Abstract
Background
Pediatric infectious disease is one of the main problems in cancerous children that treat by chemotherapy drugs. Thus, study in this regard is necessary. The aim of this study was to evaluate antimicrobial properties of ethanolic extract of Ziziphus Jujuba fruits against different infectious pathogens.
Materials and Methods
This study is descriptive. In vitro antimicrobial activity of extract was assessed on gram negative and gram positive bacteria as well as fungi. The antimicrobial activity was tested by Radial Diffusion Assay (RDA) and Minimal Inhibitory Concentration (MIC) methods.
Results
The results showed a wide antimicrobial activity of the extract against the microbes studied. Escherichia coli was the most susceptible to the extracts among tested microorganisms for which the MIC was 0.65±0.22 mg/ml. Amongst the bacterial strains investigated, Staphylococcus aureus was the most resistant strain with MIC of 2.26±0.68 mg/ml. The ethanolic extract also showed antimicrobial activity on the fungi studied as no growth was observed in 2.35±0.38 and 2.86±0.7 mg/ml concentration for Candida albicans and Aspergillus fumigatus, respectively. The results of qualitive and quantitative test are well indicative of the extract effective activity against the microbes mentioned.
Conclusion
Confirming the potential antimicrobial activities of crude extract of Ziziphus Jujuba fruits, this study suggested that ethanolic extracts of this plant is appropriate candidate for treatment of microbial infections, especially pediatric infectious diseases.
Key Words: Fungi, Pediatrics, Ziziphus
Introduction
Immune deficiencies following cancer treatment with different chemotherapy drugs such as doxorubicin lead to development of infectious diseases, especially in children (1); hence, search and discovery over antimicrobial compounds has been an active branch of medical sciences. Use of traditional antibiotics such as penicillin causes development of resistant microbial strains. This status is more than important for children’s infections. With increasing antibiotic-resistant microbial strains, the discovery of new antimicrobial compounds has been of double significance (2). Various sources of poisonous secretion of animals such as snake, scorpion, spider, amphibians, insects and etc have been discovered with diverse effects (3-6). The plants are also of special importance. Herbal plants, in particular, have a special place in traditional medicine. Antimicrobial herbal compounds are one of the valuable medical resources, and in line with the spread of pediatric infectious diseases, identification of more of these extracts and compounds will be useful in treating patients (7, 8). Plant-derived antimicrobial compounds have numerous therapeutic effects; not only they are useful for treatment of infectious diseases, but also have less side effects than most of antimicrobial compounds (9-11). Isolation of effective antimicrobial components has been made from various plants, showing a significant impact on a variety of pathogenic bacteria and fungi. Historically, plants have provided sources of antimicrobial compounds to fight infection (12, 13). It is estimated that 250000 to 500000 plant species exist on Earth, some of which are widely used in medicine (14). In recent years, the attention has been attracted to research on medicinal plants. Various activity-related aspects of plant compounds have been investigated against various bacteria, fungi, viruses and protozoa. Hexan extract of the stem bark of Amona glabra (15), alkaloid extract from dried seeds of Semecarpus anacardium (16), alcoholic extract of the stem bark of Clausena anisata (16), aqueous extracts of Azadirachta indica leaves (17) and oil extract from the foliage of Santolina chamaecyparissus and Aegle marmelos (18) have shown antimicrobial activity against various bacteria and fungi. Zizyphus jujuba has medicinal properties; it is used as antidote, diuretic, laxative and expectorant. Its dried fruits are used as sedatives, anticancer, antipyretic, analgesic, appetizer, anti-hemorrhage agents and as the tonic as well (19-21). So far, there is no study on plant extract against pediatric infection. Thus, the present study has been conducted to evaluate antimicrobial activity of raw extract of Ziziphus Jujuba fruits.
Materials and Methods
This study is descriptive. Sample extraction was carried out through Soxhlet extractor. For this purpose, 15 g powder of Zizyphus jujuba fruits was filled in the thimble and extracted with ethanol solvent in Soxhlet extractor for 48 hours (22). Then the solution was filtered by filter paper. The extract was concentrated to one-tenth of the original volume using the vacuum distillation. To investigate the antimicrobial activity, two methods, including Radial Diffusion Assay and macro broth dilution, were used. The extract was dissolved in distilled water. The antimicrobial effects of the extract were investigated using Radial Diffusion Assay (RDA) as described below.
