-coupling” of bone formation and resorption, accompanied by decreased inflammation was thought to be beneficial to bone health. However, subsequent studies [11, 12], including our own [13] have shown a 2–6% BMD decrease over the first 96 weeks of therapy, which correlates with the acceleration of bone turnover [14]. Thus, even though bone formation increases with ART-initiation, there is still a net loss of bone. It is not clear, however, whether these changes result from alterations in the inflammatory/immune environment or direct antiretroviral effects. Tenofovir (TDF) and protease inhibitors (PIs) appear to be associated with greater bone losses than other antiretrovirals [12], but the etiology behind this is not well-established.
Other cellular mechanisms may also be important mediators of bone loss and bone turnover changes with ART-initiation. Bone resorption and formation are normally coupled through the interaction of RANK (receptor activator for nuclear factor kappa β), a receptor on osteoclasts, and RANK Ligand (RANKL), an osteoblast-secreted factor. Osteoblasts also secrete osteoprotegerin (OPG) which binds to RANKL to prevent osteoclast activation. HIV viral components can upregulate RANKL [7], and certain antiretrovirals may enhance this effect [8]. Serum RANKL and OPG concentrations are associated with lower BMD in ART-treated HIV-infected patients [15], and are higher than in HIV-seronegative controls [16], despite some conflicting data [15].
To help distinguish the effects of ART and HIV infection on bone metabolism, longitudinal studies of ART-initiation are needed. To date, however, there are no such reported studies. Therefore, the purpose of this study was to evaluate changes in plasma concentrations of OPG/RANKL, bone markers, and inflammation in HIV-infected patients before and after ART-initiation. Because TDF has been implicated in the pathogenesis of reduced BMD in HIV-infected patients, our primary objective was to investigate whether changes in bone turnover markers were greater in those who initiate a TDF-containing regimen than in those receiving other nucleoside/nucleotide reverse transcriptase-inhibitors (NRTIs). Our secondary objectives were to (1) determine whether changes in bone turnover markers were associated with plasma concentrations of OPG/RANKL or markers of systemic inflammation prior to and after ART-initiation, and (2) investigate predictive factors for changes in bone turnover after ART-initiation.
METHODS
Study Subjects
Study subjects were identified from a clinical cohort at the Case Western Reserve University’s Center for AIDS Research (CFAR) in Cleveland, OH, and were eligible for enrollment if they were HIV-infected adults, 18–50 years of age, had initiated ART, and had stored plasma samples prior to and within 6–12 months after ART-initiation. Two groups were identified: those who initiated TDF-containing regimens and those who initiated non-TDF regimens. Exclusion criteria were known osteoporosis, fragility fractures, or prior therapy with bisphosphonates or other bone therapies. Demographic and clinical data were extracted from the CFAR database and clinical charts. Each subject signed written informed consent approved by the Institutional Review Board of University Hospitals Case Medical Center. Effects of efavirenz (EFV) initiation on 25-hydroxyvitamin D (25(OH)D) concentrations in this cohort has been previously described [17].
Biomarker Assays
Plasma samples were stored at −80° C until analysis without prior thawing. All assays were performed at Johns Hopkins Bayview Advanced Chemistry Laboratory, Baltimore, MD, USA. 25(OH)D was measured using radioimmunoassay (DiaSorin, Stillwater, Minnesota). Bone turnover markers included bone formation marker, osteocalcin (OC) and bone resorption marker, C-terminal telopeptide of type I collagen (CTX). CTX was measured using an enzyme-immunosorbent assay (Osteometer BioTech, Herlev, Denmark) and OC was measured using an immuno-radiometric assay (Nichols Institute Diagnostics, San Juan Capistrano, California, USA). Median intra-assay coefficients of variation were 8.15 and 2.97%, respectively, and the median inter-assay coefficients of variation were 0.01 and 6.3%, respectively. Expected normal range was 3.4–11.7 ng/mL (men) and 2.4–10.0 ng/mL (women) for OC, and 0.115–0.748 ng/ml (men), 0.142–1.351 ng/mL (post-menopausal women), and 0.112–0.738 ng/mL (pre-menopausal women) for CTX.
Plasma concentrations of OPG and total soluble RANKL (sRANKL) were determined by enzyme immunoassays (ALPCO, Salem, New Hampshire, USA) with coefficients of variations of 4.1% and 3.5% (intra-assay) and 6.2% and 9.3% (inter-assay), respectively. OPG to sRANKL ratio was then calculated. Plasma levels of inflammatory markers, including IL-6 and the soluble receptors of TNF-α, (sTNFR-I, -II) were measured via an enzyme-immunosorbent assay (R&D Systems, Minneapolis, Minnesota, USA).
Statistical Analysis
Baseline descriptive characteristics of the TDF vs. non-TDF groups were compared using Mann-Whitney U or Chi-square tests. Continuous measures are described by medians and interquartile ranges (IQR), and categorical variables are described with frequencies and percentages.
To address the primary objective, the change in biomarker levels prior to and 6–12 months after ART-initiation was compared between TDF and non-TDF groups using parametric (Student’s t test) and non-parametric (Wilcoxon rank-sum test) methods. Change in biomarker measurements from prior to ART-initiation to the on-treatment time-point within each group and with groups combined was determined by Wilcoxon testing.
