Abstract
Background:
Tissue iron deposition may disturb functions of the organs. In many diseases like thalassemia, the patients suffer from iron deposition in kidney and heart tissues. Deferoxamine (DF) is a synthetic iron chelator and silymarin (SM) is an antioxidant and also a candidate for iron chelating. This study was designed to investigate the effect of DF and SM combination against kidney and heart iron deposition in an iron overload rat model.
Methods:
Male Wistar rats were randomly assigned to 5 groups. The iron overloading was performed by iron dextran 100 mg/kg/day every other day during 2 weeks and in the 3rd week, iron dextran was discontinued and the animals were treated daily with combination of SM (200 mg/kg/day, i.p.) and DF (50 mg/kg/day, i.p.) (group 1), SM (group 2), DF (group 3) and saline (group 4). Group 5 received saline during the experiment. Finally, blood samples were obtained and kidney, heart and liver were immediately removed and prepared for histopathological procedures.
Results:
The results indicated no significant difference in kidney function and endothelial function biomarkers between the groups. However, combination of SM and DF did not attenuate the iron deposition in the kidney, liver and heart. DF alone, rather than DF and SM combination, significantly reduced the serum level of malondialdehyde (P < 0.05). Co-administration of SM and DF significantly increased the serum level of ferritin (P < 0.05).
Conclusions:
DF and SM may be potentially considered as iron chelators. However, combination of these two agents did not provide a protective effect against kidney, liver and heart iron deposition.
Keywords: Deferoxamine, heart, iron deposition, kidney, liver, silymarin
INTRODUCTION
The prevention of tissue iron deposition is extremely important for physiological functions of the organs. Mammals do not have any physiological pathway for iron excretion. Therefore, iron homeostasis is regulated by the level of iron absorption. Disorder in iron absorption leads to iron deficiency or overload.[1] Furthermore, regulation of iron is influenced in hereditary hemochromatosis or thalassemia.[1] Transfusional hemosiderosis in major thalassemia patients leads to iron overload and then iron deposition in some organs.[2] Iron excess in kidney tissue may form hydroxyl radical, which promotes organ damaging including tubular damage.[3,4] Many of the patients with kidney diseases and anemia suffer from iron deposition in kidney tissue.[5,6,7,8] Cardiac iron deposition also is the leading cause of death in the patients with sickle cell disease and thalassemia[9,10] possibly due to cell apoptosis.[11]
Many efforts have been made to prevent iron deposition in functional tissues. Deferoxamine (DF), as an iron chelator,[12] is the most common drug to prevent the iron level increase in plasma or specific organ tissues in patients,[13,14,15] and it was subject of research for such purpose in experimental animals.[16,17,18,19]
Different substances such as nitric oxide (NO),[20,21] sesame oil[15] or tocotrienols[22] had been evaluated as nephroprotectants against iron-induced injury. Alpha-tocopherol also decreases iron-induced hippocampal neuron loss.[23] Among the anti-oxidant agents, silymarin (SM) is derived from the herb milk thistle (Silybum marianum), which has the iron chelating effect,[24,25,26] and it has strong affinity with iron ions.[27] The antioxidant effect of SM in the renal ischemia/reperfusion injury[28,29] and renal toxicity[30,31] has been investigated before. Previously, we designed an intensive iron overload rat model by administration of iron dextran (200 mg/kg/every other day) for a period of 4 weeks, and the animals were treated with oral SM and injections of DF and we found that the intensity of iron overload was too high. However, SM alone reduced iron deposition in the kidney.[32] In the current study, we attempted to perform a moderate iron overload model during 2 weeks and then the role of SM and DF injections would be considered.
METHODS
Animals
A total of 30 adult male Wistar rats (178 ± 3 g) were randomly assigned to 5 groups. The rats were individually housed at a temperature of 23-25°C. Rats had free access to water and chow. The experimental procedures were in advance approved by the Isfahan University of Medical Sciences Ethics Committee.