Two species of gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) and two species of gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) were used for primary assays. An aliquot of bacteria with a titer of 4 × 106 CFU was mixed with 10 ml of medium containing 0.03% TSB and 1% agarose and was poured into a plate. Holes were then created in the medium using a punch, 10 µl of the plant extract was loaded into the wells and the plates were incubated for 3 h at 37 °C. After the three-hour incubation, the secondary medium, enriched with 6% TSB and 1% agarose was poured into the plate, and the plates were incubated at 37 ºC for 18 hours. The plate was then stained for 24 hours using a solution containing 37% formaldehyde, 15 ml; methanol, 27 ml; water, 63 ml; and Coomassie brilliant blue R-250, 2 mg. The plates were destained for approximately 10 min with an aqueous solution of 10% acetic acid and 2% dimethylsulfoxide (23).
Antifungal activity of the plant extract was tested on Candida albicans and Aspergillus fumigatus. One ml of fungal suspension was inoculated in 20 ml of Potato dextrose agar and was poured into the germ culture plates. The holes were then created by the punch in the medium and were filled by 10 µl of the plant extract. The plates were incubated for 7 days at 30°C to 35°C and the results were recorded during this period (24).
To determine the MIC, stock serial dilutions of 1 to 35 mg/ml of the extract were prepared and 20μl of the extract stocks were added to a solution containing 106 CFU/ml of bacteria, which was then poured into a plate. The microplate was incubated at 37 °C for 18 hours. After this time, the absorbance of each well was read at 630 nm using an Enzyme-Linked Immunosorbent Assay (ELISA) reader, and the results were compared to the control samples. The MIC was defined as the extract concentration at which the absorbance of the treated bacterial sample is half of that of the untreated well of bacteria. Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis, were used for MIC determination. Experiments were carried out in triplicate.
To determine the fungi-associated MIC, 180 microliters of Sabouraud Dextrose Agar culture medium along with 10 microliters of fungal suspension (106 CFU/ml) and 10 microliters of serial concentration of the plant extract were poured in microplates which were then incubated at 37 °C for 24 hours. The MIC was similarly defined as minimum concentration at which no growth was observed. Candida albicans and Aspergillus fumigatus were used for MIC determination. Experiments were carried out in triplicate (24, 25).
Results
The results showed that plant extract obtained from Zizyphus jujuba fruits has inhibitory effect on the four bacteria studied. This plant extract has also antimicrobial activity against the fungi investigated in this study.
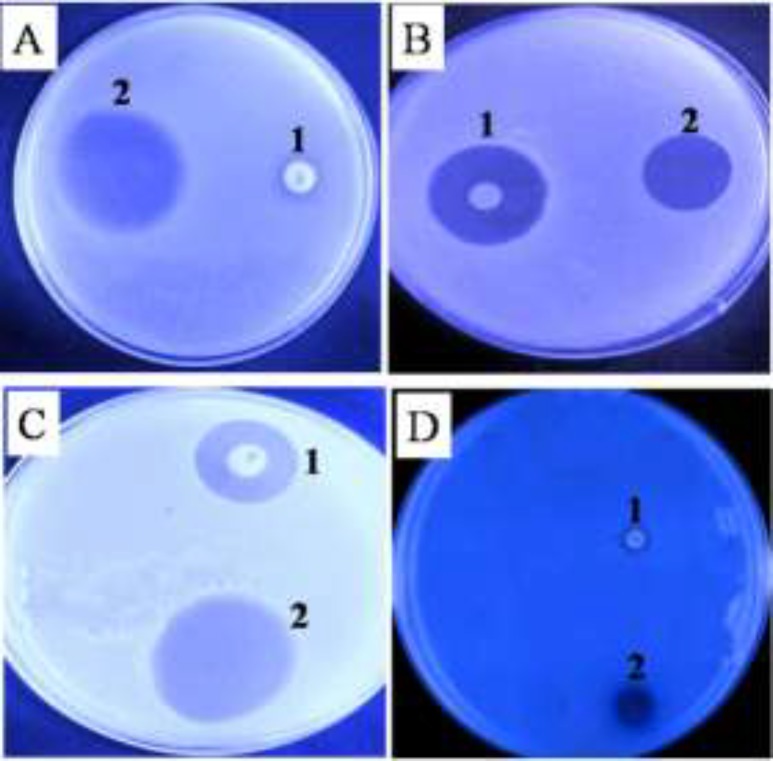
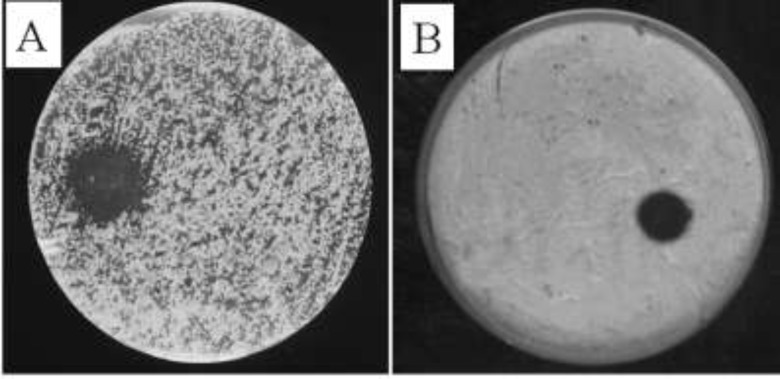
