Abstract
Recently, many of the complexities associated with cardiovascular diseases (CVD) have been unlocked. However, despite these breakthroughs, CVD and its related complications are the leading contributors of morbidity and mortality worldwide, which indicates the shortcomings of current treatment regimens and the need for continued research. Published data within the field clearly indicates that CVD are built on inflammation and autoimmune platforms, though a strong, fundamental understanding of the mechanisms remains elusive. Areas such as the mechanisms underlying increased immunogenicity of self-proteins in the cardiovascular system, the roles of immunogenic auto-antigens in eliciting inflammatory autoimmune responses, and the immunosuppressive mechanisms involved in controlling inflammatory and autoimmune cardiovascular diseases remain to be well-understood. We will delve into these topics and the advancements made within the field in this review. Specifically, we will concentrate on the innate and adaptive immune responses mediating immunogenicity; the mechanisms of inflammation and autoimmunity in atherogenesis; the mechanisms of inflammation and autoimmunity in diabetic atherosclerosis; immunogenicity and stem cell therapy; as well as immunogenicity and immunosuppression. In depth examination and comprehension of these topics will provide insight into the recent progress of the field and bring to the forefront potentially novel therapeutic avenues.
Keywords: immunogenicity, inflammation, autoimmunity, atherosclerosis, diabetes, stem cell therapy, immunosuppression
I. Introduction
A cardiovascular disease with an onset characterized by endothelial injury and lipid dysregulation, atherosclerosis was recently discovered to be driven by an inflammatory component [1,2]. Shortly thereafter, research demonstrated that both the innate and adaptive immune systems contributed to the pathogenesis of atherosclerosis. Research pursuing this breakthrough yielded fruitful results providing cellular mechanisms and new therapeutic targets. In particular, the adaptive immune system was found to contribute through a complex autoimmune process [3,4]. This finding led to development in therapeutic avenues such as vaccines [4–6]. When considering atherosclerosis as a disease it is vital to remember that lipid dysregulation, inflammation, and autoimmunity are not contending viewpoints. Rather, atherosclerosis should be thought of as a multi-faceted disease and its complexity indicates a necessity to consider multiple aspects when designing therapeutics. In this chapter, we will explore the immunogenicity of cardiovascular diseases (CVD) to better elucidate the mechanisms behind these diseases. This could provide inroads for the pioneering effort to target disease development instead of merely treating symptoms. This paper will first review the mechanisms critical to modulating immunogenicity by the adaptive and innate immune systems and then explore how these mechanisms contribute to CVD development. Finally, therapeutic strategies involving stem cell therapy, common suppressive drugs, and future therapeutic directions will be discussed to conclude this review. The progress from a variety of scientific fields will be highlighted illustrating the complexity of CVD and the requirement of multi-discipline collaboration to illuminate disease pathology and development of therapeutic strategies.
II. Innate and Adaptive Immune Responses and Immunogenicity
A concise overview of relevant immunological effectors and their influence on the adaptive and innate immune system will now be assessed to lay the foundation for further immunogenicity discussions. Consisting of a network of cells, tissues, and organs, the immune system protects its host against agents perceived as pathogens. In order to accomplish this function, the immune system must be capable of differentiating between self and non-self. Following the recognition of non-self agents, the system must also be competent in the elimination of these agents. The immune responses required for recognition and elimination can be categorized into two major classes, innate or adaptive. An innate immune response can occur instantaneously or evolve over several hours, the duration often dependent on the actions taken. The response could simply involve the recognition and removal of a foreign agent, or it may entail the recruitment and activation of other immune effectors. If a foreign agent endures the innate immune response, the adaptive immune system will be activated. Unlike the timely action of the innate system, the adaptive immune response requires several days to respond to the foreign agents [7]. The components of the adaptive immune system vary in activity with antigen-specific lymphocytes targeting unique pathogens for removal while memory cells safeguard from future infection by the same pathogen. It is important to recognize that the differences in duration and longevity between the two systems can be attributed to the unique mechanisms of each [7,8].
II.A. Activation of the Innate Immune Response
II.A.1. Innate Immune Receptors
The actions taken by the innate immune system are dependent on the specific pathogen present and the cellular receptors activated. Despite lacking the remarkable specificity of the adaptive immune system, the innate immune system is able to recognize numerous cellular patterns common among microbes and toxins through unique receptors referred to as pattern recognition receptors (PRRs) [7,8]. One such receptor, Toll-like receptor 4 (TLR4), is capable of recognizing lipopolysaccharide, which is found in the cell walls of gram negative bacteria. Similarly, TLR2 can recognize the proteoglycans of gram positive bacteria [8]. In addition to PRRs, activated phagocytic receptors can initiate the destruction of foreign agents while chemotatic receptors can mediate cytokine release causing the recruitment of effectors capable of aiding in pathogen removal. Importantly, the activation of any of these receptors not only stimulates an innate immune response but also induces the adaptive immune system [7].
II.A.2. Role of Inflammasomes in Innate Immunity
Cellular receptors, effectors, and signaling molecules work in concert to facilitate innate immune responses. Aforementioned, PRRs have the ability to identify and associate with unique pathogen-associated molecular patterns (PAMPs), leading to distinct innate immune responses [9]. One particular element, the inflammasome, vitally participates in these responses by secreting pro-inflammatory cytokines [8]. Once initiated, the assembly of this enzyme complex is dependent on individual component expression that fluctuates in a tissue-dependent fashion. To address this, a three-tier model was constructed to categorize the “inflammation privilege” of various tissues. First tier tissues constitutively express all of the inflammasome components, while the second tier expresses all but one component and the third tier lacks more than one element. The lack in constitutive expression is significant because upregulation of that particular component is required prior to inflammasome assembly [10]. Therefore, the tiers can be used to delineate the ability of specific tissues to mount acute inflammatory responses. The importance of inflammasomes in the innate immune response is highlighted by the activity of adjuvants. An adjuvant will often be co-administered with a drug or vaccine to generate a stronger immune response that enhances the potency and longevity of the drug or vaccine [11,12]. The adjuvant alum was utilized for nearly eighty years without complete understanding of its mechanism. It was eventually determined that the enhanced immune response to alum was the result of the activation of the NALP3 inflammasome [13].
II.A.3. Inflammation
The hallmarks of inflammation include swelling, redness, pain, and fever, which are all symptoms of innate immune system activity [7,14]. Swelling is the result of inflammation that occurs in response to the release of cytokines and other mediators from activated macrophages (MΦs) and other resident effectors. Functionally, swelling attracts additional effector molecules like neutrophils that are capable of eliminating perceived pathogens, acts as a physical barrier against the spread of infection, and promotes the repair of injured tissue [7]. The other hallmarks result from innate immune effects on the vasculature. Corresponding with redness and fever, an increase in vessel diameter results in augmented blood flow allowing for the rapid accumulation of inflammatory mediators while swelling and pain can be attributed to increased vascular permeability leading to exudate accumulation [7].
II.B. Innate Immune Response Effectors
II.B.1. Phagocytes
Phagocytosis is a mechanism by which an effector can eliminate a pathogen. Cells like neutrophils, MΦs, and monocytes (MCs) engulf pathogens, internalizing them within vesicles. These vesicles are then directed towards lysosomes or other enzymes that degrade the pathogens. This process is based on receptor-mediated endocytosis and can be triggered by PAMP-PRR or Fc-antibody binding [8]. Other cells, termed natural killer (NK) cells, express Fc receptors and are activated by antibody-bound pathogens. This activation leads to the subsequent destruction of the pathogen and is termed antibody-dependent cellular cytotoxicity. The regulation of NK cells is critical for normal immune functioning and is dependent on the presence of activating and inhibitory receptors. Inhibitory NK cell receptors recognize major histocompatibility complex (MHC) class I alleles presented on all nucleated cells. Following infection, a cell’s MHC class I expression is diminished leading to destruction by NK cells. In particular, NK cells are potent eliminators of tumor cells and virally-infected cells [8,15].
II.B.2. Cytokines
Cytokines play a major role in mediating the innate immune response. Both resident cells and recruited effectors are capable of releasing cytokines, which signal a myriad of effects. The diversity in roles, targets, and origins of cytokines has made sub-categorization difficult and because of this there is some debate as to whether some cytokines should be labeled as hormones and vice versa. Nevertheless, functional classifications prove useful when considering cytokines within specific contexts – e.g. pro-inflammatory vs. anti-inflammatory or type 1 vs. type 2 (pro-Th1 vs. pro-Th2, see discussion of adaptive immunity below). Cytokines act on transmembrane receptors located on target cells that typically activate the JAK-STAT pathway. Thus it can be insinuated that cytokines achieve their cellular effect via induction of new gene transcription and new protein synthesis [8,16].
II.B.3. Complement Cascade
The complement system consists of more than twenty-five proteins working in concert to form a biochemical cascade. Primarily consisting of protease zymogens, the activation of these proteins is cleavage-dependent. Thus, activation of one protein typically results in the successive cleavage of the next zymogen within the cascade. The complement system is responsible for the recruitment of effectors by inducing inflammation, identifying pathogens for removal, and eliminating certain foreign agents. These functions are carried out through three distinct, activation-dependent pathways utilizing different effectors. Capable of identifying and eliminating pathogens, the classical and lectin pathways are distinctly initiated either by antigen-antibody complexes or bacterial cell wall mannans, respectively. Meanwhile, the alternative pathway is activated spontaneously by microbial components [8,17–19].
II.C. Overview of the Adaptive Immune Response
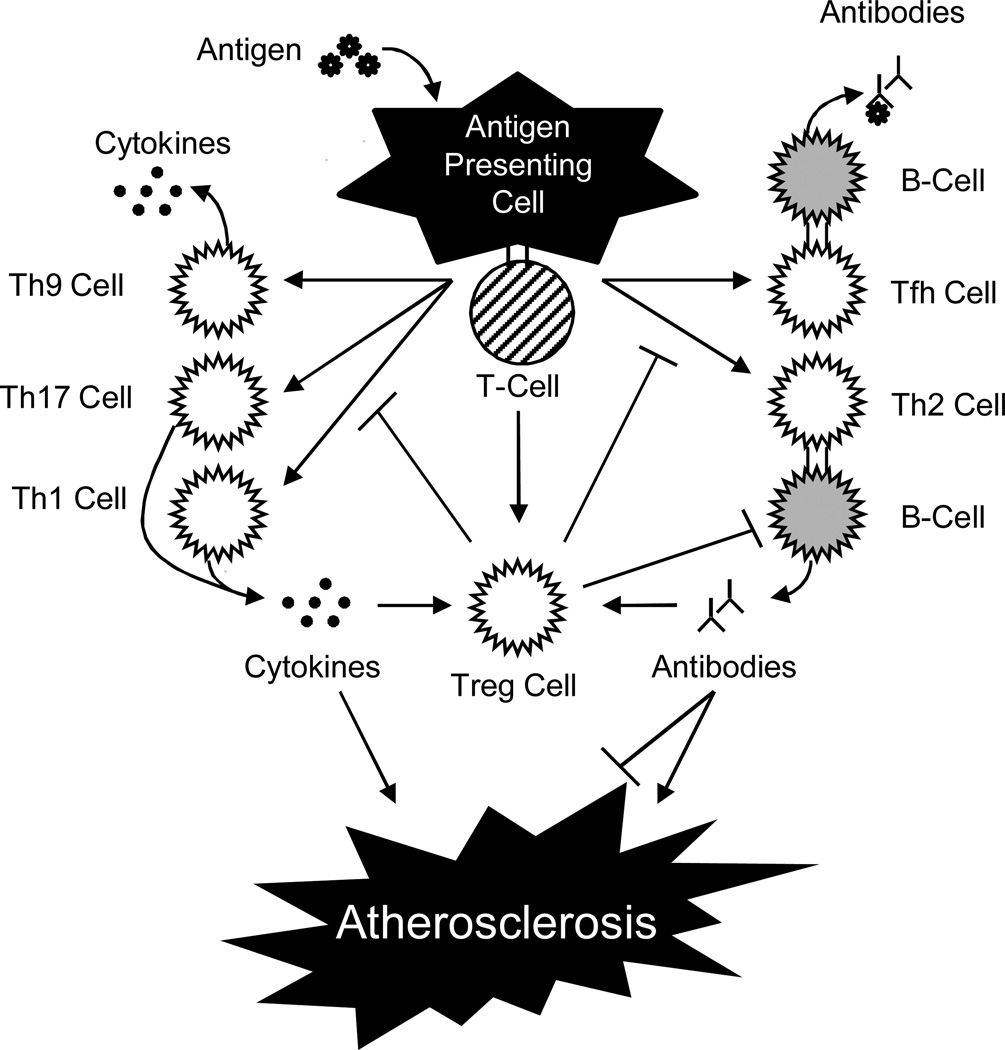
An adaptive immune response is the result of a pathogen eluding elimination by the innate immune system. The expression of MHC/antigen complexes, T-/B-cell antigen receptors, and co-stimulatory molecules on the surface of innate immune cells lead to the activation of adaptive immune lymphocyte effectors. Consisting of dendritic cells, B-cells, and MΦs, professional antigen presenting cells (APCs) activate lymphocyte effectors through antigen epitope presentation (Figure 1). B-cells are activated similarly although the presence of CD4+ T-helper (Th) cells is also required. These Th cells can be further classified into several subsets based on their cytokine secretion. Two particular subsets, Th-1 and Th-2, were the original two groups and are subsequent progenitors of CD4+ cells. Th-1 cells are generated in the presence of interferon-γ (IFN-γ) and interleukin-12 (IL-12), while IL-4 induces Th-2 cells.