Groups 1-4 received iron dextran (Vifor Inc., Switzerland) 100 mg/kg every other day during the first 2 weeks of experiment. Then, during the 3rd week, the iron dextran was discontinued and the animals were treated daily as follows:
Group 1 (n = 5) received combination of SM (200 mg/kg/day, i.p) and DF (50 mg/kg/day, i.p.); and groups 2-4 (n = 6 for each group) received SM (200 mg/kg/day, i.p.), DF (50 mg/kg/day, i.p.), or saline (0.5 ml/rat/day; positive control group), respectively.
Group 5 (n = 7) assigned as the negative control group that received saline during the 3 weeks of the experiment.
Blood samples were obtained at the end of the experiment before the animals were sacrificed humanely. The kidney, heart and liver were immediately removed and prepared for histopathological procedures.
Measurements
The levels of serum creatinine (Cr), blood urea nitrogen (BUN) and iron were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum level of nitrite (stable NO metabolite) was measured using a colorimetric enzyme-linked immunosorbent assay (ELISA) kit (Promega Corporation, USA) that involves the Griess reaction. The serum level of ferritin was measured using the enzyme immunoassay ELISA kit for rat (Immunology Consultants Laboratory Inc., USA). The serum level of malondialdehyde (MDA) was measured manually. Briefly, the samples were mixed with trichloroacetic acid and centrifuged. Thiobarbituric acid was added to the supernatant and the mixture was incubated in hot water bath. After cooling, the absorbance was measured at 532 nm. The serum concentrations of MDA were reported in μmole/l.
Histopathological procedures
The removed kidneys and heart were fixed in 10% neutral formalin solution and embedded in paraffin for staining to examine iron deposition in the kidney, heart and liver. The kidney, heart and liver iron deposition were evaluated by a pathologist who was totally blind to the study. On the basis of the intensity of tissue iron deposition and damage, the kidney and heart tissue damage scores (KTDS, HTDS, liver tissue damage score [LTDS]) were graded from 1 to 5, whereas score 0 was assigned to normal kidney tissue without any damage and iron deposition.
Statistical analysis
Data are expressed as mean ± standard error of the mean. The after-treatment serum levels of BUN, Cr, nitrite and ferritin were compared between the groups using the one-way analysis of variance. The Kruskal-Wallis or Mann-Whitney tests were applied to compare the tissue damage score between the groups. P ≤ 0.05 was considered to be statistically significant.
RESULTS
Weight change
During the overloading period (1st 2 weeks of the experiment), groups 1-4 were not significantly different with regard to weight gain (15th day weight-1st day weight), while weight gain in the normal group (group 5) was significantly higher than those in the other groups (P < 0.05). To compare the weight change during the experiment (last day weight-first day weight; ∆W), the ΔW were 12.2 ± 3.8, 24.9 ± 6.7, 14.7 ± 3.3, 12.1 ± 3.5, and 29.6 ± 5.6 g in groups 1 to 5, respectively. ΔW in the normal group (group 5) had no difference from group 2, but it was significantly greater than the values obtained for groups 1, 3, and 4 (P < 0.05).
Serum levels of BUN, Cr and nitrite and normalized kidney weight
According to the results, the groups were not significantly different in serum levels of BUN, Cr, and nitrite, as well as the normalized kidney weight [Figure 1]. Although the groups were not significantly different, co-administration of SM and DF seems to increase these parameters toward a higher value when compared with the other groups.
Figure 1.