The qualitative assessment results on antibacterial activity of the plant extract are shown in Figure1. The results indicate the effective antibacterial activity of the plant extract against the bacterial strains studied. As clear from qualitative results, Staphylococcus aureus and Escherichia coli have respectively displayed the most and the least sensitivity to the extract. As shown in Figure 1, extract-related diameter of the inhibition zone is more than that of vancomycin antibiotic, demonstrating more effective antibacterial activity of the extract. The plant extract also revealed antifungal activity, so as it led to significant fungal destruction in the area around the injected site at concentration of 1mg/ml (Figure 2).
Figure1.
Antimicrobial effects of ethanolic extract against gram-positive and gram-negative bacteria. 1, vancomycin; 2,ethanolic extract of Zizyphus jujuba fruits. A, Escherichia coli; B, Bacillus subtilis; C, Pseudomonas aeruginosa; D, Staphylococcus aureus
Figure 2.
Antimicrobial effects of ethanolic extract against fungi. A, Candida albicans; B, Aspergillus fumigatus
As mentioned before, MIC was determined for quantifying antimicrobial activity of Zizyphus jujuba fruits extract on the microbes investigated, and the results are presented in Table I. As shown at Table I, the results of the quantitative test confirmed the results of qualitative test, as Staphylococcus aureus and Escherichia coli were introduced as the most and the least sensitive bacterial strains in quantitative test. As shown in Table I, the plant extract also has potent antimicrobial activity against two fungal strains.
Table I.
The MIC values of ethanolic extract.
| microbe | MIC(mg/ml) |
|---|---|
| Bacillus cereus | 1.5±0.45 |
| Staphylococcus aureus | 2.26±0.68 |
| Escherichia coli | 0.65±0.22 |
| Pseudomonas aeruginosa | 0.76±0.32 |
| Candida albicans | 2.35±0.38 |
| Aspergillus fumigatus | 2.86±0.7 |
Discussion
Plant extracts are a complex combination of different chemical elements with different values. Due to significant alteration in these compounds, biological effects of the extracts are varied. Antimicrobial properties of some herbal extracts has been identified (26-28); regarding these properties and other biological impacts, plant extracts have been of great attention as an appropriate substitute for antibiotics for potential therapeutic targets (29, 30) and/or health-cosmetics products and food industry (31).
Zizyphus jujuba is a pharmaceutical plant with proven therapeutic potentials; investigation on antimicrobial effects of the plant essential oils has shown a highly effective antibacterial activity (19-21). The results of the present study is also demonstrating antimicrobial effects of Zizyphus jujuba fruit extract, which is in line with other researches confirming medicinal effects of this plant (32, 33). Besides the antibacterial effects, findings also revealed antifungal activity of the extract, so as it eliminated the Candida albicans and Aspergillus fumigatus completely effectively at low concentrations. The results exhibited that in addition to antimicrobial activity of jujube oils as well as therapeutic potentials of other parts of the plant such as leaves, jujube fruit can also be of biological effectiveness, particularly in terms of antibacterial and antifungal activity (33, 34).
The present study, in which macro broth dilution and disk diffusion were applied, demonstrated more effective antibacterial property of jujube fruit extract against the gram negative and gram positive bacteria strains.