Figure 1. Activation of Adaptive Immune Responses in Atherosclerosis.
This schematic depicts the roles of T-cells and B-cells in mediating immune responses to antigens in atherosclerosis.
Additionally, Th-1 cells produce IFN-γ and IL-2, whereas Th-2 cells secrete IL-4, IL-5, IL-10, and IL-13, which promote B-cell activation leading to humoral immunity [3,20]. Furthermore, these two Th subsets maintain the ability to regulate one another. Upon the discovery of IL-9-producing and IL-17-producing cells, the Th9 and Th17 cell subsets were created [21,22]. The Th9 subset promotes T-cell proliferation while the Th17 subset mediates defense against bacteria as well as a variety of autoimmune responses [21,22].
Another subset, T follicular helper cells (Tfh), was observed to target B-cell follicles where they induce B-cell antibody production [23]. All of the aforementioned effectors of the adaptive immune response function with a high degree of specificity to eliminate foreign pathogens and create immunological memory. The extent of B- and T-cell receptors, 1018 and 1014, respectively, gives rise to the adaptive immune system’s extraordinary specificity [20]. Despite this, occasionally an adaptive immune response is inadvertently mounted against self-antigens.
II.D. Pathological Consequences of Unregulated Immune Responses
The complexity of the innate and adaptive immune systems and the need for finely-tuned modulation perpetuates the opportunity for harmful effects if a malfunction in regulation occurs. Despite the beneficial need for acute inflammation to remove foreign agents, chronic inflammation is almost always detrimental [24].
Inadvertent self-targeting and tissue destruction can occur in response to overproduction of inflammatory mediators, reactive oxygen species (ROS), and complement cascade proteins. This scenario occurs in paroxysmal nocturnal hemoglobinuria where healthy red blood cells are eliminated. This ailment is the result of complement cascade hyperactivity due to a deficiency in decay-accelerating factor and CD59. Normally these factors function to protect against complement system self-activation.
Similarly, hereditary angioneurotic edema occurs as a result of C1-inhibitor deficiency. This defect leads to fluid accumulation in tissues as a result of excessive secretion of vasoactive mediators. Both of these examples highlight the vital importance of self-recognition by the immune system.
A malfunction or loss of self-recognition can have life-threatening consequences as self-antigens are marked for destruction by the adaptive immune system. Atherosclerosis, systemic lupus erythematosus (SLE), multiple sclerosis, rheumatoid arthritis, and type 1 diabetes mellitus (T1DM) all contain an autoimmune component where self-antibodies are synthesized.
Examination of autoimmune diseases in twins reveals an underlying genetic connection to disease progression. However, genetic factors do not solely affect autoimmunity; environmental factors have also been shown to have an impact [25,26]. The detailed mechanisms remain to be fully comprehended though, due to the complexity and difficulty associated with studying environmentally-induced autoimmune diseases. Despite this, it is known that environmental effectors such as drugs and medications can alter DNA methylation patterns leading to SLE-like autoimmune disease. This finding along with other similar results suggests a possible epigenetic contribution to autoimmunity.
III. Role of Inflammation and Autoimmunity in Atherogenesis
Atherosclerosis, characterized by the accumulation of plaques in large- and medium-sized arteries, frequently progresses to more severe complications like myocardial infarction (MI) or cerebrovascular accident. Atherosclerotic plaques are biologically complex consisting of altered lipids, a necrotic core, calcified regions, inflamed smooth muscle cells (SMCs), activated and dysfunctional endothelial cells (ECs), leukocytes, and foam cells [1]. The majority of atherosclerosis instances can be attributed to traditional risk factors such as age, hypertension, diabetes mellitus (DM), hyperlipidemia, sex, hyperglycemia, obesity, and cigarette smoking [27,28]. However, nontraditional risk factors like hyperhomocysteinemia and bacterial infection have also been characterized [29–32]. Whether traditional or nontraditional, atherosclerosis risk factors sustain the ability to influence inflammatory and/or autoimmune responses. These findings provide evidence of the link between immunity and atherogenesis.
III.A. Innate Immune Response Effectors and Atherogenesis
III.A.1. Monocytes and Macrophages
Contained within the heterogeneous atherosclerotic plaques are immune effectors such as MΦs, T-cells and a few B-cells [33]. Originating from MCs, MΦs partake in multiple functions such as pro-inflammatory cytokine secretion, vascular cell wall remodeling, and lipid retention. Additionally, they express immune modulating TLRs, PRRs, and scavenger receptors (SRs). The receptor expression and cellular roles of MΦs serve to bridge the gap between innate and adaptive immune interaction during atherosclerosis [34]. It is well-characterized that MΦs can become foam cells, an early indicator of atherosclerosis, as a result of lipid accumulation [35]. Also there is evidence that suggests MΦ apoptosis can affect atherogenesis. In early lesions, induction of MΦ apoptosis hinders atherogenesis, whereas impediment of MΦ apoptosis in later stages may contribute to secondary necrosis. This leads to increased pro-inflammatory responses and additional apoptotic signals for SMCs, ECs, and leukocytes within the plaques [34]. MCs are also capable of developing into dendritic cells (DCs), which may enhance naive lymphocyte recruitment into atherosclerotic vessels with the production of CC chemokine ligand 19 (CCL19) and CCL21 [36].
III.A.2. Mast Cells and Neutrophils
Mast cells and neutrophils are effectors of the innate immune system responsible for the degradation of foreign agents by lytic enzymes. This enzymatic activity may also be relevant in atherosclerosis development. Mast cells are believed to contribute to atherosclerotic plaque destabilization through the enzymatic activity of proteases such as tryptase and chymase. Their contribution is further supported by the concentrated presence of mast cells in locations of plaque rupture. Furthermore, the activation of perivascular mast cells correlates with intraplaque hemorrhage, MΦ and EC apoptosis, vascular leakage, and CXC chemokine receptor 2 (CXCR2)- and very late antigen 4 (VLA-4)-mediated recruitment of leukocytes to the plaque [37]. Mast cells also maintain the capability to affect lipid metabolism through apolipoprotein E (ApoE)- and ApoA-II-dependent cholesterol efflux [38]. Aside from mast cells, the short-lived phagocytic neutrophils possess biologically active molecules like myeloperoxidase and proteinases. When neutrophil concentration is increased in plaques it perpetuates a pro-inflammatory phenotype and apoptosis. This behavior indicates a pro-inflammatory function for neutrophils in atherosclerosis [39].
III.A.3. Platelets as Nontraditional Effectors
Platelets represent an interesting regulatory nexus for various physiological and pathological responses. Despite canonically performing protective roles, activated platelets expressing P-selectin are detected at different stages of atherosclerosis and transiently interact with the endothelium of atherosclerotic carotid arteries of ApoE−/− mice in vivo. This interaction results in the immobilization of platelet-derived CCL5 and CXC chemokine ligand 4 (CXCL4) on atherosclerotic endothelium [40,41]. Additionally, it is known that platelet glycoproteins GPIIb/IIIa, GPIb, and endothelial von Willebrand factor are at least partially responsible for these platelet-endothelial interactions. Immobilized platelets are also capable of interacting with leukocytes through P-selectin/P-selectin glycoprotein ligand (PSGL)-1 interactions that activate macrophage-1 antigen (Mac-1) and VLA-4 integrins and may facilitate firm MC adhesion [40]. Another function of platelets is to initiate the rolling of DCs through PSGL-1-dependent interactions between Mac-1 and junctional adhesion molecule C in injured carotid arteries [42]. They are also required for CX3C chemokine ligand 1 (CX3CL1)-induced leukocyte adhesion at high shear rates. Both soluble and membrane-bound CX3CL1 trigger P-selectin expression on adherent platelets, which facilitates the local accumulation of leukocytes under arterial shear stress [43]. Overall, platelets act as bridges between inflamed endothelium and circulating blood cells as well as recruiters of leukocytes to inflamed atherosclerotic endothelium.
III.B. Cytokines and Atherogenesis
With the ability to modulate immune responses, cytokines can predictably affect atherogenesis through mediation of endothelial permeability, expression of adhesion molecules, SR, lipid metabolism, as well as proliferation and migration of SMCs and ECs. Atherosclerosis-influencing cytokines can be generated by ECs, MΦs, mast cells, and platelets. ECs release IL-3, stem cell factor, granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor, and macrophage colony-stimulating factor, which all can affect hematopoietic cell proliferation [44]. Meanwhile, MΦs can secrete both pro- and anti-inflammatory cytokines when present in a hyperlipidemic state as seen in atherosclerosis. Mast cells, on the other hand, produce cytokines IL-6 and IFN-γ, which are critical for mast cell-dependent acceleration of atherosclerosis. Finally, platelets yield the pro-atherogenic mediators IL-1β, CD40L, and CXCL4 when activated. It should be noted that cytokines can also influence extracellular matrix composition by varying the expression of matrix metalloproteinases (MMP) −1, −3, −8, −9, and −12 as well as tissue inhibitors of metalloproteinases (TIMP) −1, −2, and −3 [45].
III.B.1. Pro-Inflammatory Cytokines
As a consequence of their immunological nature, pro-inflammatory cytokines typically contribute to atherogenesis. An example is TNF-α, which has been discovered to promote fatty streak formation, a characteristic of early atherosclerosis [46,47]. It is also understood that the cytokine IL-1 promotes leukocyte adhesion to ECs and leukocyte transmigration across the endothelium. Moreover, IL-1 functions as a local autocrine and paracrine stimulator of other cytokines [44]. IL-2 is known to have pro-atherogenic effects as well, which can be inhibited with IL-2 specific antibodies [48]. Similarly, IL-18 augments atherosclerosis progression via up-regulation of IFN-γ, which participates in all phases of atherogenesis [49]. When secreted by aortic plasmacytoid DCs, IFN-α has been demonstrated to enhance the expression of TNF-related apoptosis-inducing ligand on CD4+ T-cells and increase their cytolytic capacity toward SMCs [50]. In addition, IFN-α stimulates TLR4 expression on APCs thereby increasing pathogen sensitivity. Furthermore, IFN-α intensifies TNF-α, IL-12, and MMP-9 production, thus leading to further plaque destabilization [51]. Found within atherosclerotic plaques, TLR1, TLR2, TLR4, and TLR5 can influence cytokine expression following activation. In fact, it has been confirmed that atherosclerosis-associated oxidized low-density lipoprotein (oxLDL) can modulate TLR4 expression and therefore affect cytokine expression.
