Abstract
Background
Recent researches have led to find strategies to prevent relapse and to improve survival for gastric cancer patients, including preoperative neo-adjuvant approaches. However, the efficacy of some neo-adjuvant regimens including 5-fluorouracil, cisplatin, and docetaxel have been less investigated. The present study evaluated the outcome and mid-term survival of patients with gastric cancer who undergoing this regimen.
Methods
In a randomized double-blinded controlled trial performed at the Firoozgar hospital in Tehran in 2011-12, 61 patients were randomly assigned to treatment (32 to neo-adjuvant chemotherapy with docetaxel, cisplatin and 5-fluorouracil (5-FU) before surgery and 27 to surgery alone). The present study tried to assess the efficacy of neoadjuvant chemotherapy regarding improvement of mid-term survival, complications, and R0 resection status.
Results
The two groups were matched in terms of gender, mean age, cancer location, and TNM staging. However, R0 resection in the former group was 85.7%; while this indicator in the isolated surgery group was significantly lower (61.5%). Regarding WHO performance, no significant difference was observed across the two groups. Patients in neo-adjuvant chemotherapy group were followed for mean follow-up time 10.32 months and those who categorized in isolated surgery group were followed for mean follow-up time 10.88 months. Mid-term mortality rate in the two groups was 14.3% and 15.4%, respectively (p = 0.866). In this regard, 3-, 6-, and 9-month survival rate in neo-adjuvant chemotherapy group was 96.4%, 89.3%, and 85.7%, respectively. These survival rates in the surgery group were 92.3%, 88.5%, and 84.6%, respectively. Multivariable logistic regression analysis showed that among all study variables, only R0 resection status could predict mid-term mortality.
Conclusion
Neo-adjuvant chemotherapy and surgery compare to surgery alone more improve R0 resection status, but mid-term survival rate is similar in the two regiments. R0 resection status can effectively predict appropriate mid-term survival in undertreated patients.
Keywords: Gastroesophageal junction, Mortality, Chemotherapy, Surgery
INTRODUCTION
Despite its declining incidence in of gastrointestinal malignancies whole of the world, these types of cancers remain the second most common cause of cancer death.1–3 Surgical interventions are the only potentially curative approaches of localized gastric cancer and radical gastrectomy with extended lymphadenectomy is now recognized as a safe in experienced centers.4, 5 However, the prognosis for patients with locally advanced gastric cancer remains poor even after potentially curative resection with a high risk of locoregional or distant recurrence.6
For instance, more than 60% of patients undergoing an R0 resection relapse and even die due to their disease outcome and consequently, the overall 5-year survival rate of these patients range from 10% to 30%.7 Thus, recent researches have led to find strategies to prevent relapse and to improve survival for gastric cancer patients, including preoperative neo-adjuvant approaches. It has been indicated that primary chemotherapy are potentially useful for patients with advanced cancer stages that result in down-staging of the tumors and consequently improving the curative resection rate. Furthermore, a theoretical benefit of neo-adjuvant chemotherapy concerns micro-metastases that are undetectable at the start of treatment.8 A number of clinical trials have also shown that preoperative neo-adjuvant chemotherapy is able to increase the rate of R0 resection. However, the efficacy of some neo-adjuvant regimens including 5-fluorouracil, cisplatin, and docetaxel have been less investigated. The present study evaluated the outcome and mid-term survival of patients with gastric cancer who undergoing this regimen.