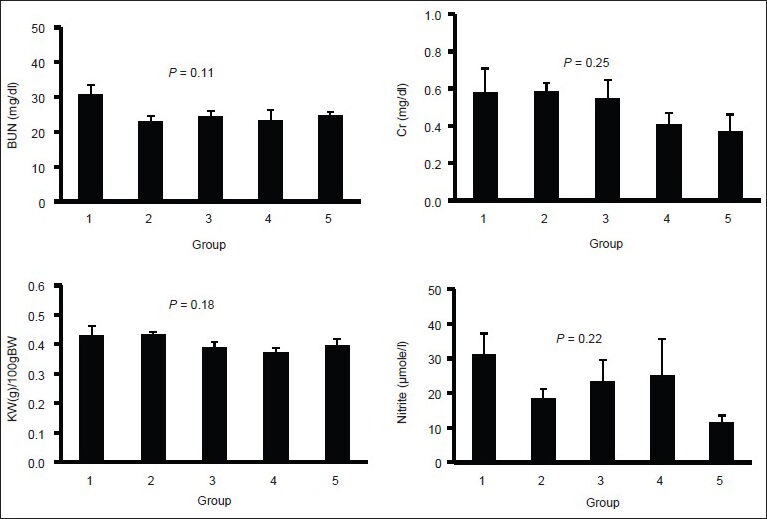
Serum levels of blood urea nitrogen, creatinine, and nitrite; and normalized kidney weight in four experimental groups. No significant differences were observed among the groups. Groups 1-4 received iron dextran for 2 weeks and then treated with combination of silymarin (SM) and deferoxamine, SM, and saline, respectively. Group 5 received saline only during the experiment. The blue color indicates iron deposition
Serum levels of MDA and ferritin and kidney, liver and heart damage
The groups were significantly different with respect to the serum levels of ferritin and MDA [Figure 2]. Treatment with DF alone potentially reduced the serum level of MDA and the intensity of heart iron deposition. However, co-administration of SM and DF significantly increased the serum level of ferritin when compared with group 4 (P < 0.05). The pathology data indicated that iron deposition in kidney and liver tissues was clearly detectable. Kidney and liver pathology damage score (KTDS and LTDS) in the iron-treated groups was significantly different from the saline-treated group (P < 0.05). The mean values for KTDS and LTDS in group 1 was greater than those for groups 2-4. However, The KTDS and LTDS obtained for groups 1-4 were not significantly different; indicating that DF, SM, or combination of both did not attenuated the iron deposition in the kidney and liver [Figure 3]. The iron deposition in heart tissue was trace, but some iron depositions were observed in the diaphragm. No difference was observed in iron deposition intensity among groups 1-4 [Figure 3].
Figure 2.
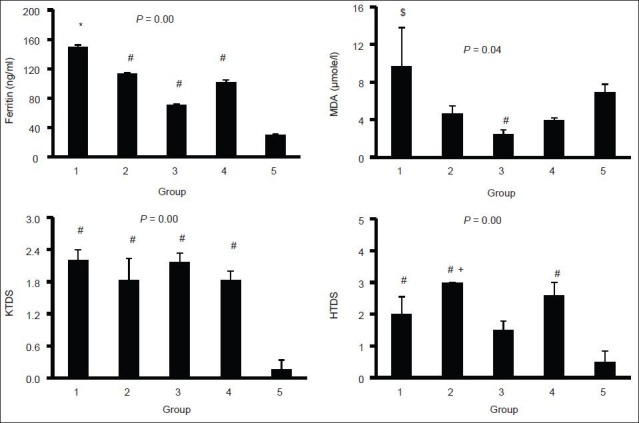
Serum levels of malondialdehyde and ferritin, and kidney and liver damage scores in five experimental groups. The statistical differences were shown by * (difference from groups 3 to 5), # (difference from group 5), $ (difference from groups 2, 3, and 4), and + (difference from group 3). Groups 1-4 received iron dextran for 2 weeks and then treated with combination of silymarin (SM) and deferoxamine (DF), SM, DF and saline, respectively. Group 5 received saline only during the experiment. The blue color indicates iron deposition
Figure 3.
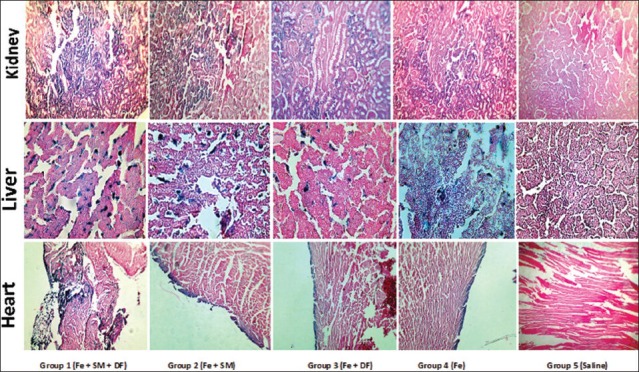
The sample images of kidney, liver, and heart tissues stained with Persian blue to examine iron deposition in the kidney, liver, and heart of five experimental groups. Groups 1-4 received iron dextran for 2 weeks and then treated with combination of silymarin (SM) and deferoxamine (DF), SM, DF and saline, respectively. Group 5 received saline only during the experiment. The dark blue color indicates iron deposition. More iron deposition was seen in group 1
DISCUSSION
The role of combination of SM and DF against deposition of iron in an iron overload rat model was investigated. The results confirmed that co-administration of SM and DF did not attenuate the intensity of iron deposition in the kidney and heart.