To address the secondary objectives, the relationships between changes in each bone turnover marker and levels of OPG/sRANKL and inflammation prior to ART-initiation and on ART were determined using Spearman Correlation Coefficients. Then, multivariable linear regression models were constructed for each dependent variable (percentage change in CTX and OC). Models included all variables P<0.10 in the univariate analysis, or in the case of the inflammatory markers, the most significant one was chosen. Each model included TDF- and PI-use as covariates, and was adjusted for potential confounding variables, including age, race, sex, pre-ART CD4 count, baseline weight, duration of ART prior to the second sample, and baseline bone marker value. Because of skewed distributions, biomarker values were natural log-transformed prior to inclusion in the regression models. The level of significance for all analyses was set at 0.05. Analyses were performed using STATA 10.1 (College Station, TX).
RESULTS
Subject Characteristics and Between Group Comparison
HIV-infected subjects who initiated ART with TDF (N=44) and non-TDF regimens (N=43) were similar except subjects in the TDF-containing group had a lower CD4 count nadir (P<0.01) and were more likely to receive a PI (P=0.03) (Table 1). Atazanavir (ATV) was the most commonly used PI in both groups (45% in TDF group vs. 14% in non-TDF group, P<0.01). All but 3 subjects (all from non-TDF group) who received a PI also received low-dose ritonavir (r). EFV was the most commonly used drug in those subjects not on a PI, with 22/23 in the TDF group and 29/32 in the non-TDF group on EFV. In the TDF group, the most commonly used NRTIs in addition to TDF were emtricitabine (FTC) (N=33) and lamivudine (3TC) (N=8). The most commonly used NRTI combinations in the non-TDF group were zidovudine (AZT)/3TC (N=27), abacavir (ABC)/3TC (N=3), and ABC/3TC/AZT (N=13).
Table 1.
Characteristics of Subjects at ART Initiation.
| Median (interquartile range),
or % (no.) |
TDF-Treated (N = 44) |
Non-TDF-Treated (N = 43) |
P-value |
|---|---|---|---|
| Age | 34 (30,41) | 37 (30, 44) | 0.14 |
| Race (% white) | 41% (18) | 33% (14) | 0.42 |
| % Male | 75% (33) | 77% (33) | 0.85 |
| Weight (kg) | 74.4 (65.9, 81.1) | 74.8 (66.1, 88.6) | 0.55 |
| Nadir CD4 cell count (cells/mm3) | 91 (20, 232) | 236 (148, 334) | <0.01 |
| Baseline HIV-1 RNA (copies/mL) | 86,400 (45,316, 147,500) | 72,759 (21,575, 112,381) | 0.28 |
| Time since HIV diagnosis (months) | 17.5 (3, 66) | 12 (5, 36) | 0.65 |
| % Protease inhibitor use | 48% (21) | 26% (11) | 0.03 |
| % Lopinavir/ritonavir | 2% (1) | 9% (4) | 0.16 |
| % Atazanavir | 45% (20) | 14% (6) | <0.01 |
| % Fosamprenavir | 0% | 2% (1) | 0.31 |
| % Zidovudine | 0% | 91% (39) | |
| % Abacavir | 0% | 40% (17) | |
| % Current smokers | 71% (31) | 56% (24) | 0.16 |
| % Current alcohol use (>2 units/day) | 7% (3) | 12% (5) | 0.46 |
| 25 hydroxyvitamin D (ng/mL) | 22.6 (12.3,31.5) | 20.7 (13.4, 26.2) | 0.50 |
ART, antiretroviral therapy; TDF, tenofovir
Pre-ART Biomarkers
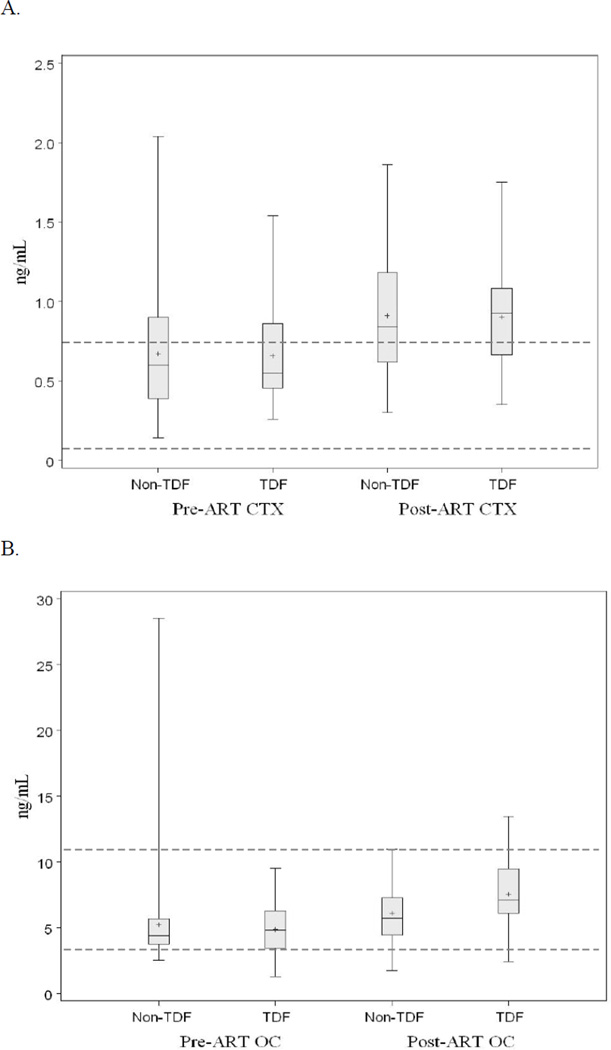
Prior to ART-initiation, median (IQR) for the bone turnover markers, CTX and OC, was 0.55 (0.46, 0.86) ng/mL and 4.8 (3.4, 6.3) ng/mL, respectively for the TDF group, and 0.60 (0.39, 0.9) ng/mL and 4.4 (3.7, 5.7) ng/mL, respectively for the non-TDF group (Figure 1).