Findings also showed effective antimicrobial activity of the extract on the four bacteria studied in comparison with Vancomycin antibiotic. In this study, it has been observed that jujube extract has less antimicrobial effect on gram positive bacteria. This data are similar with other articles that examine similar extract (35, 36). Fungi also are sensitive to plant extract. In general, herbal products contribute to cytoplasm granulation (37), cytoplasmic membranes rupture (38) and deactivation or inhibition of intracellular and intercellular enzyme activity, cell walls disintegration and the destruction of electron transport system (39); these cellular events can independently or simultaneously reach to a maximum level while preventing the mycelial growth (3). Mechanism of antifungal activity of jujube fruit extract may be owing to any of the reasons mentioned. Crude Extracts from Ziziphus Jujuba Fruits has effective antimicrobial impact against gram negative and gram positive bacteria as well as fungi, so as it revealed more effective antimicrobial activity compared to common antibiotic like Vancomycin. The results of the present study have clearly showed acceptable antimicrobial effect of this plant extract against fungi in addition to gram positive and gram negative bacteria. Thus, this plant extract may be suitable for treatment of infectious diseases, particularly for Pediatric infection.
Acknowledgements
This work was supported by Young Researchers and Elite Club from Islamic Azad University, Yazd, Iran.
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Kantar M, Çetingül N, Kansoy S, Kütükçüler N, Aksub G. Immune Deficiencies following Cancer Treatment in Children. Journal of Tropical Pediatrics. 2003;49(5):286–90. doi: 10.1093/tropej/49.5.286. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. International Journal of Antimicrobial Agents. 2004;24(6):536–47. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Lee W-H, Li Y, Lai R, Li S, Zhang Y, Wang W. Variety of antimicrobial peptides in the Bombina maxima toad and evidence of their rapid diversification. European Journal of Immunology. 2005;35(4):1220–9. doi: 10.1002/eji.200425615. [DOI] [PubMed] [Google Scholar]
- 4.Bulet P, Hetru C, Dimarcq J-L, Hoffmann D. Antimicrobial peptides in insects; structure and function. Developmental & Comparative Immunology. 1999;23(4–5):329–44. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 5.Chou H-T, Kuo T-Y, Chiang J-C, Pei M-J, Yang W-T, Yu H-C, et al. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. International Journal of Antimicrobial Agents. 2008;32(2):130–8. doi: 10.1016/j.ijantimicag.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Toke O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Peptide Science. 2005;80(6):717–35. doi: 10.1002/bip.20286. [DOI] [PubMed] [Google Scholar]
- 7.Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian Journal of Microbiology. 2000;31:247–56. [Google Scholar]
- 8.Efange SMN. Chapter 5 Natural products: a continuing source of inspiration for the medicinal chemist. In: Maurice MI, Jacqueline CW, editors. Advances in Phytomedicine. Elsevier; 2002. pp. 61–9. [Google Scholar]
- 9.Ibrahim D, Osman H. Antimicrobial activity of Cassia alata from Malaysia. Journal of Ethnopharmacology. 1995;45(3):151–6. doi: 10.1016/0378-8741(94)01200-j. [DOI] [PubMed] [Google Scholar]
- 10.Jeevan Ram A, Bhakshu LM, Venkata Raju RR. In vitro antimicrobial activity of certain medicinal plants from Eastern Ghats, India, used for skin diseases. Journal of Ethnopharmacology. 2004;90(2–3):353–7. doi: 10.1016/j.jep.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 2005;100(1–2):80–4. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Marcus JP, Goulter KC, Green JL, Harrison SJ, Manners JM. Purification, Characterisation and cDNA Cloning of an Antimicrobial Peptide from Macadamia Integrifolia. European Journal of Biochemistry. 1997;244(3):743–9. doi: 10.1111/j.1432-1033.1997.00743.x. [DOI] [PubMed] [Google Scholar]
- 13.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends in Microbiology. 2000;8(9):402–10. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 14.Borris RP. Natural products research: perspectives from a major pharmaceutical company. Journal of Ethnopharmacology. 1996;51(1–3):29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 15.Padmaja V, Thankamany V, Hara N, Fujimoto Y, Hisham A. Biological activities of Annona glabra. Journal of Ethnopharmacology. 1995;48(1):21–4. doi: 10.1016/0378-8741(95)01277-k. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty A, Chowdhury BK, Bhattacharyya P. Clausenol and clausenine—two carbazole alkaloids from Clausena anisata. Phytochemistry. 1995;40(1):295–8. doi: 10.1016/0031-9422(95)00047-b. [DOI] [PubMed] [Google Scholar]