SM has antioxidant properties, and it has been known as an iron chelator.[24,25,26] DF also is avidly used in clinic as an iron chelator, too. We expected to have a strong iron chelator by combination of SM and DF. Nevertheless, our expectation was not confirmed in the present animal model. At this stage, pharmacokinetic interaction of the agents is the only possible mechanism to explain such unexpected results. We found similar results in our previous study when the dose of iron dextran and the duration of overloading were almost twice.[32] Najafzadeh et al. reported a positive role of iron chelator for SM when the agent was administrated as prophylaxis.[17] Furthermore, oral administration of SM alone (200 mg/kg) was found to be kidney protective against KID while the dose of 400 mg/kg was not kidney protective.[32] Therefore, the route of administration and the model of iron overload are the possible factors, which could disturb the results.
By considering other findings in the current study, the serum levels of ferritin and MDA in SM or DF alone groups were less than those for the group that received combination of SM and DF. This also verifies that both SM and DF alone may reduce the level of iron or oxidative stress in the subjects, but by unknown mechanism the combination may reverse the expected results.
Based on the serum levels of BUN and Cr, no renal dysfunction occurred. Together with pathological data, the KID did not disturb kidney functions. It is reported that a single dose of 600 mg/kg iron dextran for 24 h increased the serum level of BUN and Cr.[33] Such observation was not seen in our study possibly first due to continuous administration of iron dextran and second we measured the serum levels of BUN and Cr 2 weeks after discontinuation of iron overloading.
With regard to the serum level of NO, no significant difference was observed among the groups. NO is a common biomarker to evaluate the endothelial function, and it is reported that increase in iron level disturbs endothelial function in patients[34,35] while iron chelators may improve the endothelial function.[36,37] In this study, since nitrite is a NO metabolite, no endothelial dysfunction occurred. However, nitrite is not the only NO metabolite. Another metabolite of NO is nitrate. We did not measure the serum nitrate level in the current study since nitrite is a valid marker to determine NO production from endothelium. Plasma nitrate levels are influenced by a variety of NO synthase independent factors.[38,39,40] Therefore, 2 weeks of iron overload did not lead to endothelial dysfunction.
However, many flavonoids are poorly absorbed.[41] The poor absorption of flavonoid nutrients is likely result from two factors. Firstly, they are multiple-ring molecules too large to be absorbed by simple diffusion, while they are not absorbed actively, as occurs with some vitamins and minerals. Secondly, flavonoid molecules generally have poor miscibility with oils and other lipids, severely limiting their ability to pass across the lipid-rich outer membranes of the enterocytes of the small intestine.
Water-soluble flavonoid molecules can be converted into lipid-compatible molecular complexes, aptly called phytosomes. Phytosomes are better able to transit from a hydrophilic environment into the lipid-friendly environment of the enterocyte cell membrane and from there into the cell, finally reaching the blood.[42]
CONCLUSIONS
Co-administration of SM and DF did not attenuate the serum level of iron reservoir such as ferritin and combination of these agents was not protective against iron deposition in kidney and heart. However, according to the current study and the work done by others,[13,14,15,24,25,26,27] it seems that SM or DF alone demonstrated a better result to protect organ against iron disorder.
ACKNOWLEDGMENT
This research was supported by Isfahan University of Medical Sciences (grant #291294).
Footnotes
Source of Support: This research was supported by Isfahan University of Medical Sciences (grant #291294)
Conflict of Interest: None declared.