Figure 1.
Box and whisker plots depict the distribution of bone turnover marker values (A. CTX, and B. OC) for TDF and non-TDF groups at pre-ART and post-ART time points. Center lines for each plot represent the median; whereas, top and bottom lines represent the 25th and 75th percentiles, respectively. Plus symbols (+) represent means. Dotted lines represent the approximate upper and lower range of normal for CTX or osteocalcin. CTX, C-terminal telopeptide of collagen type I; OC, osteocalcin; TDF, tenofovir; ART, antiretroviral therapy
Groups were similar for both markers (P=0.94 and 0.77, respectively). For the entire cohort, 34.5% had CTX values and 1% had OC values above the normal range. In contrast, none of the subjects had CTX values below the normal range, whereas 13% of the cohort had low OC values.
Pre-ART levels of OPG and sRANKL, as well inflammatory cytokines are shown in Table 2. There were no differences between groups with the exception of sRANKL which was higher in the TDF-group, making the OPG/sRANKL ratio in this group significantly lower.
Table 2.
Bone Markers and Inflammatory Markers Pre- and Post-ART Initiation. Values Expressed as Median (Interquartile Range).
| Total Cohort (N = 87) |
TDF-Treated (N = 44) |
Non-TDF Treated (N = 43) |
P-value† | P-value†† | ||
|---|---|---|---|---|---|---|
| Bone Markers | ||||||
| OPG | Pre-ART | 4.5 (3.7, 5.6) | 4.4 (3.7, 5.1) | 4.9 (3.7, 5.6) | 0.31 | 0.49 |
| Post-ART | 4.1 (3.4, 5.5)* | 4.0 (3.1, 5.0) | 4.2 (3.7, 5.6) | 0.10 | ||
| sRANKL | Pre-ART | 0.1 (0.07, 0.18) | 0.13 (0.9, 0.25) | 0.09 (0.05, 0.13) | <0.001 | 0.03 |
| Post-ART | 0.08 (0.06, 0.13)* | 0.08 (0.07, 0.18)* | 0.07 (0.03, 0.10) | 0.04 | ||
| OPG/sRANKL | Pre-ART | 44.9 (22.7, 76.9) | 31.1 (18.1, 55.0) | 58.8 (37.5, 96.7) | <0.001 | 0.83 |
| Post-ART | 52.4 (32.5, 78.9) | 43.4 (22.4, 65.2) | 59.7 (41, 51) | <0.001 | ||
| Inflammatory Markers | ||||||
| IL-6 (pg/mL) | Pre-ART | 1.45 (0.74, 2.12) | 1.65 (0.92, 2.21) | 1.31 (0.72, 2.02) | 0.29 | <0.01 |
| Post-ART | 0.85 (0.55, 1.58)* | 0.73 (0.52, 1.32)* | 1.11 (0.64, 1.88) | 0.07 | ||
| sTNFR-I (pg/mL) | Pre-ART | 2494 (2009,3424) | 2432 (2007, 3161) | 2741 (2119, 3875) | 0.25 | 0.23 |
| Post-ART | 2230 (1797, 3018)* | 2024 (1601, 2762)* | 2700 (1995, 3122) | 0.01 | ||
| sTNFR-II (pg/mL) | Pre-ART | 7537 (6292, 11228) | 7802 (6682, 11243) | 7537 (5982, 11163) | 0.36 | 0.20 |
| Post-ART | 4883 (3834, 6593)* | 4965 (3952, 6463)* | 4489 (3797, 6603)* | 0.55 | ||
ART, antiretroviral therapy; TDF, tenofovir; osteoprotegerin, OPG; sRANKL, soluble receptor activator for nuclear factor kappa β ligand; IL-6, interleukin-6; sTNFR-I, -II, soluble tumor necrosis factor-α receptor I, -II
P<0.05 for within-group change from pre- to post-ART concentration;
P-value for between-group difference at the pre-ART or post-ART concentration
P-value for between-group difference in changes from pre-ART to post-ART time point
Changes in Cohort Characteristics after ART-initiation
During the study period, 4 subjects from the TDF group changed ART regimens: one changed the individual PI from ATV/r to lopinavir/r and changed 3TC to FTC, another subject changed from ATV to EFV, and one subject changed from 3TC to FTC. No subject in the TDF group stopped TDF. In the non-TDF group, 3 subjects changed regimens: one subject discontinued AZT from a regimen which contained ABC/3TC/EFV, another subject switched from AZT/3TC/EFV to TDF/3TC/ATV/r after 133 days, and one subject switched ABC for TDF after 58 days. Similar number of subjects from each group had HIV-1 RNA levels <400 copies/mL after ART-initiation (TDF: 98% vs. non-TDF: 95%; P=0.54). Post-ART CD4 levels increased similarly in both groups (TDF: +240 vs. non-TDF: +222 cells/mm3), but the TDF-group still had a lower median CD4 cell count level than the non-TDF group (331 vs. 458 cells/mm3, respectively; P<0.01).
Post-ART Biomarkers
Both groups had significant increases in their CTX and OC values (CTX: P<0.0001; OC: P<0.0001). For the TDF group, median CTX and OC increased by 0.38 and 2.2 ng/mL, respectively (post-ART median (IQR): CTX 0.93 (0.67, 1.08) ng/mL; OC 7.0 (6.1, 9.5) ng/mL).