PATIENTS AND METHODS
In a randomized double-blinded controlled trial performed at the Firoozgar hospital in Tehran in 2011-12, patients of any age who had final diagnosis of adenocarcinoma of the stomach or esophagogastric junction (EGJ) or lower third of the esophagus that was considered to be at least stage IB, based on clininal staging (abdominopelvic CT scan, endoscopic ultrasonography) before surgery, and with a World Health Organization (WHO) performance status of 0 or 1 were eligible in the study. Patients were not included if they had any evidence of distant metastases, locally advanced inoperable disease, cancer with other origins, previous history of cytotoxic chemotherapy or radiotherapy, creatinine clearance of 60 ml per minute or less, uncontrolled cardiac disease, active liver disease (bilirubin > 1.5, AST > 60, or ALT more than two times of normal level). The protocol was approved by the relevant ethics committees at the Tehran University of Medical Sciences, and patients gave written informed consent for participation in this trial. Baseline characteristics and medical history were collected from the recorded files or interviewing by the patients. Pre-treatment and post-treatment of tumor staging were determined from resected specimens were examined at local pathology laboratories according to a standard protocol that used the tumor–node–metastasis (TNM) classification. Patients were randomly assigned to either perioperative chemotherapy or surgical resection (the perioperative-chemotherapy group) or to surgical resection alone (the surgery group) by computerized randomization table. Chemotherapy was administered for three cycles preoperatively consisted of docetaxel (75 mg per square meter by intravenous infusion in 500cc normal saline at 1 hour), cisplatin (75 mg per square meter by intravenous infusion in 1000cc normal saline at 3 hours), and fluorouracil (750 mg per square meter by continuous intravenous infusion in 1000cc normal saline at 24 hours). Before each cycle of chemotherapy, a complete blood count was obtained and blood urea nitrogen, electrolyte, and serum creatinine levels and liver function were determined. All patients in chemotherapy group were taken granulocyte colony stimulating factor (GCSF) 24 hours after chemotherapy, daily for at least 3 days. Surgery was scheduled to take place within three to four weeks after completion of the third cycle of chemotherapy in the perioperative chemotherapy group. The matched control group received no chemotherapy and surgery was scheduled to take place within four weeks after randomization. Both groups recieved 5-FU based chemoradiation with consideration of pathologic TNM classification within 3-4 weeks after surgery. The severity of adverse effects, defined according to the National Cancer Institute Common Toxicity Criteria, and performance status were assessed every three weeks.
Results were reported as mean ± standard deviation (SD) for the quantitative variables and percentages for the categorical variables. The groups were compared using the Student's t-test or one-way ANOVA test for the continuous variables and the chi-square test (or Fisher's exact test if required) for the categorical variables. Predictors exhibiting a statistically significant relation with mid-term survival in the two groups were taken for a multivariable logistic regression analysis to investigate their independence as predictors. The survival analysis was performed using the Kaplan-Mayer Curve. P values of 0.05 or less were considered statistically significant. All the statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) and SAS version 9.1 for windows (SAS Institute Inc., Cary, NC, USA).
RESULTS
61 patients were assigned to treatment (32 to perioperative chemotherapy and surgery and 27 to surgery alone). Among them, 4 subjects in the first group and 1 patient in the second group refused the complete treatment regimen because of intolerance and thus were excluded from the study. Finally, 28 patients in the neo-adjuvant chemotherapy group and 26 patients in isolated surgery group were studied. The two groups were matched in terms of gender, mean age, cancer location, and TNM classification (Table 1). However, R0 resection in the former group was 85.7%, while this indicator in the isolated surgery group was significantly lower (61.5%). Regarding, chemotherapy-related complications in the first group, two patients had anemia, two of them had neutropenia, one had thrombocytopenia, and another one skin lesions. Regarding WHO performance, no significant difference was observed across the two groups. In the chemotherapy group, the considered treatment schedule resulted in decreasing TNM classification that T3 class was changed from 39.3% to 28.6% and T4 class was modified from 39.3% to 0.0% (Table 2). Also, regarding nodal involvement, N2 was reduced from 28.6% to 7.1% (down staging of tumor).
Table 1.
Baseline Characteristics and Clinical Data of Study Subjects
| Variable | neo-adjuvant surgery group(n = 28) | Isolated surgery group(n = 26) | P-value |
|---|---|---|---|
| Male gender | 82.1% | 84.6% | 0.999 |
| Age, yr | 62.63 ± 7.50 | 61.22 ± 6.26 | 0.437 |
| Site of tumor: | 0.890 | ||
| Stomach | 60.7% | 57.7% | |
| Lower esophagus | 21.4% | 19.1% | |
| Esophagogastric junction | 17.9% | 23.1% | |
| WHO performance status: | 0.779 | ||
| 0 | 67.9% | 65.4% | |
| 1 | 32.1% | 34.6% | |
| Size of tumor (T staging) | 0.434 | ||
| 1 | 3.6% | 0.0% | |
| 2 | 17.9% | 19.2% | |
| 3 | 39.3% | 50.0% | |
| 4 | 39.3% | 30.8% | |
| Lymph node involvement: | 0.789 | ||
| 0 | 7.1% | 7.7% | |
| 1 | 64.3% | 53.8% | |
| 2 | 28.6% | 38.5% |
Table 2.