REFERENCES
- 1.Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202:199–211. doi: 10.1016/j.taap.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu VP, Prabhu RS. Iron overlod in beta thalassemia-A review. J Biosci Technol. 2009;1:20–31. [Google Scholar]
- 3.Kondo A, Deguchi J, Okada S. Intranuclear iron deposition in hepatocytes and renal tubular cells in mice treated with ferric nitrilotriacetate. Virchows Arch. 1998;433:543–8. doi: 10.1007/s004280050287. [DOI] [PubMed] [Google Scholar]
- 4.Sponsel HT, Alfrey AC, Hammond WS, Durr JA, Ray C, Anderson RJ. Effect of iron on renal tubular epithelial cells. Kidney Int. 1996;50:436–44. doi: 10.1038/ki.1996.334. [DOI] [PubMed] [Google Scholar]
- 5.Madhusudhan KS, Oberoi R. Renal iron deposition in aplastic anemia: Magnetic resonance imaging appearance. Indian J Nephrol. 2011;21:134–5. doi: 10.4103/0971-4065.82145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakow-Penner R, Glader B, Yu H, Vasanawala S. Adrenal and renal corticomedullary junction iron deposition in red cell aplasia. Pediatr Radiol. 2010;40:1955–7. doi: 10.1007/s00247-010-1824-2. [DOI] [PubMed] [Google Scholar]
- 7.Schein A, Enriquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: A marker of chronic hemolysis. J Magn Reson Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Nishiya K, Ito H, Hosokawa T, Hashimoto K, Moriki T. Iron deposition in renal biopsy specimens from patients with kidney diseases. Am J Kidney Dis. 2001;38:1038–44. doi: 10.1053/ajkd.2001.28593. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim el-SH, Rana FN, Johnson KR, White RD. Assessment of cardiac iron deposition in sickle cell disease using 3.0 Tesla cardiovascular magnetic resonance. Hemoglobin. 2012;36:343–61. doi: 10.3109/03630269.2012.679376. [DOI] [PubMed] [Google Scholar]
- 10.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: Pathophysiology, diagnosis, and treatment. J Card Fail. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wu M, Al-Rousan R, Liu H, Fannin J, Paturi S, et al. Iron-induced cardiac damage: Role of apoptosis and deferasirox intervention. J Pharmacol Exp Ther. 2011;336:56–63. doi: 10.1124/jpet.110.172668. [DOI] [PubMed] [Google Scholar]
- 12.Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood. 2012;120:3657–69. doi: 10.1182/blood-2012-05-370098. [DOI] [PubMed] [Google Scholar]
- 13.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M, Gandini G, de Gironcoli M, Vassanelli A, Borgna-Pignatti C, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload. Blood. 2000;95:2776–9. [PubMed] [Google Scholar]
- 15.Franchini M, Gandini G, Veneri D, Aprili G. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload: An update. Blood. 2004;103:747–8. doi: 10.1182/blood-2003-10-3373. [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Wang P, Zhong ML, Wang T, Huang XS, Li JY, et al. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int. 2013;62:165–72. doi: 10.1016/j.neuint.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Najafzadeh H, Jalali MR, Morovvati H, Taravati F. Comparison of the prophylactic effect of silymarin and deferoxamine on iron overload-induced hepatotoxicity in rat. J Med Toxicol. 2010;6:22–6. doi: 10.1007/s13181-010-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obejero-Paz CA, Yang T, Dong WQ, Levy MN, Brittenham GM, Kuryshev YA, et al. Deferoxamine promotes survival and prevents electrocardiographic abnormalities in the gerbil model of iron-overload cardiomyopathy. J Lab Clin Med. 2003;141:121–30. doi: 10.1067/mlc.2003.18. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Hu R, Li M, Li F, Meng H, Zhu G, et al. Deferoxamine attenuates iron-induced long-term neurotoxicity in rats with traumatic brain injury. Neurol Sci. 2013;34:639–45. doi: 10.1007/s10072-012-1090-1. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Sharma S, Chopra K. Reversal of iron-induced nephrotoxicity in rats by molsidomine, a nitric oxide donor. Food Chem Toxicol. 2008;46:537–43. doi: 10.1016/j.fct.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Li YH, Chien SP, Chu PY, Liu MY. Prophylactic and therapeutic effects of a subcutaneous injection of sesame oil against iron-induced acute renal injury in mice. JPEN J Parenter Enteral Nutr. 2012;36:344–8. doi: 10.1177/0148607111415530. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Chopra K. Effect of tocotrienols on iron-induced renal dysfunction and oxidative stress in rats. Drug Chem Toxicol. 2009;32:319–25. doi: 10.1080/01480540903130633. [DOI] [PubMed] [Google Scholar]