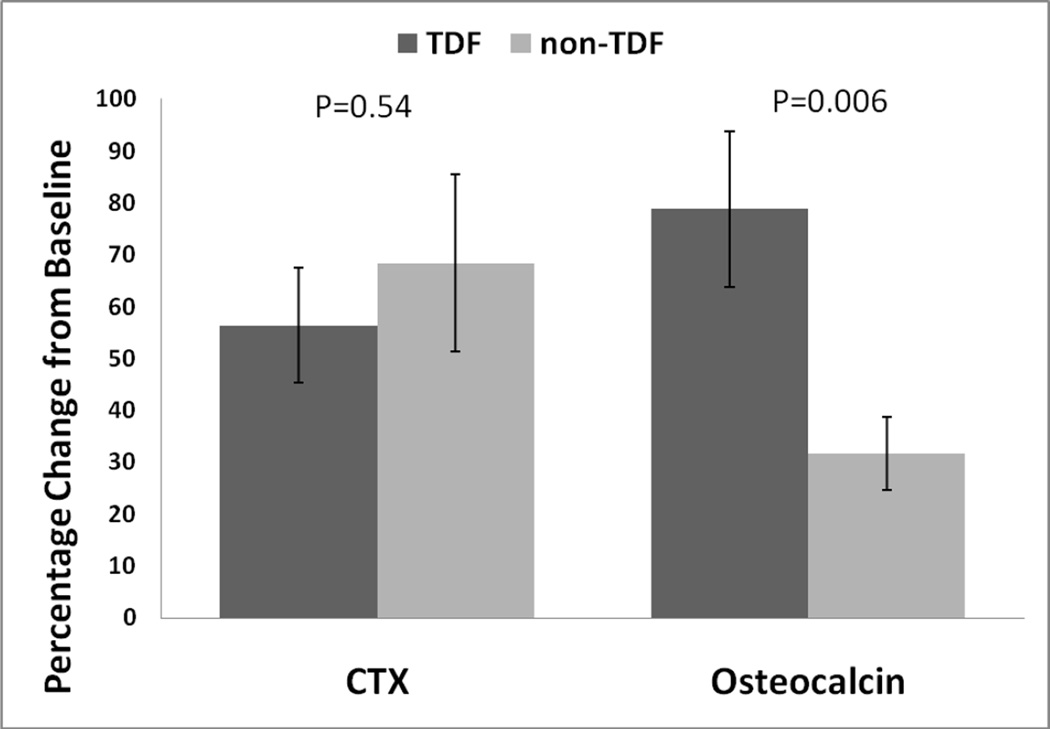
For the non-TDF group, CTX and OC medians increased by 0.24 and 1.3 ng/mL, respectively (post-ART median (IQR): CTX 0.84 (0.62, 1.18) ng/mL; OC: 5.7 (4.4, 7.2) ng/mL) (Figure 1). Between-group changes were significant for OC (P=0.03), i.e. the TDF group had greater increases in OC after ART-initiation than the non-TDF group, but changes in CTX were not different between groups (P=0.73). For the entire cohort, CTX increased by 41% (IQR: 5%, 87%) and OC increased by 33% (IQR: 8%, 88%). The mean percentage change in CTX was similar between groups, but the percentage change in OC was greater in the TDF group than the non-TDF group (Figure 2).
Figure 2.
Mean percentage change in C-telopeptide (CTX) and osteocalcin in tenofovir (TDF)-and non-TDF groups 6–12 months after initiation of antiretroviral therapy. P< 0.001 for all changes from baseline. Error bars represent standard errors. P values represent difference between TDF and non-TDF groups.
Post-ART levels of OPG, sRANKL, and inflammatory markers are shown in Table 2. In the total cohort, both OPG and sRANKL decreased significantly with ART-initiation, but their ratio remained unchanged. Whereas the change in OPG with ART-initiation was similar in the two treatment groups, the decrease in sRANKL in the TDF group was greater than the non-TDF group (P=0.03). IL-6, sTNFR-I, and sTNFR-II decreased significantly in the total cohort with ART-initiation. The percentage decrease in IL-6 tended to be larger in the TDF group (mean difference ± standard error from non-TDF group: −48 ± 27%, P=0.08) after adjustment for age, race, sex, pre-ART CD4 cell count, baseline weight, duration of ART prior to the second sample, PI-use, and baseline IL-6 concentration. The median percentage change in 25(OH)D was similar between the TDF and non-TDF groups, −10.2% vs −12.7%, P=0.63).
Relationship between OPG, RANKL, Inflammatory Markers and Bone Turnover Markers with ART-initiation
Pre-ART levels of OPG, sRANKL, and inflammatory markers, and changes in these markers were correlated with percent change in OC and CTX using Spearman Correlation. OPG and sRANKL at pre-ART and changes in these markers from pre-ART to post-ART were not related to percent change of OC or CTX. Pre-ART levels of IL-6, sTNFR-I, and sTNFR-II were not related to the percent change in CTX, and changes in these markers were not related to either ART-related changes in OC or CTX. Only pre-ART inflammatory markers were significantly related to the percent change in OC (IL-6: Spearman’s rho=0.32; P<0.01; sTNFR-I: rho=0.21, P=0.05; sTNFR-II: rho=0.27, P=0.01). Pre-ART 25(OH)D or the percent change in 25(OH)D were not correlated to the percent change in OC or CTX (data not shown).
Regression Analysis
Biomarkers which were significant in the univariate analysis, and variables thought to potentially affect results, including age, race, sex, pre-ART CD4 cell count, baseline weight, duration of ART prior to the second sample, TDF-use, PI-use, and baseline values of the bone turnover markers were included in the multivariable regression analyses with percent change in OC and CTX from the pre-ART to post-ART time-points as dependent variables. Only baseline CD4 count and pre-ART CTX levels were associated with percent change in CTX (Table 3A). Age, TDF-use, PI-use, pre-ART sTNFR-I and pre-ART OC were all associated with percent change in OC (Table 3B).