Changes in TN Classification in Chemotherapy Group
| TN class | Before treatment | After treatment | P-value |
|---|---|---|---|
| T class | 0.001 | ||
| 1 | 3.6% | 21.4% | |
| 2 | 17.9% | 50.0% | |
| 3 | 39.3% | 28.6% | |
| 4 | 39.3% | 0.0% | |
| N class | 0.015 | ||
| 0 | 7.1% | 64.3% | |
| 1 | 64.3% | 28.6% | |
| 2 | 28.6% | 7.1% |
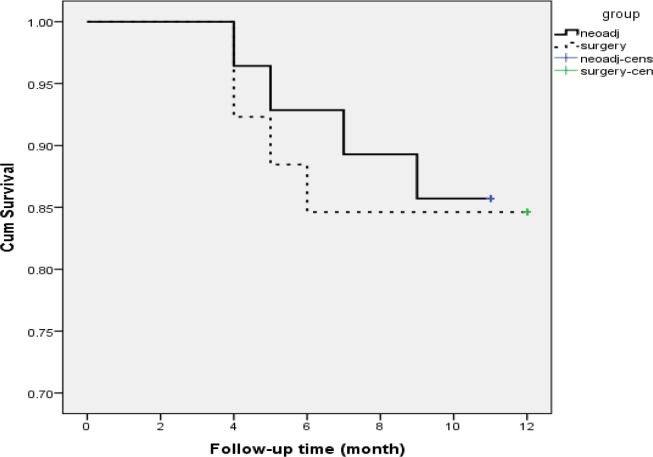
Patients in neo-adjuvant chemotherapy group were followed for 1 to 11 months (mean follow-up time 10.32 months) and those who categorized in isolated surgery group were followed for 3 to 12 months (mean follow-up time 10.88 months). Mid-term mortality rate in the two groups was 14.3% and 15.4%, respectively (p = 0.866). In this regard, 3-, 6-, and 9-month survival rate in neo-adjuvant chemotherapy group was 96.4%, 89.3%, and 85.7%, respectively. These survival rates in the surgery group were 92.3%, 88.5%, and 84.6%, respectively (Figure 1).
Figure 1.
Survival rate in neo-adjuvant chemotherapy and surgery groups
Multivariable logistic regression analysis showed that among all study variables, only R0 resection status could predict mid-term mortality (Table 3).
Table 3.
Multivariate Regression Analysis for Determining Indicators of Survival
| P-value | 95% | Confidence Interval | Odds Ratio | Indicator |
|---|---|---|---|---|
| 0.333 | 34.749 | 0.301 | 3.232 | Type of regimen |
| 0.669 | 35.404 | 0.101 | 1.893 | Male gender |
| 0.629 | 1.230 | 0.882 | 1.042 | Age |
| 0.657 | 2.597 | 0.016 | 0.206 | Site of tumor |
| 0.538 | 3.369 | 0.098 | 0.573 | T stage |
| 0.342 | 2.877 | 0.048 | 0.370 | N stage |
| 0.033 | 0.798 | 0.004 | 0.056 | R0 resection |
| 0.186 | 2.447 | 0.010 | 0.156 | WHO performance |
DISCUSSION
The present study tried to compare of two groups including neo-adjuvant chemotherapy-surgery and isolated surgery group regarding improvement of mid-term survival, complications, and R0 resection status. Our study could demonstrate higher efficacy of the former regimen to improve R0 resection indicator (85.7% versus 65.5%), however the beneficial effects of the two regimens on mid-term survival rate were similar. Also, among all probable determinants of mid-term survival rate, only R0 resection status could predict survival rate in the follow-up patients. Reviewing the findings of most previous studies were similar and in parallel with our results. According to the results of Ychou et al., study, neo-adjuvant chemotherapy could increase the rate of complete tumor resections, combat systemic metastases, and prolong survival in patients with gastric cancer. The available data suggest that the sequence of treatments is the main responsible for the benefit of perioperative therapy.9 Several small phase II trials with different cisplatin-based neo-adjuvant chemotherapy regimens have reported response rate between 40% and 60% and R0 resection rates up to 80%. In the small Dutch randomized trial, however, 59 patients were randomly assigned to receive the FAMTX regimen before surgery or to surgery alone. Complete or partial response was registered in 32% of the FAMTX group and there was no difference in terms of resectability. With a median follow-up of 83 months, the overall survival since randomization was 18 months in the FAMTX-treated patients versus 30 months in the surgery-alone group. This trial did not show a beneficial effect