III.B.2. Anti-Inflammatory Cytokines
As opposed to pro-inflammatory cytokines, it can be generalized that anti-inflammatory cytokines typically have anti-atherogenic effects. This idea has been supported by experimental conclusions. The anti-inflammatory cytokine IL-10 is generated by MCs, Th2 cells, B-cells, and MΦs. Its presence reduces vascular inflammation and inhibits lesion progression by inducing an anti-inflammatory MΦ phenotype [52]. Additionally, IL-10 maintains the delicate balance between Th1 and Th2 responses. If IL-10 expression is knocked down in mice, a phenotype characterized by T-cell accumulation, increased IFN-γ production and limited collagen in atherosclerotic vessels is witnessed [44]. IL-33, another anti-inflammatory cytokine, also provides beneficial effects. Experimentally, IL-33 has been demonstrated to significantly reduce atherosclerosis in ApoE−/− mice. This physiological result can be attributed to increases in IL-4, IL-5, and IL-13 production, reduction of pro-inflammatory cytokines, and diminished IFN-γ secretion. These alterations lead to the alteration of the Th1 response to a Th2 response [53]. In addition, it is hypothesized that IL-33 can counteract harmful oxLDL through IL-5-dependent secretion of oxLDL antibodies [53]. In addition, there is supporting data for the beneficial effects of TGF-β in atherosclerosis by modulating SMC and EC phenotypes, as well as Th1 functions.
III.B.3. Chemokines
Chemokines are a subdivision of cytokines possessing the ability to influence chemotaxis. In correlation to atherosclerosis they play an important function in directing leukocytes into atherosclerosis-prone vessels. The first chemokine to be associated with atherosclerosis, CCL2 and its receptor CC chemokine receptor 2 (CCR2), integrally participate in MC recruitment from the bone marrow into the arterial wall [54 55].
Likewise, platelets facilitate MC and T-cell recruitment into the endothelium through the secretion of pro-inflammatory chemokines CCL5 and CXCL4 [40]. The expression of CXCL4 can perpetuate atherosclerosis by potentially activating ECs through NF-κB activation, inducing E-selectin expression, and advocating oxLDL binding to ECs [56]. Another chemokine with implications in atherosclerosis development is CXCL8. It can promote both migration and proliferation of SMCs and ECs as well as affect neovascularization. Oppositely, the chemokine CXCL1 initiates MC arrest by increasing production of VLA-4 integrin [57]. In low stress regions, CCL2, CXCL10, CXCL1 and CX3CL1 are present with CX3CL1 being solely observed in areas with thinner fibrous caps and larger necrotic cores [58].
In atherosclerotic plaques, CX3CL1 adopts a transmembrane form that can attract CX3C chemokine receptor 1 (CX3CR1) positive NK cells, subsets of T-cells, and MCs [59]. A different chemokine, CXCL10, can regulate the balance between the effector and regulatory arms of the immune system in aortas by reducing regulatory T-cell (Treg) expression. Chemokines also act as potent mitogenic and chemotactic factors for SMCs [60]. In research conducted with migration inhibitory factor (MIF−/−) and low-density liproprotein receptor (Ldlr−/−), dual knockouts indicate a correlation of MIF with atherosclerosis.
This link is found in the regulation of lipid deposition, protease expression, and intimal thickening [61]. Additionally, when MIF binds to the CXCR2 and CD74 receptor complex, elevated MC targeting of atherosclerotic endothelium was observed [62].
III.C. Atherogenesis and Inflammatory Mediators
Once an innate immune response has been mounted and inflammation has been induced, a number of pro-inflammatory mediators are expressed that can impact the development of atherosclerosis. Leukotrienes, inflammatory lipid mediators, are a component of the 5-lipoxygenase (5-LO) pathway with pro-atherogenic aptitude [63]. Furthermore, plaque instability is augmented with the up-regulation of 5-LO and leukotriene A4. In response to LDL oxidation another inflammatory mediator, heme oxygenase (HO)-1, is induced leading to a reduction in MC chemotaxis [64]. Therapeutically, HO-1 has demonstrated protection from atherosclerosis in an antioxidant-dependent manner [65].
This result is substantiated by the fact that excessive generation of ROS can induce atherosclerosis through the activation of the vascular endothelium and components of the immune system. One of the ways a superoxide can affect atherogenesis is through the activation of SMC mitogenic signaling pathways [66]. Furthermore, NADPH-induced superoxide has been shown to enhance platelet recruitment [67]. oxLDL is tightly linked to atherosclerosis and can influence the migration of MCs to the aortic wall by alternating from CCR2 to CX3CR1 expression through a peroxisome proliferator-activated receptor-γ (PPAR-γ)-dependent pathway [68].
III.D. Atherogenesis and the Adaptive Immune Response
III.D.1. T-Cells
Experimentation in T- and B-cell deficient hypercholesterolemic mice has been conducted to confirm the role of adaptive immunity in atherogenesis. It was observed in these mice that disease progression was significantly attenuated [69–72]. Furthermore, lesion formation was resurrected when CD4+ T-cells were transferred into these deficient mice [73]. Aforementioned, atherosclerotic plaques are comprised of numerous cells that secrete IFN-γ, IL-12, IL-15, IL-18, and TNF with only a few cells that produce IL-4 [74–76]. This finding implies a role for Th1 cells and not Th2 cells in promoting atherosclerosis. This notion was confirmed in studies where lesion reduction was witnessed when mediators contributing to T-cell differentiation into Th1 cells were suppressed [77–82]. Moreover, administration of Th1 differentiating IFN-γ expectantly induces atherosclerosis in mice whereas a reduction in atherosclerosis incidence was observed when Th1 cells were pharmacologically inhibited [83,84]. Contrary to Th1 cells, Th2 cells reveal anti-atherosclerotic effects in animal studies. Mice genetically altered to yield Th2 immune responses (as opposed to Th1 immune responses) showed reduced fatty streak formation. This finding could be reversed by inhibiting T-cell differentiation into Th2 cells [85,86]. Additionally, over-expression in hypercholesterolemic mice of the Th2 cytokine IL-10 led to reduced lesion size by 50% [87]. Conversely, a deficiency in the IL-5, another Th2 cytokine, resulted in an increase in atherosclerosis [88]. Moreover, studies involving over-expression of the Th2-inducing cytokine IL-4 show inconsistent results [89,90]. Thus, it can be concluded that Th1 cells have a clear pro-atherogenic role, whereas Th2 cells have a more complex role in atherosclerosis that is yet to be fully elucidated.
Th1 cytokines primarily encourage atherosclerosis by perpetuating the inflammatory mechanisms discussed previously (Figure 1). Located within atherosclerotic plaques, the pro-inflammatory cytokines IL-12, IL-18, IFN-γ, and TNF, induce Th1 differentiation resulting in further cytokine production and activate both MΦs and vascular cells. Individually, IFN-γ is capable of activating MΦs while simultaneously inhibiting EC and SMC [91,92]. These actions result in the secretion of pro-inflammatory cytokines, pro-thrombotic factors, and vasoactive mediators. Meanwhile, TNF can stimulate the NF-κB pathway in vascular cells furthering inflammation and causing the production of ROS, proteolytic enzymes, and pro-thrombotic factors [93–95]. The activation of these two pathways provides strong evidence that Th1 adaptive immune responses participate in atherosclerosis. It should be noted that a correlation between atherosclerosis and other pro-atherosclerotic pathways associated with Th2, Th9, Th17, Tfh, and CD8+ T-cells, has yet to be established [4,96]. However, it is believed that these cells participate in modulating pro- and anti-atherosclerotic pathways as a result of their common lineage. This implies that the nuances governing their differentiation ultimately affect pro- or anti-atherosclerotic responses. Tfh cells maintain the ability to regulate B-cell response and antibody production while Th2 cells modulate Th1 cell differentiation and activity. In addition, Th17 cells can modulate immunosuppressive Tregs [22,96] and secrete IL-17, which has been indicated in affecting autoimmune responses in atherosclerosis (Figure 1) [52,97,98].
III.D.2. Tregs
CD4+CD25high Tregs are capable of suppressing both the innate and adaptive immune systems [99]. Expectedly, the loss of Treg function has been found to perpetuate autoimmune responses (Figure 1) [100,101]. The identification of a Treg-specific apoptotic pathway that relies on IL-2 participation provides evidence that Treg apoptotic/survival pathways can serve as therapeutic targets in Treg-based immunotherapy. It has also been demonstrated that inhibition of Treg results in accelerated vascular inflammation. This heightened inflammation can understandably contribute to atherosclerosis exacerbation [102–104]. For activation, Tregs depend on T-cell receptor activity and co-stimulation similar to effector T-cells. Tregs and effector T-cells differ however, in the fact that a distinguished pathway that exclusively regulates Tregs has yet to be identified [105]. Superagonistic CD28 antibody was attributed with preferentially-activating Tregs in animal models but phase I clinical trials identified a lack of specificity that triggered a cytokine storm [106–108]. Despite this, direct Treg transplant has generated positive results [109].
This line of therapy could prove useful in treating atherosclerosis where plaques contain a depleted number of Tregs (1%–5% of all T-cells versus normally 25% of all T-cells) as well as other T-cell dysfunction ailments [110]. It is important to note when exploring Treg therapies that excessive T-cell suppression can compromise cellular defense systems. The development of Treg-based atherosclerosis therapies should ideally target specific autoantigens both involved in atherogenesis and located in atherosclerotic plaques.
III.D.3. B-Cells
In addition to T-cell mediated cellular responses, humoral responses modulated by B-cells are also part of the adaptive immune response. Experiments with T- and B-cell deficient mice revealed a pro-atherogenic function of these mediators [69–72]. Data exists where splenectomy and consequent loss of some T- and B-cells led to increased atherosclerosis. Furthermore, this finding could be reversed through adoptive transfer of B-cells [111–113]. These conflicting results demonstrate the complex nature of B-cell activity. It is postulated that the principal role of B-cells in atherosclerosis is the production of antibodies, which may be either pro- or anti-atherogenic [3,96]. Moreover, B-cells contain the ability to secrete cytokines and serve as APCs. This capacity suggests the ability of B-cells to modulate T-cell adaptive immune response; however, this function has not been adequately examined in atherosclerosis. A limiting factor in fully comprehending B-cell mediated cellular responses is the inability to completely characterize B-cell subsets [114,115]. Discovered in mice, specific B-cell subsets have yet to be concretely identified in humans [115]. However, it is postulated, that a B-cell subset termed B1-cells produce natural antibodies of the IgM subtype in a T-cell and antigen independent manner [116]. The B1-cells function as a first line of defense against foreign pathogens with their primary location in the peritoneal cavity [114,116]. Interestingly, oxLDL-specific IgM antibodies can be detected in the circulation of individuals with minimal levels of atherosclerosis [117].
III.D.4. oxLDL as an Autoantigen
CD4+ T-cells located within atherosclerotic plaques exhibit specificity for exogenous and endogenous antigens such as oxLDL and heat shock protein 60 (HSP60) [75,118–120]. A well characterized biomarker, oxLDL is the preeminent autoantigen associated with atherosclerosis. [121]. Atherogenesis is first initiated as LDL particles embedded within the arterial intima become oxidized leading to the accumulation of fatty streaks [122–124]. The oxLDL is then taken up by APCs via the SR pathway. At this point the oxLDL molecules undergo proteolytic processing and then bind to MHC class II molecules for presentation to T-cells [3]. Since native LDL components do not elicit a T-cell response it is postulated that oxidation of LDL is responsible for the loss of immunogenic tolerance. Compounding the issue, hypercholesterolemia is thought to impair DC mobility causing diminished immunogenic tolerance due to inhibition of clearance functions [4,125–127]. Furthermore, the existence of oxLDL specific antibodies provides support that an adaptive immune response to oxLDL occurs during atherosclerosis [119]. This response is believed to be a balance between pathological autoimmunity and protective immunity [4].
III.D.5. HSP as an Autoantigen
Primarily functioning as chaperones, HSPs are highly conserved and abundantly expressed. Despite their conservation they have also been implicated in autoimmune atherosclerosis [128]. The notion that HSPs may have negative health implications developed from observations of elevated expression in patients with systemic hypertension, coronary artery disease, carotid atherosclerosis, MI, and ischemia [6].
Additionally, high levels of antibodies against mycobacterial HSP65 independently predict stroke, MI, and cardiovascular death [129]. The cross-reactivity of HSPs with other bacterial HSPs (e.g., Mycobacteria, Chlamydia) and the detection of bacterial HSPs by antibodies suggest that HSPs may function as autoantigens [6].
In fact, Chlamydia pneumoniae have HSPs discovered to be located in atherosclerotic plaques [6,29]. Meanwhile, other infectious agents such as herpes simplex and cytomegalovirus have also been found in atherosclerotic lesions [20]. Even though these infections do not directly contribute to atherosclerosis, their presence can have indirect effects by activating PRRs leading to immune responses. This indirect effect is coined “molecular mimicry” [130]. Comprehensive evidence of this can be seen with the activation of TLR9 located on DCs in atherosclerotic plaques. This activation results in an autoimmune T-cell attack on vascular SMCs [50] and clearly reveals how an unassociated pathogen can generate a pro-atherogenic autoimmune response.