- 17.Polaquini SRB, Svidzinski TIE, Kemmelmeier C, Gasparetto A. Effect of aqueous extract from Neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Archives of Oral Biology. 2006;51(6):482–90. doi: 10.1016/j.archoralbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Rana BK, Singh UP, Taneja V. Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. Journal of Ethnopharmacology. 1997;57(1):29–34. doi: 10.1016/s0378-8741(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 19.Hung C-F, Hsu B-Y, Chang S-C, Chen B-H. Antiproliferation of melanoma cells by polysaccharide isolated from Zizyphus jujuba. Nutrition. 2012;28(1):98–105. doi: 10.1016/j.nut.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Al-Reza SM, Yoon JI, Kim HJ, Kim J-S, Kang SC. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food and Chemical Toxicology. 2010;48(2):639–43. doi: 10.1016/j.fct.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Al-Reza SM, Rahman A, Lee J, Kang SC. Potential roles of essential oil and organic extracts of Zizyphus jujuba in inhibiting food-borne pathogens. Food Chemistry. 2010;119(3):981–6. [Google Scholar]
- 22.Gürkan T, Gürer S. Investigation of parameters affecting atmospheric extraction yields and extract qualities of two Turkish lignites. Fuel Processing Technology. 1985;10(1):19–31. [Google Scholar]
- 23.Lehrer RI, Rosenman M, Harwig SSSL, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. Journal of Immunological Methods. 1991;137(2):167–73. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 24.Zare-Zardini H, Amiri A, Shanbedi M, Memarpoor-Yazdi M, Asoodeh A. Studying of antifungal activity of functionalized multiwalled carbon nanotubes by microwave-assisted technique. Surface and Interface Analysis. 2012;45(3):751. [Google Scholar]
- 25.Zare-Zardini H, Amiri A, Shanbedi M, Maghrebi M, Baniadam M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids and Surfaces B. Biointerfaces. 2012;92:196–202. doi: 10.1016/j.colsurfb.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Thompson KD. Herbal extracts and compounds active against herpes simplex virus. In: Mahmud THK, Arjumand A, editors. Advances in Phytomedicine. Elsevier; 2006. pp. 65–86. [Google Scholar]
- 27.Hamill FA, Apio S, Mubiru NK, Mosango M, Bukenya-Ziraba R, Maganyi OW, et al. Traditional herbal drugs of southern Uganda, I. Journal of Ethnopharmacology. 2000;70(3):281–300. doi: 10.1016/s0378-8741(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 28.Oh J, Jo H, Cho AR, Kim S-J, Han J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control. 2013;31(2):403–9. [Google Scholar]
- 29.Hudson JB, Towers GHN. Therapeutic potential of plant photosensitizers. Pharmacology & Therapeutics. 1991;49(3):181–222. doi: 10.1016/0163-7258(91)90055-q. [DOI] [PubMed] [Google Scholar]
- 30.Vaishnav P, Demain AL. Industrial Biotechnology, (overview) In: Moselio S, editor. Encyclopedia of Microbiology. Oxford: Academic Press; 2009. pp. 335–48. [Google Scholar]
- 31.Antignac E, Nohynek GJ, Re T, Clouzeau J, Toutain H. Safety of botanical ingredients in personal care products/cosmetics. Food and Chemical Toxicology. 2011;49(2):324–41. doi: 10.1016/j.fct.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Yan J, Cao J, Jiang W, Zhao Y. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Scientia Horticulturae. 2012;142(0):196–204. [Google Scholar]
- 33.Kamilolu Ö, Ercisli S, Sengül M, Toplu C, Serçe S. Total phenolics and antioxidant activity of jujube (Zizyphus jujube Mill.) genotypes selected from Turkey. African Journal of Biotechnology. 2012;8(2):303–307. [Google Scholar]
- 34.Cruz ZN, Rodríguez P, Galindo A, Torrecillas E, Ondoño S, Mellisho CD, et al. Leaf mechanisms for drought resistance in Zizyphus jujuba trees. Plant Science. 2012;197:77–83. doi: 10.1016/j.plantsci.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiology. 2004;21(1):33–42. [Google Scholar]
- 36.Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) Journal of Applied Microbiology. 2000;88(1):170–5. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 37.Omidbeygi M, Barzegar M, Hamidi Z, Naghdibadi H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 2007;18(12):1518–23. [Google Scholar]
- 38.Caccioni DRL, Guizzardi M, Biondi DM, Agatino R, Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. International Journal of Food Microbiology. 1998;43(1–2):73–9. doi: 10.1016/s0168-1605(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 39.Tassou C, Koutsoumanis K, Nychas GJE. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food research international (Ottawa, Ont) 2000;33(3/4):273–80. [Google Scholar]