Table 3.
Factors Associated with the Change in Bone Turnover Markers with ART-Initiation
| A. Factors Associated with % Change in CTX | |||
|---|---|---|---|
| β | SE | P | |
| Pre-ART CD4 cell count <50 cells/mm3 | 41 | 18 | 0.03 |
| Pre-ART CTX (per log difference) | −126 | 16 | <0.01 |
| B. Factors Associated with % Change in OC | |||
|---|---|---|---|
| β | SE | P | |
| Age (per year difference) | −1.8 | 0.8 | 0.04 |
| TDF (vs. no TDF use) | 32 | 14 | 0.03 |
| PI (vs. no PI use) | 31 | 14 | 0.03 |
| Pre-ART sTNFRI (per log difference) | 52 | 16 | <0.01 |
| Pre-ART OC (per log difference) | −116 | 15 | <0.01 |
Models adjusted for age, race, sex, pre-ART CD4 cell count, baseline weight, duration of ART prior to the second sample, TDF-use, PI-use, and pre-ART value of the bone turnover marker
ART, antiretroviral therapy; CTX, C-terminal telopeptide of type I collagen; OC, osteocalcin; TDF, tenofovir; PI, protease; sTNFRI, soluble tumor necrosis factor-α receptor-I
DISCUSSION
In this longitudinal study, we found that bone resorption and formation markers significantly increased 6–12 months after ART-initiation, whereas OPG, sRANKL, and inflammatory markers decreased over the same period. Increases in OC were independently associated with TDF-use, PI-use, and pre-ART levels of sTNFR-I, whereas greater increases in CTX were associated with lower nadir CD4 count. Although the OPG/RANK/RANKL system is thought to play a major role in the coupling of osteoblast and osteoclast activity [18], the acceleration in bone turnover with ART-initiation was unrelated to pre-ART levels of OPG, sRANKL, or their changes after ART-initiation. These findings underscore the complicated interaction between ART, bone turnover, inflammation, and immune status, which extend beyond the OPG/RANK/RANKL system.
The skeleton undergoes constant remodeling with osteoclasts resorbing older bone and osteoblasts laying down new bone. This process is normally tightly coupled, such that osteoclast and osteoblast activity is matched and the total amount of bone remains constant. The actions of osteoclasts and osteoblasts can be assessed in vivo using markers of bone turnover which can be quantified in the serum, urine, or plasma. CTX is a breakdown product of type 1 collagen whose circulating concentrations represent osteoclast activity [19] and increase during conditions marked by high bone resorption, such as menopause and hyperparathyroidism [20, 21], and decrease by 50% or more during antiresorptive therapy with bisphosphonates [22]. Osteocalcin is a polypeptide of unknown function, secreted primarily by osteoblasts into the bone matrix. Circulating osteocalcin represents the 10–40% of secreted osteocalcin that is not adsorbed to hydroxyapatite [23]. Owing to its osteoblast origin, osteocalcin is considered a marker of bone formation. However, it should be noted that osteocalcin increases in conditions of high bone resorption, such as estrogen deficiency [24], albeit to lesser extent than markers of bone resorption, and decreases with bisphosphonate therapy [25].
Our study provides insight into the balance of osteoclast and osteoblast activity before and after ART-initiation. In the pre-ART sample, we observed an uncoupling of bone resorption and formation. Whereas 34.5% had CTX levels above the normal range, OC was relatively suppressed, as previously observed [4], and is consistent with in vitro models demonstrating direct effects of HIV-1 and its proteins on increasing osteoclastogenesis and impairing osteoblast function [7]. Similar to other studies [4, 14], we observed a rapid acceleration in bone turnover with ART-initiation. We speculate that since osteoclast activity was already increased prior to ART-initiation and that the increase in osteoclast activity was greater than the osteoblast activity (ΔCTX, 41% vs. ΔOC, 33%), the acceleration in bone turnover with ART-initiation favors bone resorption and accounts for the 2–6% bone loss seen with ART-initiation [12, 13, 26]. Indeed, the ASSERT study recently showed that a greater acceleration of bone turnover with initiation of TDF/FTC/EFV or ABC/3TC/EFV was associated a more severe loss in BMD after 48 weeks of treatment [14].
We specifically designed the study to compare bone turnover in patients receiving TDF compared to other NRTIs. Several studies in ART-naïve subjects have demonstrated decreases in BMD with initiation of TDF [14], and a recent study in virologically-suppressed HIV-infected subjects found that those who were randomized to switch to TDF/FTC had a significant decrease in BMD after 96 weeks compared to those randomized to ABC/3TC, confirming an independent effect of TDF on bone [27]. We hypothesized, therefore, that those initiating TDF would have more accelerated bone turnover compared to non-TDF treated subjects. Interestingly, although the increase in bone resorption was similar in the two groups, the median increase in osteocalcin in the TDF-treated subjects was greater than in the non-TDF group, which was also observed in the ASSERT study [14].