III.E. Atherogenesis Resulting from Other Pathologies
As the connections between atherosclerosis, inflammation, and autoimmunity are unearthed it can be postulated that chronic inflammation and other autoimmune diseases will have an impact on atherosclerosis progression. This hypothesis is supported by an increase in atherosclerosis progression seen in the presence of rheumatoid arthritis [131], SLE, systemic sclerosis [132], Wegener’s granulomatosis [133], and antiphospholipid syndrome [134].
In addition, diabetic mice develop atherosclerosis at an accelerated rate with diabetes, glucose, and products of glucose metabolism believed to induce atherosclerosis under hyperlipidemic conditions. Furthermore, the symptoms associated with a pre-diabetic state – abdominal obesity, elevated LDL cholesterol, and increased blood pressure – are all positively correlated with CVD. Finally, experimental mice expressing human aldose reductase, which leads to elevated glucose levels, develop atherosclerosis at an accelerated rate compared with controls [135].
IV. Inflammation and Autoimmunity in Diabetic Atherosclerosis
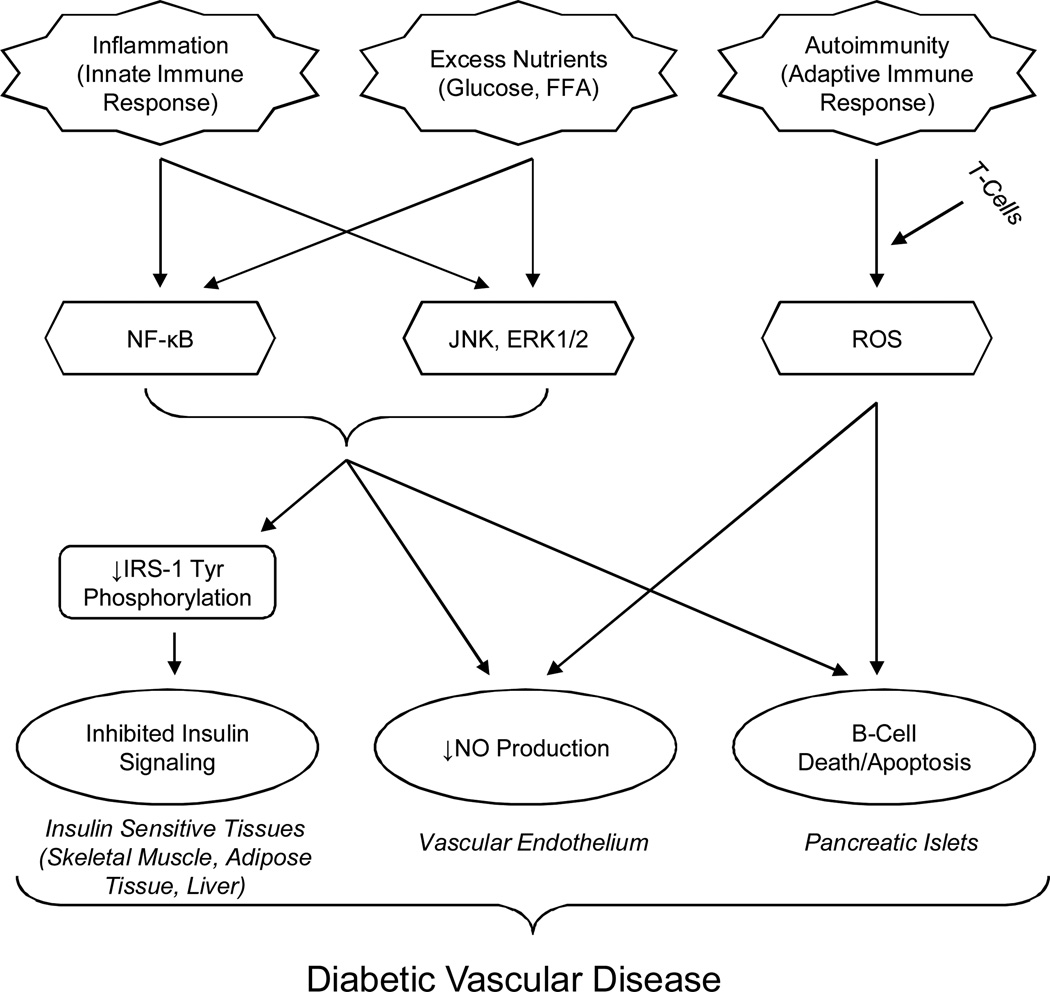
DM is defined by elevated blood glucose levels due to either insufficient pancreatic insulin production or insufficient insulin use. T1DM is an absolute loss of insulin secretion from autoimmune-mediated destruction of pancreatic β-cells. On the other hand, type 2 DM (T2DM) is a relative insulin deficiency occurring because of defective β-cell insulin secretion due to peripheral insulin resistance (IR) (Figure 2) [136]. Diabetes is a growing pandemic currently afflicting more than 220 million people worldwide and predicted to ail twice as many individuals by 2030 [137]. These overwhelming values indicate the necessity of determining the mechanism of IR so novel therapeutic targets can be identified.
Figure 2. The Immunopathology of Type 2 Diabetes Mellitus.
This flow chart shows how both innate and adaptive immune responses contribute to T2DM pathology.
IV.A. Insulin Resistance
Insulin is a mediator of fuel homeostasis stimulating glucose uptake and suppressing the release of stored lipid from adipose tissue. Publications have positively linked the advancement of IR with hepatic steatosis, abdominal obesity, and chronic subclinical inflammation [138–142]. This suggests that elevated cytokine levels may contribute to peripheral IR as well as decreasing β-cell function and mass. Pro-inflammatory cytokine levels can affect susceptible tissue like blood, brain, adipocytes, endothelium, liver, muscle, pancreatic islet cells, and muscle, which are used as diagnostic markers for T2DM and metabolic syndrome [138,140]. Moreover, inflammatory cytokines and free fatty acids (FFA) can spur IR and activate IKK-β (inhibitor of NF-κB kinase subunit β) in the presence of inflammatory stimuli. IKK-β is capable of activating NF-κB and inhibiting insulin signaling through the restraint of insulin receptor substrate (IRS) tyrosine phosphorylation. Furthermore, suppression of IKK-β protects against the deleterious effects of TNF-α and FFA on insulin signaling. Studies show that overexpression of a constitutively active version of IKK-β in the liver of rodents causes IR and diabetes in liver and muscle [143–148]. Interestingly, obesity or a high fat diet has been demonstrated to cause activation of NF-κB and its pro-inflammatory targets in the liver. In addition, an obese or diabetic state can lead to elevated levels of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) activity in several tissues under metabolic and inflammatory stress [149–153]. The above findings indicate that prolonged exposure to lipids and other metabolic fuels can affect IR development through the alteration of cytokine production within the liver, adipose tissue, and infiltrating immune cells.
IV.B. β-Cell Destruction
The graded serum levels of acute phase reactants (e.g. CRP, sialic acid) and their cytokine mediators (e.g. TNF-α, IL-1, IFN-γ) in correlation with diabetes, obesity, metabolic syndrome, hypertension, and atherosclerosis development suggests a role in disease pathogenesis [140,154–156]. A similar mechanism may occur in the pancreatic islet cells of people with T2DM [157,158]. While T1DM is immune-mediated and based upon pancreatic islet inflammation [159,160], T2DM is defined by inadequate insulin production in the face of IR [161]. With insulin secretion dependent on pancreatic β-cell function and mass, the pancreas is remarkably capable of adapting to conditions that require elevated insulin production such as obesity, pregnancy, and growth hormone excess, by augmenting its β-cell mass [162]. However, in T2DM, β-cell function declines with disease progression [14,162] and β-cell dysfunction was found to be the major contributor in the transition from normoglycemia to DM in an experimental population of Pima Indians [163]. Despite etiological and pathogenic differences between T1DM and T2DM, pancreatic β-cell demise by apoptosis is a common cellular denominator. Eventually, in both situations, β-cell destruction occurs leading to clinical manifestations of absolute or relative insulin deficiency [164]. As hyperglycemia develops, additional processes like islet inflammation, O-linked glycosylation, and amyloid deposition accelerate β-cell demise. This results in a deterioration of β-cell function and a loss of controlled glucose stimulated insulin secretion [162,165]. The chronic hyperglycemia then leads to ROS production, which induces pancreatic β-cell oxidative damage. Additionally, both ROS and IL-1β activate NF-κB, a critical mediator of inflammatory responses. Apoptosis in the presence of pro-inflammatory cytokines like IL-1β and TNF-α can provoke additional immune responses. Therefore, the chronic increase in inflammatory mediators observed in T2DM and the elevated secretion of TNF-α, IL-6, and IL-1β by adipose tissue [166] could have a significant effect on noninsulin-sensitive cells.
IV.C. Diabetic Atherosclerosis
Atherosclerosis is a frequent complication of DM but despite strong epidemiologic evidence of their correlation, the mechanism connecting the two has yet to be determined. Postulations made from early studies suggest that DM exacerbates many traditional risk factors for atherosclerosis [167] and in fact, DM patients often exhibit elevated levels of oxLDL, hyperinsulinemia, oxidative stress, hyperglycemia, and increased FFA, which all are associated with endothelial dysfunction [167–172]. Another possible contributor to the accelerated atherosclerosis development is increased LDL, reduced high-density lipoprotein, and increased triglycerides connected to diabetic dyslipidemia [167]. Furthermore, individuals with DM have hypercoagulability, impaired fibrinolysis and platelet hyperaggregability, which all augment thrombogenicity and contribute to atherosclerosis [173–175]. Another supporting piece of evidence is an oxidative stress mechanism unique to DM with atherosclerosis effects. It involves the formation of advanced glycation endproducts (AGE) with altered structure and function. The receptor for AGE (RAGE) is expressed by ECs, SMCs, MCs, and lymphocytes with enhanced expression in atherosclerotic lesions. The administration of soluble recombinant RAGE neutralizes AGE, thereby reducing NF-κB induction, vascular cell adhesion molecule 1 (VCAM-1), and tissue factor expression. This in turn causes a reduction in atherosclerotic lesion burden [176]. The AGE-RAGE pathway is known to elevate oxidative stress, endothelial dysfunction, expression of adhesion molecules, as well as the generation of inflammatory cytokines and tissue factor. The modulation of these cellular factors can ultimately effect atherosclerosis progression [177].
IV.D. Unifying Immune Responses of DM and CVD
The role of the immune system in modulating diabetic atherosclerosis has revealed some mechanistic associations. S100A9+ MΦs are pro-inflammatory biomarkers for cardiovascular events and are recruited to atherosclerotic lesions in T1DM during plaque disruption [178]. In addition, T1DM conditions can induce S100A9+ MΦ differentiation. Meanwhile, other findings indicate that high concentrations of glucose can induce MΦ CD36 expression [179]. Significantly, CD36 is a MΦ SR that mediates oxLDL uptake and subsequent foam cell development. Moreover, studies with CD36 have demonstrated that oxLDL promotes assembly of a TLR4/TLR6 heterodimer [180]. These conclusions provide a direct mechanistic relationship between DM and atherosclerosis with immune mediation.