of preoperative FAMTX.10 The most important large phase III study is the UK Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial that was also the first well-powered phase III neo-adjuvant chemotherapy study to assess the efficacy of perioperative chemotherapy. Five hundred and three patients with potentially resectable gastric cancer were randomly assigned to both preoperative and postoperative ECF chemotherapy versus surgery alone. ECF regimen consisted of epirubicin (50 mg/m2) and cisplatin (60 mg/m2) administered on day 1 and protracted venous infusion of 5-fluorouracil (5-FU; 200 mg/m2/day) on days 1–21, administered every 3 weeks for three cycles before and after surgery. The results of this trial demonstrated a statistically significant improvement of the study arm in progression-free survival (PFS) [hazard ratio (HR) 0.66; 95% confidence interval (CI) 0.53–0.81; P < 0.001] and overall survival (OS) compared with surgery alone (HR 0.75; 95% CI 0.60–0.93; P = 0.009; 5-year survival rate, 36% versus 23%). The resected tumors were significantly smaller and less advanced in the perioperative chemotherapy group.11
Totally, it can be concluded that although neo-adjuvant chemotherapy-surgery regimen may not be effective to improve mid-term survival of patients, but it can result in more improvement in R0 resection status.
REFERENCES
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Levi F, Lucchini F, Negri E, et al. Cancer mortality in Europe, 1995–1999, and an overview of trends since 1960. Int J Cancer. 2004;110:155–169. doi: 10.1002/ijc.20097. [DOI] [PubMed] [Google Scholar]
- 3.Levi F, Lucchini F, Gonzalez JR, et al. Monitoring falls in gastric cancer mortality in Europe. Ann Oncol. 2004;15:338–345. doi: 10.1093/annonc/mdh057. [DOI] [PubMed] [Google Scholar]
- 4.Engstrom PF, Lavin DT, Douglass HO, Brunner KW. Postoperative adjuvant 5-fluorouracil plus methyl-CCNU therapy for gastric cancer patients. Eastern Cooperative Oncology Group (EST 3275) Cancer. 1985;55:1868–1873. doi: 10.1002/1097-0142(19850501)55:9<1868::aid-cncr2820550904>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Coombes RC, Schein PS, Chilvers CED, et al. A randomized trial comparing adjuvant fluorouracil, doxorubicin and mitomycin with no treatment in operable gastric cancer. J Clin Oncol. 1990;8:1362–1369. doi: 10.1200/JCO.1990.8.8.1362. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Fuchs CS. Adjuvant therapy in gastric cancer: can we prevent recurrences? Oncology. 2003;17:714–722. [PubMed] [Google Scholar]
- 7.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 8.Landry J, Tepper JE, Wood WC, Moulton EO, Koerner F, Sullinger J. Patterns of failure following curative resection of gastric carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:1357–1362. doi: 10.1016/0360-3016(90)90344-j. [DOI] [PubMed] [Google Scholar]
- 9.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011 May 1;29(13):1715–21. doi: 10.1200/JCO.2010.33.0597. Epub 2011 Mar 28. [DOI] [PubMed] [Google Scholar]
- 10.Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH. Cooperating Investigators of The Dutch Gastric Cancer Group Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004 Aug;30(6):643–9. doi: 10.1016/j.ejso.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006 Jul 6;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]