The mechanisms underlying this observation are unclear and deserve further exploration. It is possible that the observed BMD decrease with TDF may represent abnormal bone mineralization (i.e. osteomalacia) rather than loss of bone volume or structure which is characteristic of osteoporosis. At high doses, TDF exposure in SIV-infected rhesus monkeys has been associated with impaired bone mineralization [28] and there have been rare case reports of severe osteomalacia in HIV-infected persons treated with TDF [29, 30]. However, subclinical phosphate wasting, perhaps contributing to osteomalacia, is common in TDF-treated patients [31]. In the Swiss HIV Cohort Study, the prevalence of excessive renal phosphate wasting was 2.4–3.4-fold higher in those receiving TDF [32], and was associated with high bone turnover [33]. Although bone alkaline phosphatase is considered a more sensitive marker of osteomalacia[34], osteocalcin has also been showed to be increased in conditions associated with impaired bone mineralization [35], including excessive urinary phosphate losses [36]. Therefore, our finding of greater increases in osteocalcin with TDF exposure may reflect a mineralization deficit. Alternatively, osteocalcin secretion is stimulated by both vitamin D (1,25(OH)2D) and parathyroid hormone (PTH) [23], which are both increased with initiation of TDF [37]. Because PTH secretion, vitamin D metabolism, phosphate homeostasis, and bone turnover are closely inter-related, further longitudinal studies are required to understand the pathophysiological mechanisms underlying TDF’s effect on bone.
We also found that PI-use was independently associated with greater increases of osteocalcin with ART-initiation. PIs have been implicated in HIV-related bone loss previously [38], and recently ART-naïve patients randomized to ATV showed greater BMD decreases in the lumbar spine than those on efavirenz [12]. This last study is particularly relevant since the majority of subjects on PIs in our study were taking ATV/r. Ritonavir also has been shown to have in vitro effects on bone; however, data are conflicting as to its overall effect on osteoclast and osteoblast activity [39, 40]. As with TDF, the mechanisms underlying the PI effect on osteocalcin require further investigation. While high bone turnover leading to reductions in bone mass and architecture would be consistent with our data, our study was limited by not including assessment of bone density or architecture to evaluate this relationship. Impaired mineralization from phosphate wasting or abnormalities in vitamin D metabolism are also potential explanations for increases in osteocalcin [23]. In vitro studies have shown that certain PIs can affect vitamin D bioactivation [41] and PI-use was associated with proximal tubule dysfunction in the Swiss HIV Cohort Study [32]. PI-use has also been associated with increases in PTH in the MEDICLAS study [11], which may lead to increased osteocalcin.
Baseline inflammation was also associated with OC increases with ART-initiation. In the general population, inflammation has been implicated in the pathogenesis of osteoporosis and bone fractures, and IL-6 and TNF-α are potent stimulators of osteoclast activity, leading to uncoupled bone resorption in some people with osteoporosis [42]. However, the role of inflammation in HIV-related bone loss is not clear. In treatment-naïve subjects, TNF-α activity is associated with decreased bone formation and increased bone resorption [43]. We found that ART-initiation was associated with decreases in IL-6 and TNF-α activity, consistent with previous studies [13, 43]. We also found that pre-ART cytokine levels, but not the extent of their decrease, were associated with greater increases in osteocalcin. It is possible that this association represents the release of osteoblast inhibition with suppression of HIV and reduction of systemic inflammation. We have previously shown that higher baseline TNF-α was associated with more profound reductions in BMD, but this effect was no longer significant after adjustment for baseline CD4 count [13]. In contrast, the relationship between baseline sTNFR-1 and the change in osteocalcin observed in our study was independent of baseline CD4 count. Further work is needed to understand the relationship between inflammation, ART, and bone turnover. In many inflammatory conditions, such as rheumatoid arthritis, suppression of TNF-α activity leads to improvements in BMD [9, 10]. This is in contrast to what is observed during the first 48 weeks after ART-initiation when the marked suppression of systemic inflammation is associated the acceleration of bone turnover and the loss of bone mineral density.
Along with the increases in osteocalcin, CTX, a sensitive measure of bone resorption, increased by 41% after ART-initiation. However, in contrast to the findings with osteocalcin, only more advanced HIV disease was associated with greater increases in bone resorption after ART-initiation. This finding is consistent with other studies [11] and suggests that immune modulation plays a role in HIV-related bone loss and earlier ART-initiation may attenuate the acceleration in bone turnover and reduce the burden of osteoporosis in HIV-infected patients.
The actions of osteoclasts and osteoblasts are normally coupled through the OPG/RANK/RANKL system [18]. RANKL and OPG are members of the TNF receptor superfamily which are produced by osteoblasts to induce and inhibit, respectively, the differentiation and activation of osteoclasts. The ratio of OPG/RANKL is widely used in clinical studies as a parameter to measure balance in the OPG/RANK/RANKL system [44, 45]. Previous work has implicated the dysregulation of OPG/RANK/RANKL system in the pathogenesis of reduced bone density among HIV-infected patients [7, 8, 15]. We hypothesized that the acceleration in bone turnover characteristic of ART-initiation would be associated with changes in systemic concentrations of OPG and RANKL. We found that both OPG and RANKL decreased significantly with ART-initiation, while their ratio remained constant. Furthermore, we did not find any association between baseline concentrations in OPG or RANKL or their change with ART-initiation and markers of bone turnover. While these findings may suggest that the changes in bone turnover with ART-initiation are independent of the OPG/RANK/RANKL system, there are significant limitations to this interpretation. First, these biomarkers are difficult to measure which may account for their inconsistent association with post-menopausal osteoporosis [46]. Second, their plasma concentrations may not reflect their concentrations in the bone micro-environment and soluble levels of RANKL may not correlate with the activity of membrane RANKL, which has been shown to be more potent in stimulating osteoclastogenesis in vitro [47]. Next, production of OPG and RANKL are not specific to osteoblasts. Among other cell types, activated T-cells are an important source of RANKL and activated B-cells produce OPG [18] and both of these cell types undergo significant changes during ART-initiation [48]. Furthermore, changes in OPG and RANKL during ART-initiation may be modified by concomitant decreases in systemic inflammation, as many cytokines have been shown to affect OPG and RANKL concentrations [49]. Therefore, any changes in circulating OPG and RANKL related to bone turnover in our study might have been obscured by alterations in these biomarkers caused by changes in immune function during ART-initiation. Investigation of the specific effect of an antiretroviral medication, such as TDF, on the OPG/RANK/RANKL system would best be carried out in ART-treated HIV-infected persons switching ART regimens or in HIV-uninfected persons receiving ART for the prevention of HIV infection. In this way, the specific effect of the ART component can be evaluated independently of its effect on immune function.