IV.E. Endothelial Dysfunction as an Ancestor for DM and CVD
Development of CVD is the principal complication in T2DM but it can precede its development as well [181]. This intertwined relationship lends itself to the idea of mutual ancestors. While IR is an accepted factor in the pathogenesis of T2DM its link to CVD has not been well defined [182]. However, inflammation is a common tie being a precursor of CVD, associated with IR, and preceding the progression of T2DM [156,183]. Inflammatory mediators are known to function pathogenically by enhancing systemic endothelial dysfunction, leading to hindered NO and vascular hemostatic agent production [184]. Imbalance of the vasodilatory agent NO and the vasoconstrictor endothelin-1 (ET-1) can further contribute to endothelial dysfunction [185]. Noteworthy is the fact insulin can stimulate production of NO from the endothelium and cause vasodilation through the binding and activation of the insulin receptor. This mechanism is similar to metabolic insulin signaling in skeletal muscle and adipose tissue [186–188]. Once the insulin receptor is bound, tyrosine phosphorylation of IRS occurs resulting in activation of both the phosphoinositide 3-kinase (PI3K)-Akt pathway and the mitogen-activated protein kinase (MAPK) pathway [189,190]. A key feature of IR is the alteration of the PI3K-Akt pathway, though other insulin-signaling branches, including Ras/MAPK-dependent pathways, remain unmediated [191]. As a result, hyperinsulinemia will lead to an imbalance between PI3K- and MAPK-dependent pathways. This imbalance leads to elevated vascular inflammation via enhanced expression of VCAM-1, intracellular adhesion molecule 1, E-selectin, monocyte chemotactic protein 1, and IL-6. Additionally, the imbalance will lead to a reduction in available NO in the endothelium [191,192]. This results in diminished eNOS activity, decreased NO generation, and increased ET-1 secretion, a characteristic of endothelial dysfunction. The elevated levels of ET-1 increase serine phosphorylation of IRS-1, leading to a reduction of PI3K activity in vascular SMCs and hindering insulin-stimulated translocation of glucose transporter type 4 in adipocytes [193]. It can be concluded that IR-mediated reduction of NO in the endothelium contributes to accelerated atherosclerosis through the regulation of vasoconstriction, inflammation, and thrombosis [194,195]. The categorization of endothelial dysfunction as a T2DM precursor may provide additional vantages for diabetes prevention and treatment [196,197]. Moreover, therapies targeting endothelial dysfunction may prove vital in preventing IR development and therefore T2DM [198–200].
IV.F. Therapeutic Strategies
IR is a leading factor in T2DM and is the central component of metabolic syndrome [163,201]. Recent studies suggest that pro-inflammatory cytokines, adipocytokines, and transcription factors are involved in the pathogenesis of IR. This claim is supported by clinical observations where patients with metabolic syndrome and T2DM had a positive correlation between IR and chronic inflammation. Based on these data, methods to attenuate IR by inflammation modulation were investigated. The insulin sensitizer thiazolidinedione exerts its effects through PPAR-γ by enhancing the effects of insulin in metabolic tissues and by directly decreasing peripheral IR [187,202]. Also, the receptor of interest is expressed in all major cell types involved in vascular lesions – MCs, MΦs, ECs and vascular SMCs. Additionally, the PPAR-γ agonist glitazone has numerous beneficial effects like improvement of glycemic control, decreased levels of circulating FFA, and anti-inflammatory effects on EC and leukocyte function [203,204]. Other beneficial anti-atherogenic effects have also been reported in several studies using PPAR-γ ligands [205,206]. Potent inhibition of inflammation, blockade of MΦs differentiation, and cytokine secretion [207] as well as inhibition of vascular SMC proliferation and migration have been shown [208]. In addition, treatment with PPAR-γ agonists in T2DM patients reduces plasma levels of inflammatory biomarkers that are predictive of CVD [209]. These results encourage and suggest that anti-inflammatory agents can improve tissue susceptibility to insulin and are promising for the treatment of metabolic syndrome and T2DM.
V. Immunogenicity and Stem Cell Therapy
Blood transfusions can be considered the first primitive form of cell therapy. Since then the concept of cell therapy has been refined to include only essential components needed to restore function or repair injury. These components may undergo modifications altering their functionality or enhancing their versatility. This ability to control cell functionality is one reason why cell therapy is an attractive avenue. Other reasons include the relative abundance of cells from countless donors and the inclusion of cellular features that are advantageous for targeting. Moreover, specific cell types demonstrate tremendous versatility and can be genetically modified to meet specific therapeutic demands [210]. At the forefront, stem cells contain immense cell therapeutic potential with their self-renewing ability and capacity to differentiate into more specialized cell types. We will outline key therapeutic concerns and discuss some progress in the stem cell therapeutic field.
V.A. Immunogenicity of Stem Cells
V.A.1. Antigen Matching
The idea of stem cell therapy in CVD is promising though apprehension regarding inflammation and immune responses needs to be addressed [211,212]. Allogeneic cells, tissues, and organs regularly elicit host immune responses. The host immune system “recognizes” certain protein antigens as foreign, leading to graft destruction. Due to their participation in tissue compatibility these molecules are named alloantigens or histocompatibility antigens. In allogeneic transplantations three distinct classes of antigens exist. The first class consists of MHC antigens, also termed human leukocyte antigens (HLA), which are the major contributors of tissue compatibility. The other two groups, minor histocompatibility complex (mHC) antigens and ABO blood group antigens, play a relatively minor role in tissue compatibility compared to MHC antigens [213,214]. Host T-cell recognition of MHC antigens from the donor is the primary cause for the rejection of an allogeneic transplant. To alleviate this issue, HLA matching between the donor and the recipient is conducted but is extremely difficult. A high number of polymorphisms results in hundreds of alleles for some HLA genes [215]. Also, the combination of the alleles can reach in the billions due to the two sets of HLA genes with six alleles per set [216]. This variability makes it virtually impossible to find the perfect match in genetically unrelated individuals.
V.A.2. Stem Cell Antigen Expression
In order to determine the feasibility of stem cell therapy it is critical to evaluate the MHC antigen cell surface expression to alleviate the risk of graft reject. Utilizing flow cytometry, it was characterized that undifferentiated human embryonic stem cells (ESCs) have very low MHC class I antigen expression [217,218]. Inciting human ESCs to differentiate into embryonic bodies or teratomas increases MHC class I antigen expression 2–4 fold and 8–10 fold, respectively [218]. Further, supporting studies in mice revealed that MHC class I antigens are not expressed in undifferentiated mouse ESCs and are only present at low levels following prolonged differentiation [219]. It was also discovered that epitope processing by immunoproteasomes is accelerated in the presence of IFN-γ [220]. This suggests that increased immunogenicity of stem cells may be related to stimuli such as stress, cytokines, and drugs that hasten self-antigen epitope processing [221]. These findings suggest ESC therapy of CVD could be feasible; however, elevated levels of IFN-γ and similar cytokines could result in amplified MHC expression and therefore needs to be characterized.
V.A.3. Immunogenicity of Self Antigens
Aside from alloantigens it is also necessary to comprehend the mechanism of how non-mutated self-protein antigens obtain immunogenicity and elicit immune responses [222]. The most common mechanism for non-mutated self antigen immunogenicity is the abnormal overexpression of antigens in tumors [223]. This elevated expression can be a product of either transcriptional or post-transcriptional mechanisms and can be identified using serological analysis of recombinant tumor cDNA expression libraries (SEREX). This technique involves the screening of patient’s sera [224] for the presence of autoantigens [225]. Additionally, a stimulation-responsive splicing model for autoantigen and self-tumor antigen selection has been established [221]. Utilization of several strategies [226–231] has been employed to identify immunogenic antigen alleles like modified SEREX [224], database-mining [232], and immunogenic isoform mapping. Despite investigation, the detailed antigen repertoire of normal stem cells remains to be well-defined. Gaining insight into these autoantigens and self-tumor antigens would prove vital in designing more effective cell replacement therapeutics.
V.B. Stem Cell Subset Selection
V.B.1. Embryonic Stem Cells
An important consideration when developing cell therapies is the selection of cell subsets. Each has its advantages and disadvantages such as ESCs that maintain the flexibility to differentiate into any cell type within the adult body. It could also be rationalized that the reduction in MHC I expression of ESCs would reduce the likelihood of tissue rejection. However, the rates of tissue rejection in recipient MHC−/− mice are comparable to wild-type control groups [233]. This suggests that selection of ESCs to limit immunological rejection may not be practical. Additionally, ESCs show a propensity to develop into teratomas when injected in vivo [234,235]. In order to alleviate this risk, in vitro differentiated ESCs could be selected for therapy, though the gain of specificity and control comes at the expense of flexibility and a gain in immunogenicity [236,237]. Further elucidation of ESC differentiation mechanisms could prove fruitful in fully understanding ESC differentiation.
V.B.2. Adult Stem Cells
Over the past decade the majority of CVD stem cell therapy has focused on adult stem cells. The utilization of adult stem cell subsets allows for the circumvention of graft rejection through autologous harvest. Another benefit of adult stem cells is the avoidance of in vitro expansion before use in transplantation. This alleviates the possibility of abnormal growth under culturing conditions. Thus far adult stem cell therapy has focused on the use of skeletal myoblasts (SkMbs), bone marrow mononuclear cells (BMCs), and endothelial progenitor cells (EPCs).
V.B.3. Skeletal Myoblasts
SkMbs were the first adult cell type to be transplanted into cardiovascular tissue. They were successfully transferred into recipients with injured hearts [238] and shown to restore myocardial function in animal models [239]. However, the mechanisms leading to improved heart function are still unknown. Working with SkMbs is advantageous due to their resistance to ischemia and their ability to differentiate into myotubes, which can potentially supplement injured myocardium. However, it should be noted that these myotubes are incapable of electrically integrating with the myocardium and are associated with arrhythmogenesis after transplantation [240].
V.B.4. Bone Marrow Mononuclear Cells
BMCs consist of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and EPCs, all of which can independently improve cardiac function. In fact, a unique subset of BMCs was found to differentiate into cardiomyocytes in mouse models [241]. However, from a therapeutic standpoint this finding was irrelevant due to the exceptionally low rate of differentiation [242]. Additional investigations in animal models also revealed the inability of HSCs to differentiate into cardiomyocytes. This was further supported in clinical studies where the effect of BMCs on cardiac function had little or no improvement [243]. On the other hand MSCs, unique from other BMCs, demonstrated the ability to differentiate into cardiomyocytes under stringent in vitro conditions [244,245]. This capacity was also observed in vivo though at an extremely low rate. A potential advantage for the use of MSCs in therapy is their relatively low immunogenicity compared with other stem cells [246]. Despite these findings there is little concrete evidence that BMCs can differentiate into cardiomyocytes at a physiological rate in vivo.
V.B.5. “Cardiac Stem Cells”
Recent publications have characterized a subset of endogenous cardiac progenitor cells that are capable of differentiating into various cardiac cells including cardiomyocytes [247,248]. These epicardial resident progenitor cells had previously been shown to generate SMC, EC, and fibroblasts; however, until recently their ability to produce cardiomyocytes had not been demonstrated. The discovery of this capability should provide new avenues for cardiovascular stem cell therapy. The use of endogenous progenitors is advantageous because it will allow the circumvention of possible graft rejection. Also, the ability to manipulate these “cardiac stem cells” may provide a novel approach to the restoration of depleted cardiomyocytes following ischemia or MI [249].
V.C. Stem Cell Therapy
V.C.1. Vascular Therapy
Endothelial injury and dysfunction are common contributors to vascular diseases. A promising candidate for use in vascular cell therapy, EPCs are capable of combating this complication. After being discovered in 1997 by Asahara et al. studies involving EPCs have yielded promising results in the cell therapy field [250]. It was indicated that an age-related reduction in progenitor cells contributes to the development of atherosclerosis [251]. This idea was experimentally verified by transferring EPCs from young non-atherosclerotic mice to older hypercholesterolemic mice, which led to atherosclerosis development inhibition via mechanisms of vascular repair and inflammation attenuation [251,252]. Despite the success of this model, additional experimentation with other subsets has led to differentiation into cell types that contribute to atherosclerosis development [253]. It should also be noted that while stem cell therapy in the treatment of atherosclerosis demonstrates important scientific principles it may be an unnecessarily complicated approach [254,255].
V.C.2. Cardiac Therapy
A focal point of cardiovascular stem cell therapy is the regeneration of cardiac myocytes following ischemia or MI. This is important due to the inadequate regenerative capacity of the human cardiac muscle in compensating for the substantial loss of the myocardium by MI or other CVD. While SkMbs in mammals are capable of sufficiently regenerating after widespread or repeated injury [256,257], mammalian cardiomyocytes rarely divide either before or after injury [258,259]. However, it should be noted that differentiation of cardiomyocytes can be accelerated through the over-expression of specific transgenic genes in mouse models [259]. The identification of an ideal stem cell subset for cardiac therapy is critical for successful therapy. The cell subset should be immunologically innate, capable of reconstituting the infarct zone with cardiomyocytes, pro-angiogenic, able to repair damaged vasculature, and electrically integrable with surviving myocardium.