- 23.Bostanci MO, Bas O, Bagirici F. Alpha-tocopherol decreases iron-induced hippocampal and nigral neuron loss. Cell Mol Neurobiol. 2010;30:389–94. doi: 10.1007/s10571-009-9461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borsari M, Gabbi C, Ghelfi F, Grandi R, Saladini M, Severi S, et al. Silybin, a new iron-chelating agent. J Inorg Biochem. 2001;85:123–9. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 25.Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: A comparison with desferrioxamine. Eur J Pharmacol. 2008;589:1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson C, Bomford A, Geissler CA. The iron-chelating potential of silybin in patients with hereditary haemochromatosis. Eur J Clin Nutr. 2010;64:1239–41. doi: 10.1038/ejcn.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gharagozloo M, Karimi M, Amirghofran Z. Immunomodulatory effects of silymarin in patients with β-thalassemia major. Int Immunopharmacol. 2013;16:243–7. doi: 10.1016/j.intimp.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Senturk H, Kabay S, Bayramoglu G, Ozden H, Yaylak F, Yucel M, et al. Silymarin attenuates the renal ischemia/reperfusion injury-induced morphological changes in the rat kidney. World J Urol. 2008;26:401–7. doi: 10.1007/s00345-008-0256-1. [DOI] [PubMed] [Google Scholar]
- 29.Turgut F, Bayrak O, Catal F, Bayrak R, Atmaca AF, Koc A, et al. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40:453–60. doi: 10.1007/s11255-008-9365-4. [DOI] [PubMed] [Google Scholar]
- 30.Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2010;28:703–13. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 31.Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49:1082–90. doi: 10.3109/13880209.2011.568506. [DOI] [PubMed] [Google Scholar]
- 32.Nematbakhsh M, Pezeshki Z, Moaeidi BA, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Protective role of silymarin and deferoxamine against iron dextran-induced renal iron deposition in male rats. Int J Prev Med. 2013;4:286–92. [PMC free article] [PubMed] [Google Scholar]
- 33.Kadkhodaee M, Gol A. The role of nitric oxide in iron-induced rat renal injury. Hum Exp Toxicol. 2004;23:533–6. doi: 10.1191/0960327104ht485oa. [DOI] [PubMed] [Google Scholar]
- 34.Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, et al. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40:2189–94. doi: 10.1016/s0735-1097(02)02611-6. [DOI] [PubMed] [Google Scholar]
- 35.Mascitelli L, Pezzetta F. High iron stores and impaired endothelial function in prediabetic subjects. Am J Cardiol. 2006;97:1550. doi: 10.1016/j.amjcard.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Mascitelli L, Pezzetta F, Sullivan JL. Iron chelation by green tea flavonoids and improved endothelial function in chronic smokers. Circ J. 2006;70:1523. doi: 10.1253/circj.70.1523. [DOI] [PubMed] [Google Scholar]
- 37.Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF, Jr, et al. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 38.Metzger IF, Sertorio JT, Tanus-Santos JE. Relationship between systemic nitric oxide metabolites and cyclic GMP in healthy male volunteers. Acta Physiol (Oxf) 2006;188:123–7. doi: 10.1111/j.1748-1716.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 39.Kelm M, Preik-Steinhoff H, Preik M, Strauer BE. Serum nitrite sensitively reflects endothelial no formation in human forearm vasculature: Evidence for biochemical assessment of the endothelial L-arginine-NO pathway. Cardiovasc Res. 1999;41:765–72. doi: 10.1016/s0008-6363(98)00259-4. [DOI] [PubMed] [Google Scholar]
- 40.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 42.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10:193–203. [PubMed] [Google Scholar]