Our study had additional limitations. First, we did not include an otherwise similar HIV-uninfected control arm. This group would be useful to understand whether biomarkers levels were within normal range prior to ART-initiation and to provide context for the observed changes with ART-initiation. Second, the allocation of TDF or PIs was not randomized and therefore these groups may have differed with respect to unmeasured confounders that could have affected the results. In particular, nadir CD4 was lower in the TDF group compared to the non-TDF group. Although we adjusted for this variable in the analyses, it is possible that residual confounding based on HIV disease severity, not captured by CD4 cell count, contributed to the observed effect of TDF on bone turnover. We believe that such residual confounding is not likely since our results regarding the effect of TDF on bone markers are similar to the ASSERT study, where HIV disease severity was balanced between the groups through randomization [14]. Next, we did not have measures of PTH, 1,25 dihydroxyvitamin D or other vitamin D metabolites, or fractional excretion of phosphate, which may have helped clarify the inter-relationships between vitamin D and phosphate metabolism, TDF, and bone turnover. Finally, without BMD assessment or bone histomorphometry, we were unable to investigate how the bone turnover marker changes related to changes in BMD, bone structure, or mineralization. This is particularly important in order to determine the mechanisms underlying the observed effect of TDF and PIs seen in this study. Nevertheless, these limitations do not detract from the novel findings in this study, which provide a substrate for future research. It is clear that there are complex interplays between ART, inflammation, immune modulation, bone turnover, and the OPG/RANK/RANKL system in HIV-infected individuals, and it is imperative to further clarify these relationships.
ACKNOWLEDGEMENTS
This study was partially supported by a research grant from GlaxoSmithKline.
Footnotes
Sources of support:
Disclosure statement.
ACR has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline. All other authors: no conflicts.
DISCLOSURE STATEMENT
TTB serves as a consultant and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, ViiV Healthcare, Tibotec, and Abbott. GAM serves as a consultant and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, and Abbott. GAM currently chairs a DSMB for Pfizer-funded study. ACR has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline. All other authors: no conflicts.
REFERENCES
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.Dao C, Young B, Buchacz K, Baker R, Brooks J HOPS. Higher and increasing rates of fracture among HIV-infected persons in the HIV outpatient study (HOPS) compared to the general US population, 1994 to 2008. 17th Conference on Retroviruses and Opportunistic Infections. 2010 [Google Scholar]
- 3.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. Journal of Clinical Endocrinology & Metabolism. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aukrust P, Haug CJ, Ueland T, Lien E, Muller F, Espevik T, Bollerslev J, Froland SS. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999;84:145–150. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 5.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, Hoffmann M, Tebas P. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 6.Moore AL, Vashisht A, Sabin CA, Mocroft A, Madge S, Phillips AN, Studd JW, Johnson MA. Reduced bone mineral density in HIV-positive individuals. AIDS. 2001;15:1731–1733. doi: 10.1097/00002030-200109070-00019. [DOI] [PubMed] [Google Scholar]
- 7.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150:67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 8.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL crosstalk. J Biol Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 9.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med. 2009;122:599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook P. Tumour necrosis factor blockade and the risk of osteoporosis: back to the future. Arthritis Res Ther. 2007;9:107. doi: 10.1186/ar2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vonderen MG, Lips P, van Agtmael MA, Hassink EA, Brinkman K, Geerlings SE, Sutinen J, Ristola M, Danner SA, Reiss P. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23:1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 12.McComsey G, Kitch D, Daar E, Tierney C, Jahed N, Tebas P, Myers L, Sax P A5224 aAS. Bone and Limb Fat Outcomes of ACTG A5224s, a Substudy of ACTG A5202: A Prospective, Randomized, Partially Blinded Phase III Trial of ABC/3TC or TDF/FTC with EFV or ATV/r for Initial Treatment of HIV-1 Infection. The 17th Conference on retroviruses and Opportunistic Infections. 2010 [Google Scholar]
- 13.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 14.Moyle G, Givens N, Pearce H, Compston J ASSERT. Effect of ART on bone turnover markers and bone density in HIV infected patients. 11th International Workshop on Adverse Drug Reactions and Co-Morbidities in HIV. 2009 [Google Scholar]
- 15.Seminari E, Castagna A, Soldarini A, Galli L, Fusetti G, Dorigatti F, Hasson H, Danise A, Guffanti M, Lazzarin A, Rubinacci A. Osteoprotegerin and bone turnover markers in heavily pretreated HIV-infected patients. HIV Med. 2005;6:145–150. doi: 10.1111/j.1468-1293.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Sala N, Bricalli D, Zuin G, Chiumello G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001;15:1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 17.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 15:425–429. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 18.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eastell R, Hannon RA. Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 2008;67:157–162. doi: 10.1017/S002966510800699X. [DOI] [PubMed] [Google Scholar]