VI. Immunogenicity and Immunosuppression
Thus far, the content of this review has concentrated on the role of the immune system in mediating CVD. The final section will focus on the treatment and neutralization of undesired immune responses. Controlled suppression of the immune system is a delicate endeavor requiring precision to prevent unwanted effects. Original immunosuppressants like corticosteroids have been used to effectively suppress the innate immune response but lacked specificity leading to the consequent stemming of the adaptive immune response. Drugs were then developed with more specific activity capable of targeting distinct mediators of the adaptive immune system. However, it is important to consider that any immunosuppression, specific or broad, inhibits not only the harmful activity but the protective mechanisms of the immune system as well. An ideal immunosuppressive agent would effectively mitigate the harmful mechanisms of the immune system with little or no effect on the protective effects. Agent specificity is vital in accomplishing this. Fortunately, the adaptive immune system relies heavily on specificity for its regular function. Therefore, the use of antibodies for therapeutic inhibition of immune responses has been heavily investigated.
VI.A. Anti-Inflammatory Drugs
The activation of the adaptive immune system following innate immune activity has several therapeutic implications. It explains why the co-administration of adjuvants optimizes the potency and longevity of vaccines and it elucidates why the administration of innate immunosuppressants can inherently eliminate the adaptive immune response. This is exemplified by corticosteroids, which have potent anti-inflammatory effects due to diminished IL-1, IL-3, IL-4, IL-5, IL-8, TNF-α, and GM-CSF expression [7]. Since corticosteroids bind to intracellular receptors in almost every cell type, their effect is broad with both beneficial and toxic results. Fortunately, this negative effect can be mitigated through combinational therapy allowing for lower therapeutic doses, thereby accentuating the favorable effects while diminishing the toxic effects. Corticosteroids are often co-administered with cytotoxic drugs like azathioprine and cyclophosphamide, a class of immunosuppressive drugs that interferes with DNA synthesis. In small doses these drugs target rapidly dividing cells like lymphocytes, thus suppressing the adaptive immune response. Another class of immunosuppressive drugs target T-cell proliferation through the inhibition of IL-2 induction [7]. Even though this class of agents is effective, its insufficient specificity makes standard use unreasonable. In fact, the harmful side effects restrict use to extreme circumstances like organ transplant, graft rejection, and various autoimmune diseases.
VI.B. Immunosuppressive Antibodies
Immunosuppressive antibodies have been employed in two capacities to ensure a high level of specificity. Depleting antibodies, the first subset, associate with lymphocytes by forming a complex that is later eliminated by NK cells. The second group is blocking antibodies, which inhibit the activity of the target protein but do not cause the protein’s destruction [8].
VI.B.1. Receptor Antibodies
Certain antibodies elicit their physiological effect through interactions with receptor complexes. Exemplifying this interaction, anti-CD3-specific antibodies act on the CD3 antibody/T-cell receptor complex to inhibit immunological responses. These antibodies have been administrated therapeutically to prevent short-term allograft rejection while their use in non-obese diabetic (NOD) mice resulted in the long-term remission of autoimmune diabetes [260]. Furthermore, the use of anti-CD3 antibodies in Ldlr−/− mice on a high cholesterol diet led to the inhibition of additional plaque formation as well as the halted progression of previously established lesions. Interestingly, an increase in both anti-inflammatory cytokine TGF-β in lymph node cells and the expression of the Treg marker Foxp3 in spleen cells was reported. This finding suggests that short-term treatment with an anti-CD3 antibody leads to the induction of a Treg phenotype, known to aid in the restoration of self-tolerance in a mouse model of atherosclerosis [261].
VI.B.2. Co-Stimulatory Signal Antibodies
Required for T-cell activation, co-stimulatory signals can be considered targets for immunosuppressive therapy. CD40 and its ligand CD40L have been demonstrated to be co-expressed on ECs, MΦs, SMCs, and T-cells present in atherosclerotic lesions. The inhibition of CD40L-CD40 signaling with antibodies results in the reduction of experimental autoimmune diseases like collagen-induced arthritis, lupus nephritis, acute or chronic graft-versus-host disease, multiple sclerosis, and thyroiditis. Additionally, the use of an anti-CD40L antibody in Ldlr−/− mice on a high cholesterol diet led to a decline in aortic atherosclerotic lesion area. Furthermore, a reduction in lesion lipid content and the presence of MΦs and lymphocytes was also reported [262]. In spite of these findings, it should be stated that treatment in Ldlr−/− or ApoE−/− mice with established atherosclerosis did not cause atherosclerotic lesion decline. It did, however, spur a change in lesion composition resulting in a reduction of lipids and MΦs while increasing the content of collagen and SMCs [90,263]. Ultimately, these modifications contributed to a more stable plaque phenotype. Although therapeutics involving CD40 and its ligand were not further pursued due to the potential risk of thrombotic complications caused by CD40L expression on platelets, it provides a useful example of how co-stimulatory signal antibodies can be used in immunosuppressive therapy.
VI.C. Tolerance
The ability to restore immune tolerance is a major goal in immunotherapy – particularly in regard to autoimmune diseases. With an early onset, most atherosclerosis therapies are directed at reducing progression by limiting innate and adaptive immune responses instead of prevention. Oral or intranasal immunization is a common method utilized to develop tolerance [264]. Administration of autoantigens by these routes has been shown to reduce organ-specific inflammation and disease expression in several models of autoimmunity. Mucosal antigens are capable of restoring tolerance through the induction of antigen-specific T-cell immune responses characterized by the secretion of IL-4, IL-10, and TGF-β [265]. Tolerance is gained mechanistically through antigen site-directed bystander suppression by the T-cell response effectors [266,267]. Mucosal tolerance treatments have already shown their validity in the treatment of HSPs and oxLDL in murine atherosclerosis models. The success of mucosal tolerance treatments has led to human clinical trials [265].
VI.C.1. HSPs
Thought to contribute to atherogenesis, HSPs are expressed by ECs under duress typically associated with classic atherosclerosis risk factors such as high blood pressure, smoking, DM, and chemically altered lipoproteins [268,269]. These risk factors may lead to EC activation and death therefore resulting in the release of HSPs into intercellular spaces. These soluble HSPs (sHSPs) are pro-atherogenic in nature, causing the secretion of pro-inflammatory cytokines by MΦs and the expression of adhesion molecules by ECs [270]. In addition, MΦs present antigens to B-cells, which produce HSP autoantibodies. These autoantibodies interact with HSPs expressed on activated ECs further exacerbating the situation. The elevated expressions of both HSPs and HSP autoantibodies have been positively correlated with atherosclerosis [269,271,272]. Interestingly, the nasal and oral administrations of HSP65 in Ldlr−/− mice seem to cause an immunization effect, resulting in a reduction of atherosclerotic lesions induced by a high cholesterol diet [273,274]. Maron et al. observed a decrease in the proliferation of splenocytes and a decline in the total anti-HSP65 IgG titers with both administration routes. Meanwhile, nasal administration also resulted in decreased MΦand T-cell lesion numbers, reduced expression of IFN-γ, increased IL-10 expression, and increased Th2 dependent anti-HSP65 IgG1 titers [274]. Additional research was conducted with full length HSP60 or a 16aa HSP60 fragment (253 to 268) in Ldlr−/− mice that were subsequently fed a Western type diet [275]. These oral treatments both resulted in an 80% reduction of lesion formation in carotid arteries following collar placement and a 27% reduction in plaque area at the aortic root of HSP60-treated mice. Furthermore, augmented populations of CD4+CD25+Foxp3+ Tregs in lymphoid organs and blood were found. In vitro follow-up revealed that HSP60 treatment of lymph node cells from HSP60-tolerized mice secreted additional levels of IL-10 and TGF-β. Meanwhile, endogenous anti-HSP60 antibody levels were unaffected.
VI.C.2. oxLDL
Although the induction of immune responses against oxLDL by active immunization strategies can mediate atheroprotection as discussed above, pro-atherogenic responses specific for oxLDL have been suggested to exist as well. For example, transfer of CD4+ T-cells from mice immunized with malondialdehyde-modified LDL (MDA-LDL) induced significantly more atherosclerosis in recipient ApoE−/− SCID mice than T-cells from control mice. This response is thought to be the result of IFN-γ-secreting Th1 cells that are believed to mediate pro-inflammatory effects in lesions. To resolve this, an oral tolerance strategy using human copper-oxidized LDL (Cuox-LDL) and MDA-LDL was employed. Interestingly, only Ldlr−/− mice that received Cuox-LDL through oral administration developed significantly less atherosclerosis, while MDA-LDL feeding did not have an effect. Meanwhile, oral administration of Cuox-LDL led to a significant increase in TGF-β production and levels of CD4+CD25+Foxp3+ cells in the spleen, mesenteric lymph nodes, and in lymph node cells of tolerized mice [276]. No effects were observed in antibody titers to Cuox-LDL. Thus, similar to mucosal tolerance approaches with HSP60/65, the induction of Treg populations is associated with the decrease in atherogenesis, though direct immunological suppression of endogenous responses has not been well documented.
Conclusion
In this review, we thoroughly examined the role of immunogenicity in the development, participation, and exacerbation of CVD. A brief overview of immunogenicity and the innate and adaptive responses were discussed to provide the necessary foundation required for the comprehension of the concepts discussed. The recently established involvement of inflammatory/autoimmune mechanisms in atherogenesis has spurred the attention of researchers from a variety of scientific fields leading to an escalation of knowledge and novel findings. These advances will prove valuable in other fields that share etiology with atherogenesis such as diabetes. In addition, the elucidation of immunogenicity in atherosclerogenesis has led to the investigation of novel therapies. Potential treatment with stem cells has come to the forefront and requires judicious review due to their impending ability to trigger immune responses. Despite this possible complication, stem cells offer vast potential in future therapy and for the understanding of unknown mechanisms of pathophysiology. The examination of selective immune suppression concluded this review, providing possible insight into the immunogenic mechanisms that govern atherogenesis and the development of prospective therapeutics. Undeniable are the substantial advances that have been made in the field of CVD as the scientific disciplines of vascular biology, immunology, epidemiology, and bioinformatics become interpolated in the quest for a more comprehensive knowledge of CVD.
Table 1.
Abbreviations.
| AGE – advanced glycation endproduct |
| APC – antigen presenting cell |
| Apo – apolipoprotein |
| BMC – bone marrow mononuclear cell |
| CCL – CC chemokine ligand |
| CCR – CC chemokine receptor |
| Cuox–LDL – copper-oxidized low-density lipoprotein |
| CVD – cardiovascular disease |
| CX3CL – CX3C chemokine ligand |
| CX3CR – CX3C chemokine receptor |
| CXCL – CXC chemokine ligand |
| CXCR – CXC chemokine receptor |
| DC – dendritic cell |
| DM – diabetes mellitus |
| EC – endothelial cell |
| EPC – endothelial progenitor cell |
| ERK – extracellular signal-regulated kinase |
| ESC – embryonic stem cell |
| ET – endothelin |
| FFA – free fatty acid |
| GM-CSF – granulocyte macrophage colony-stimulating factor |
| HLA – human leukocyte antigens |
| HO – heme oxygenase |
| HSC – hematopoietic stem cell |
| IFN – interferon |
| IKK-β – inhibitor of NF-κB kinase subunit β |
| IL – interleukin |
| IR – insulin resistance |
| IRS – insulin receptor substrate |
| JNK – c-Jun N-terminal kinase |
| LDL – low-density liproprotein |
| LO – lipoxygenase |
| MΦ – macrophage |
| Mac-1 – macrophage-1 antigen |
| MAPK – mitogen-activated protein kinase |
| MC – monocyte |
| MDA–LDL – malondialdehye-modified low-density lipoprotein |
| MHC – major histocompatibility complex |
| mHC – minor histocompatibility complex |
| MI – myocardial infarction |
| MIF – migration inhibitory factor |
| MMP – matrix metalloproteinase |
| MSC – mesenchymal stem cell |
| NK – natural killer |
| NOD – non-obese diabetic |
| oxLDL – oxidized low-density lipoprotein |
| PAMP – pathogen-associated molecular |
| PI3K – phosphoinositide 3-kinase |
| PPAR – peroxisome proliferator-activated |
| PRR – pattern recognition receptor |
| PSGL – P-selectin glycoprotein ligand |
| RAGE – receptor for advanced glycation |
| ROS – reactive oxygen species |
| SEREX – serological analysis of recombinant tumor cDNA expression libraries |
| sHSP – soluble heat shock protein |
| SkMb – skeletal myoblast |
| SLE – systemic lupus erythematosus |
| SMC – smooth muscle cell |
| SR – scavenger receptor |
| T1DM – type 1 diabetes mellitus |
| Tfh – T follicular helper cells |
| Th – T-helper |
| Th1 – type 1 T-helper |
| Th2 – type 2 T-helper |
| TIMP – tissue inhibitor of metalloproteinases |
| TLR – toll-like receptor |
| TNF – tumor necrosis factor |
| Treg – regulatory T-cell |
| VCAM – vascular cell adhesion molecule |
| VLA – very late antigen |
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Harker L. Hyperlipidemia and atherosclerosis. Science. 1976;193:1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? J. Intern. Med. 2008;263:464–478. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Jan M, Meng S, Chen NC, Mai J, Wang H, Yang XF. Inflammatory and autoimmune reactions in atherosclerosis and vaccine design informatics. J. Biomed Biotechnol. 2010;2010:459798. doi: 10.1155/2010/459798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley E, Dasari V, Frishman WH, Sperber K. Vaccines in development to prevent and treat atherosclerotic disease. Cardiol. Rev. 2008;16:288–300. doi: 10.1097/CRD.0b013e3181885933. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. ed 6th. New York: Garland Science Publishing; 2005. [Google Scholar]
- 8.Chaplin DD. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, Yan Y, Jiang X, et al. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int. J. Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Wack A, Rappuoli R. Vaccinology at the beginning of the 21st century. Curr. Opin. Immunol. 2005;17:411–418. doi: 10.1016/j.coi.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv. Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 16.Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J. Allergy Clin. Immunol. 2010;125:S138–S149. doi: 10.1016/j.jaci.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 17.Botto M, Kirschfink M, Macor P, Pickering MC, Wurzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol. Immunol. 2009;46:2774–2783. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Carroll MC. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 19.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check--a new role for complement regulators? Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 21.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 22.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 23.King C. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 24.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 25.Doevendans PA, Jukema W, Spiering W, Defesche JC, Kastelein JJ. Molecular genetics and gene expression in atherosclerosis. Int. J. Cardiol. 2001;80:161–172. doi: 10.1016/s0167-5273(01)00466-1. [DOI] [PubMed] [Google Scholar]
- 26.Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. J. Autoimmun. 2009;33:3–11. doi: 10.1016/j.jaut.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 28.Tegos TJ, Kalodiki E, Sabetai MM, Nicolaides AN. The genesis of atherosclerosis and risk factors: a review. Angiology. 2001;52:89–98. doi: 10.1177/000331970105200201. [DOI] [PubMed] [Google Scholar]
- 29.Campbell LA, Kuo CC. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat. Rev. Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 30.Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009;60:39–54. doi: 10.1146/annurev.med.60.041807.123308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Jiang X, Yang F, et al. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101:3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Jiang X, Fang P, et al. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 34.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 35.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erbel C, Sato K, Meyer FB, et al. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res. Cardiol. 2007;102:123–132. doi: 10.1007/s00395-006-0636-x. [DOI] [PubMed] [Google Scholar]
- 37.Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 38.Lee M, Calabresi L, Chiesa G, Franceschini G, Kovanen PT. Mast cell chymase degrades apoE and apoA-II in apoA-I-knockout mouse plasma and reduces its ability to promote cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2002;22:1475–1481. doi: 10.1161/01.atv.0000029782.84357.68. [DOI] [PubMed] [Google Scholar]
- 39.Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 40.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 41.Schober A, Manka D, von Hundelshausen P, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 42.Langer HF, Daub K, Braun G, et al. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb. Vasc. Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 43.Schulz C, Schafer A, Stolla M, et al. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: a critical role for P-selectin expressed on activated platelets. Circulation. 2007;116:764–773. doi: 10.1161/CIRCULATIONAHA.107.695189. [DOI] [PubMed] [Google Scholar]
- 44.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 45.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 46.Canault M, Peiretti F, Poggi M, et al. Progression of atherosclerosis in ApoE-deficient mice that express distinct molecular forms of TNF-alpha. J. Pathol. 2008;214:574–583. doi: 10.1002/path.2305. [DOI] [PubMed] [Google Scholar]
- 47.Xiao N, Yin M, Zhang L, et al. Tumor necrosis factor-alpha deficiency retards early fatty-streak lesion by influencing the expression of inflammatory factors in apoE-null mice. Mol. Genet Metab. 2009;96:239–244. doi: 10.1016/j.ymgme.2008.11.166. [DOI] [PubMed] [Google Scholar]
- 48.Upadhya S, Mooteri S, Peckham N, Pai RG. Atherogenic effect of interleukin-2 and antiatherogenic effect of interleukin-2 antibody in apo-E-deficient mice. Angiology. 2004;55:289–294. doi: 10.1177/000331970405500308. [DOI] [PubMed] [Google Scholar]
- 49.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ. Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 50.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 51.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 52.Taleb S, Romain M, Ramkhelawon B, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 55.Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu G, Rux AH, Ma P, Bdeir K, Sachais BS. Endothelial expression of E-selectin is induced by the platelet-specific chemokine platelet factor 4 through LRP in an NF-kappaB-dependent manner. Blood. 2005;105:3545–3551. doi: 10.1182/blood-2004-07-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huo Y, Weber C, Forlow SB, et al. The chemokine, KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J. Clin. Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng C, Tempel D, van Haperen R, et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. J. Clin. Invest. 2007;117:616–626. doi: 10.1172/JCI28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraaijeveld AO, de Jager SC, van Berkel TJ, Biessen EA, Jukema JW. Chemokines and atherosclerotic plaque progression: towards therapeutic targeting? Curr. Pharm. Des. 2007;13:1039–1052. doi: 10.2174/138161207780487584. [DOI] [PubMed] [Google Scholar]
- 60.Heller EA, Liu E, Tager AM, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 61.Pan JH, Sukhova GK, Yang JT, et al. Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:3149–3153. doi: 10.1161/01.CIR.0000134704.84454.D2. [DOI] [PubMed] [Google Scholar]
- 62.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 63.Radmark O, Samuelsson B. 5-lipoxygenase: regulation and possible involvement in atherosclerosis. Prostaglandins Other Lipid Mediat. 2007;83:162–174. doi: 10.1016/j.prostaglandins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J. Clin. Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu BJ, Kathir K, Witting PK, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J. Exp. Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 67.Freedman JE. Oxidative stress and platelets. Arterioscler .Thromb. Vasc. Biol. 2008;28:s11–s16. doi: 10.1161/ATVBAHA.107.159178. [DOI] [PubMed] [Google Scholar]
- 68.Barlic J, Zhang Y, Foley JF, Murphy PM. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation. 2006;114:807–819. doi: 10.1161/CIRCULATIONAHA.105.602359. [DOI] [PubMed] [Google Scholar]
- 69.Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl .Acad. Sci. USA. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daugherty A, Pure E, Delfel-Butteiger D, et al. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J. Clin. Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reardon CA, Blachowicz L, White T, et al. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 72.Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J. Clin. Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 74.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 75.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Clin. Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc. Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 78.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 80.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. J. Am. Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elhage R, Jawien J, Rudling M, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 82.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laurat E, Poirier B, Tupin E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 84.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 86.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 87.Pinderski LJ, Fischbein MP, Subbanagounder G, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 88.Binder CJ, Hartvigsen K, Chang MK, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J. Clin. Invest. 2004;114:427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler Thromb. Vasc. Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 90.Lutgens E, Cleutjens KB, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJ. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc. Natl. Acad. Sci. USA. 2000;97:7464–7469. doi: 10.1073/pnas.97.13.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friesel R, Komoriya A, Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon. J. Cell Biol. 1987;104:689–696. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J. Exp. Med. 1989;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee E, Vaughan DE, Parikh SH, et al. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ. Res. 1996;78:44–49. doi: 10.1161/01.res.78.1.44. [DOI] [PubMed] [Google Scholar]
- 94.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J. Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 95.van Hinsbergh VW, van den Berg EA, Fiers W, Dooijewaard G. Tumor necrosis factor induces the production of urokinase-type plasminogen activator by human endothelial cells. Blood. 1990;75:1991–1998. [PubMed] [Google Scholar]
- 96.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2009;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie JJ, Wang J, Tang TT, et al. The Th17/Treg functional imbalance during atherogenesis in ApoE(−/−) mice Th17/Treg imbalance in atherogenesis. Cytokine. 2009 doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Yang XF, Yin Y, Wang H. Vascular Inflammation and Atherogenesis Are Activated Via Receptors for PAMPs and Suppressed By Regulatory T Cells. Drug Discov. Today Ther Strateg. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat. Rev. Immunol. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 101.Yan Y, Chen Y, Yang F, et al. HLA-A2.1-restricted T cells react to SEREX-defined tumor antigen CML66L and are suppressed by CD4+CD25+ regulatory T cells. Int. J. Immunopathol Pharmacol. 2007;20:75–89. doi: 10.1177/039463200702000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Xiong Z, Song J, Yan Y, et al. Higher expression of Bax in regulatory T cells increases vascular inflammation. Front Biosci. 2008;13:7143–7155. doi: 10.2741/3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiong Z, Yan Y, Song J, et al. Expression of TCTP antisense in CD25(high) regulatory T cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 106.Beyersdorf N, Gaupp S, Balbach K, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J. Exp. Med. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin T, Hu J, Wang D, Stocco DM. Interferon-gamma inhibits the steroidogenic acute regulatory protein messenger ribonucleic acid expression and protein levels in primary cultures of rat Leydig cells. Endocrinology. 1998;139:2217–2222. doi: 10.1210/endo.139.5.6006. [DOI] [PubMed] [Google Scholar]
- 108.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 109.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler. Thromb Vasc. Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 113.Witztum JL. Splenic immunity and atherosclerosis: a glimpse into a novel paradigm? J. Clin. Invest. 2002;109:721–724. doi: 10.1172/JCI15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Leeuwen M, Damoiseaux J, Duijvestijn A, Tervaert JW. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmun Rev. 2009;9:53–57. doi: 10.1016/j.autrev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 116.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 117.Shaw PX, Horkko S, Tsimikas S, et al. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler. Thromb Vasc Biol. 2001;21:1333–1339. doi: 10.1161/hq0801.093587. [DOI] [PubMed] [Google Scholar]
- 118.de Boer OJ, van der Wal AC, Houtkamp MA, Ossewaarde JM, Teeling P, Becker AE. Unstable atherosclerotic plaques contain T-cells that respond to Chlamydia pneumoniae. Cardiovasc. Res. 2000;48:402–408. doi: 10.1016/s0008-6363(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 119.Palinski W, Rosenfeld ME, Yla-Herttuala S, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17. doi: 10.1161/01.atv.20.1.10. [DOI] [PubMed] [Google Scholar]
- 121.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 123.Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 124.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 125.Ait-Oufella H, Kinugawa K, Zoll J, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–2177. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- 126.Angeli V, Llodra J, Rong JX, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 127.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N. Engl. J. Med. 2003;349:160–169. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 128.Gupta M, Vavasis C, Frishman WH. Heat shock proteins in cardiovascular disease a new therapeutic target. Cardiol. Rev. 2004;12:26–30. doi: 10.1097/01.crd.0000090894.70619.fa. [DOI] [PubMed] [Google Scholar]
- 129.Veres A, Fust G, Smieja M, et al. Relationship of anti-60 kDa heat shock protein and anti-cholesterol antibodies to cardiovascular events. Circulation. 2002;106:2775–2780. doi: 10.1161/01.cir.0000038890.39298.8d. [DOI] [PubMed] [Google Scholar]
- 130.Bachmaier K, Penninger JM. Chlamydia and antigenic mimicry. Curr. Top Microbiol. Immunol. 2005;296:153–163. doi: 10.1007/3-540-30791-5_9. [DOI] [PubMed] [Google Scholar]
- 131.Szekanecz Z, Kerekes G, Der H, et al. Accelerated atherosclerosis in rheumatoid arthritis. Ann. N Y Acad Sci. 2007;1108:349–358. doi: 10.1196/annals.1422.036. [DOI] [PubMed] [Google Scholar]