- 20.Kushida K, Takahashi M, Kawana K, Inoue T. Comparison of markers for bone formation and resorption in premenopausal and postmenopausal subjects, and osteoporosis patients. J Clin Endocrinol Metab. 1995;80:2447–2450. doi: 10.1210/jcem.80.8.7629240. [DOI] [PubMed] [Google Scholar]
- 21.Seibel MJ, Gartenberg F, Silverberg SJ, Ratcliffe A, Robins SP, Bilezikian JP. Urinary hydroxypyridinium cross-links of collagen in primary hyperparathyroidism. J Clin Endocrinol Metab. 1992;74:481–486. doi: 10.1210/jcem.74.3.1740480. [DOI] [PubMed] [Google Scholar]
- 22.Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 23.Lee AJ, Hodges S, Eastell R. Measurement of osteocalcin. Ann Clin Biochem. 2000;37((Pt 4)):432–446. doi: 10.1177/000456320003700402. [DOI] [PubMed] [Google Scholar]
- 24.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 25.Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 26.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 27.DA Cooper, M Bloch, A Humphries, J Amin, D Baker, S Emery., editors. 16th Conference on Retroviruses and Opportunistic Infections. Montreal, Canada: 2009. Simplification with fixed-dose tenofovir-emtricitaine or abacavir-lamivudine in adults with suppressed HIV replication (The Steal Study): A randomized, open-label, 96-week, non-inferiority trial. [Google Scholar]
- 28.Castillo AB, Tarantal AF, Watnik MR, Martin RB. Tenofovir treatment at 30 mg/kg/day can inhibit cortical bone mineralization in growing rhesus monkeys (Macaca mulatta) J Orthop Res. 2002;20:1185–1189. doi: 10.1016/S0736-0266(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 29.Wanner DP, Tyndall A, Walker UA. Tenofovir-induced osteomalacia. Clin Exp Rheumatol. 2009;27:1001–1003. [PubMed] [Google Scholar]
- 30.Perrot S, Aslangul E, Szwebel T, Caillat-Vigneron N, Le Jeunne C. Bone pain due to fractures revealing osteomalacia related to tenofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patient. JCR: Journal of Clinical Rheumatology. 2009;15:72–74. doi: 10.1097/RHU.0b013e31819c20d8. [DOI] [PubMed] [Google Scholar]
- 31.Badiou S, De Boever CM, Terrier N, Baillat V, Cristol JP, Reynes J. Is tenofovir involved in hypophosphatemia and decrease of tubular phosphate reabsorption in HIV-positive adults? J Infect. 2006;52:335–338. doi: 10.1016/j.jinf.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Fux C, Opravil M, Cavssini M, Calmy A, Spycher B, Flepp M, Hasse B, Schmid P, Stockle M, Gurtner-de la Fuente V, Rauch A, Furrer H. The 16th Conference on retroviruses and Opportunistic Infections. Montreal, Canada: 2009. Tenofovir and protease inhibitor use are associated with an increased prevalence of proximal tubule dysfunction in the Swiss Cohort Study. [Google Scholar]
- 33.Fux C, Hasse B, Opravil M, Cavssini M, Calmy A, Gurtner-de la Fuente V, Schmid P, Stockle M, Flepp M, Furrer H. The 17th Conference on retroviruses and Opportunistic Infections. San Francisco, CA: 2010. Bone turnover, in particular osteoclast activity in patients with confirmed proximal tubulopathy within the Swiss Cohort Study. [Google Scholar]
- 34.Daniels ED, Pettifor JM, Moodley GP. Serum osteocalcin has limited usefulness as a diagnostic marker for rickets. Eur J Pediatr. 2000;159:730–733. doi: 10.1007/pl00008336. [DOI] [PubMed] [Google Scholar]
- 35.Demiaux B, Arlot ME, Chapuy MC, Meunier PJ, Delmas PD. Serum osteocalcin is increased in patients with osteomalacia: correlations with biochemical and histomorphometric findings. J Clin Endocrinol Metab. 1992;74:1146–1151. doi: 10.1210/jcem.74.5.1569162. [DOI] [PubMed] [Google Scholar]
- 36.Tsilchorozidou T, Yovos JG. Hypophosphataemic osteomalacia due to de Toni-Debre-Fanconi syndrome in a 19-year old girl. Hormones (Athens) 2005;4:171–176. doi: 10.14310/horm.2002.11156. [DOI] [PubMed] [Google Scholar]
- 37.Viread® (tenofovir disoproxil fumarate) (prescribing information) Gilead Sciences. Foster City; California: Mar, 2010. [Google Scholar]
- 38.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 39.Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, Teitelbaum SL, Ross FP. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses. 2007;23:243–250. doi: 10.1089/aid.2006.0084. [DOI] [PubMed] [Google Scholar]
- 41.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Aukrust P, Muller F, Lien E, Nordoy I, Liabakk NB, Kvale D, Espevik T, Froland SS. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 44.Racz JG. Drug use by the members of youth subcultures in Hungary. Int J Addict. 1992;27:289–300. doi: 10.3109/10826089209068743. [DOI] [PubMed] [Google Scholar]
- 45.Skoumal M, Kolarz G, Haberhauer G, Woloszczuk W, Hawa G, Klingler A. Osteoprotegerin and the receptor activator of NF-kappa B ligand in the serum and synovial fluid. A comparison of patients with longstanding rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2005;26:63–69. doi: 10.1007/s00296-004-0579-1. [DOI] [PubMed] [Google Scholar]
- 46.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007;92:4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- 47.Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J Biol Chem. 2006;281:36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 48.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]