- 132.Sherer Y, Cerinic MM, Bartoli F, et al. Early atherosclerosis and autoantibodies to heat-shock proteins and oxidized LDL in systemic sclerosis. Ann. N Y Acad. Sci. 2007;1108:259–267. doi: 10.1196/annals.1422.028. [DOI] [PubMed] [Google Scholar]
- 133.de Leeuw K, Kallenberg C, Bijl M. Accelerated atherosclerosis in patients with systemic autoimmune diseases. Ann. N Y Acad. Sci. 2005;1051:362–371. doi: 10.1196/annals.1361.078. [DOI] [PubMed] [Google Scholar]
- 134.Belizna CC, Richard V, Thuillez C, Levesque H, Shoenfeld Y. Insights into atherosclerosis therapy in antiphospholipid syndrome. Autoimmun. Rev. 2007;7:46–51. doi: 10.1016/j.autrev.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 135.Vikramadithyan RK, Hu Y, Noh HL, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J. Clin. Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stumvoll M. Type 2 diabetes : principles of pathogensis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 137.Diabetes. World Health Organization. 2009 Nov
- 138.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr. Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 139.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 140.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 141.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 142.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome, X. Diabetologia. 1997;40:1286–1292. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 143.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 144.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gual P, Gremeaux T, Gonzalez T, Le Marchand-Brustel Y, Tanti JF. MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia. 2003;46:1532–1542. doi: 10.1007/s00125-003-1223-4. [DOI] [PubMed] [Google Scholar]
- 146.Rohl M, Pasparakis M, Baudler S, et al. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J. Clin. Invest. 2004;113:474–481. doi: 10.1172/JCI18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wunderlich FT, Luedde T, Singer S, et al. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc. Natl. Acad. Sci. USA. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 149.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 150.Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J. Biol. Chem. 1995;270:23780–23784. doi: 10.1074/jbc.270.40.23780. [DOI] [PubMed] [Google Scholar]
- 151.Kwon G, Xu G, Marshall CA, McDaniel ML. Tumor necrosis factor alpha-induced pancreatic beta-cell insulin resistance is mediated by nitric oxide and prevented by 15-deoxy-Delta12,14-prostaglandin J2 and aminoguanidine. A role for peroxisome proliferator-activated receptor gamma activation and inos expression. J. Biol. Chem. 1999;274:18702–18708. doi: 10.1074/jbc.274.26.18702. [DOI] [PubMed] [Google Scholar]
- 152.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch. Med. Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 153.Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol. 1999;10:19–29. doi: 10.1006/scdb.1998.0273. [DOI] [PubMed] [Google Scholar]
- 154.Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol. Ther. 2006;8:7–17. doi: 10.1089/dia.2006.8.7. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez-Real JM, Pickup JC. Innate immunity, insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 2008;19:10–16. doi: 10.1016/j.tem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 157.Dandona P, Aljada A. A rational approach to pathogenesis and treatment of type 2 diabetes mellitus, insulin resistance, inflammation, and atherosclerosis. Am. J. Cardiol. 2002;90:27G–33G. doi: 10.1016/s0002-9149(02)02556-0. [DOI] [PubMed] [Google Scholar]
- 158.Marette A. Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr. Opin. Clin. Nutr .Metab Care. 2002;5:377–383. doi: 10.1097/00075197-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 159.Eizirik DL, Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 160.Winer S, Tsui H, Lau A, et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat. Med. 2003;9:198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 161.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension. 2005;45:828–833. doi: 10.1161/01.HYP.0000163475.04421.e4. [DOI] [PubMed] [Google Scholar]
- 162.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 163.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Taylor SI, Accili D, Imai Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes. 1994;43:735–740. doi: 10.2337/diab.43.6.735. [DOI] [PubMed] [Google Scholar]
- 165.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 166.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: mechanisms and management. Ann. Intern. Med. 2003;139:824–834. doi: 10.7326/0003-4819-139-10-200311180-00010. [DOI] [PubMed] [Google Scholar]
- 168.Anderson TJ, Meredith IT, Charbonneau F, et al. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation. 1996;93:1647–1650. doi: 10.1161/01.cir.93.9.1647. [DOI] [PubMed] [Google Scholar]
- 169.Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 170.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 171.Steinberg HO, Tarshoby M, Monestel R, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 173.Davi G, Catalano I, Averna M, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N. Engl. J. Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 174.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 175.Thogersen AM, Jansson JH, Boman K, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 176.Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 177.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 178.Johansson F, Kramer F, Barnhart S, et al. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Griffin E, Re A, Hamel N, et al. A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nat. Med. 2001;7:840–846. doi: 10.1038/89969. [DOI] [PubMed] [Google Scholar]
- 180.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- 182.Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation. 1998;97:996–1001. doi: 10.1161/01.cir.97.10.996. [DOI] [PubMed] [Google Scholar]
- 183.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 184.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler. Thromb. Vasc. Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 185.Pirola L, Johnston AM, Van Obberghen E. Modulation of insulin action. Diabetologia. 2004;47:170–184. doi: 10.1007/s00125-003-1313-3. [DOI] [PubMed] [Google Scholar]
- 186.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 188.Semenkovich CF. Insulin resistance and atherosclerosis. J. Clin. Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Montagnani M, Golovchenko I, Kim I, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J. Biol. Chem. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 190.Montagnani M, Quon MJ. Insulin action in vascular endothelium: potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab. 2000;2:285–292. doi: 10.1046/j.1463-1326.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 191.Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiang ZY, Zhou QL, Chatterjee A, et al. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120–1130. doi: 10.2337/diabetes.48.5.1120. [DOI] [PubMed] [Google Scholar]
- 194.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 195.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J. Am. Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 196.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes. Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 197.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. Jama. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 198.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 199.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 200.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 201.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 202.Kota BP, Huang TH, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005;51:85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 203.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 204.Michalik L, Auwerx J, Berger JP, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol.Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 205.Collins AR, Meehan WP, Kintscher U, et al. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb. Vasc. Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 206.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 207.Marx N, Kehrle B, Kohlhammer K, et al. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ. Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab. Vasc. Dis. Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 209.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 210.Gulati R, Simari RD. Defining the potential for cell therapy for vascular disease using animal models. Dis. Model. Mech. 2009;2:130–137. doi: 10.1242/dmm.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat. Rev. Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 212.Yang XF. Immunology of stem cells and cancer stem cells. Cell Mol Immunol. 2007;4:161–171. [PubMed] [Google Scholar]
- 213.Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002;190:86–94. doi: 10.1034/j.1600-065x.2002.19007.x. [DOI] [PubMed] [Google Scholar]
- 214.Watkins WM. The ABO blood group system: historical background. Transfus Med. 2001;11:243–265. doi: 10.1046/j.1365-3148.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 215.Schreuder GM, Hurley CK, Marsh SG, et al. The HLA Dictionary 2001: a summary of HLA-A, -B, -C, -DRB1/3/4/5 and -DQB1 alleles and their association with serologically defined HLA-A, -B, -C, -DR and -DQ antigens. Eur. J. Immunogenet. 2001;28:565–596. doi: 10.1046/j.0960-7420.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 216.Rubinstein P. HLA matching for bone marrow transplantation--how much is enough? N. Engl. J. Med. 2001;345:1842–1844. doi: 10.1056/NEJM200112203452511. [DOI] [PubMed] [Google Scholar]
- 217.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J. Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Drukker M, Katz G, Urbach A, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tian L, Catt JW, O'Neill C, King NJ. Expression of immunoglobulin superfamily cell adhesion molecules on murine embryonic stem cells. Biol. Reprod. 1997;57:561–568. doi: 10.1095/biolreprod57.3.561. [DOI] [PubMed] [Google Scholar]
- 220.Yang XF, Mirkovic D, Zhang S, et al. Processing sites are different in the generation of HLA-A2.1-restricted, T cell reactive tumor antigen epitopes and viral epitopes. Int. J. Immunopathol Pharmacol. 2006;19:853–870. doi: 10.1177/039463200601900415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang F, Chen IH, Xiong Z, Yan Y, Wang H, Yang XF. Model of stimulation-responsive splicing and strategies in identification of immunogenic isoforms of tumor antigens and autoantigens. Clin. Immunol. 2006;121:121–133. doi: 10.1016/j.clim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 222.Yang F, Yang XF. New concepts in tumor antigens: their significance in future immunotherapies for tumors. Cell Mol Immunol. 2005;2:331–341. [PubMed] [Google Scholar]
- 223.Zinkernagel RM, Hengartner H. Regulation of the immune response by antigen. Science. 2001;293:251–253. doi: 10.1126/science.1063005. [DOI] [PubMed] [Google Scholar]
- 224.Sahin U, Tureci O, Schmitt H, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chen YT. Identification of human tumor antigens by serological expression cloning: an online review on SEREX. Cancer Immunity. 2004 [Google Scholar]
- 226.Xiong Z, Yan Y, Liu E, et al. Novel tumor antigens elicit anti-tumor humoral immune reactions in a subset of patients with polycythemia vera. Clin. Immunol. 2007;122:279–287. doi: 10.1016/j.clim.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu CJ, Yang XF, McLaughlin S, et al. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J. Clin. Invest. 2000;106:705–714. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xiong Z, Liu E, Yan Y, et al. An unconventional antigen translated by a novel internal ribosome entry site elicits antitumor humoral immune reactions. J. Immunol. 2006;177:4907–4916. doi: 10.4049/jimmunol.177.7.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xiong Z, Liu E, Yan Y, et al. A novel unconventional antigen MPD5 elicits anti-tumor humoral immune responses in a subset of patients with polycythemia vera. Int. J. Immunopathol. Pharmacol. 2007;20:373–380. doi: 10.1177/039463200702000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yang XF, Wu CJ, Chen L, et al. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517–5522. [PMC free article] [PubMed] [Google Scholar]
- 231.Yang XF, Wu CJ, McLaughlin S, et al. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc. Natl. Acad. Sci. USA. 2001;98:7492–7497. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ng B, Yang F, Huston DP, et al. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J. Allergy Clin. Immunol. 2004;114:1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Grusby MJ, Auchincloss H, Jr, Lee R, et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 235.Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 236.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp. Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tomescot A, Leschik J, Bellamy V, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in postmyocardial infarcted rats. Stem Cells. 2007;25:2200–2205. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- 238.Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann. Thorac. Surg. 1995;60:12–18. [PubMed] [Google Scholar]
- 239.Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 240.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc. Natl. Acad. Sci. USA. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 242.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch. Intern. Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 244.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 245.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ. Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 246.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cai CL, Martin JC, Sun Y, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhou B, Ma Q, Rajagopal S, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kubo H, Jaleel N, Kumarapeli A, et al. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 251.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 252.Hibbert B, Olsen S, O'Brien E. Involvement of progenitor cells in vascular repair. Trends Cardiovasc Med. 2003;13:322–326. doi: 10.1016/j.tcm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 253.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 254.Libby P. Bone marrow: a fountain of vascular youth? Circulation. 2003;108:378–379. doi: 10.1161/01.CIR.0000084801.04026.7B. [DOI] [PubMed] [Google Scholar]
- 255.SoRelle R. Aging of progenitor cells contributes to atherosclerosis. Circulation. 2003;108:e9006–e9007. doi: 10.1161/01.CIR.0000089325.27670.85. [DOI] [PubMed] [Google Scholar]
- 256.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 257.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 258.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol. Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu. Rev. Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 260.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Steffens S, Burger F, Pelli G, et al. Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation. 2006;114:1977–1984. doi: 10.1161/CIRCULATIONAHA.106.627430. [DOI] [PubMed] [Google Scholar]
- 262.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 263.Schonbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl. Acad. Sci. USA. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–S95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 265.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv. Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 266.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 267.Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 1991;174:791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Amberger A, Maczek C, Jurgens G, et al. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:ceoive>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wick G, Knoflach M, Kind M, Henderson B, Bernhard D. Heat shock proteins and stress in atherosclerosis. Autoimmun. Rev. 2004;3(Suppl 1):S30–S31. [PubMed] [Google Scholar]
- 270.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Invest. 1999;103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schett G, Xu Q, Amberger A, et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J. Clin. Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Xu Q, Schett G, Perschinka H, et al. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- 273.Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J. Am. Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/s0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- 274.Maron R, Sukhova G, Faria AM, et al. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 275.van Puijvelde GH, van Es T, van Wanrooij EJ, et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 276.van Puijvelde GH, Hauer AD, de Vos P, et al. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]