Abstract
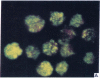
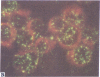
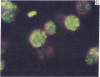
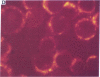
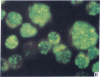
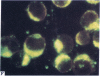
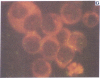
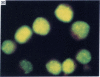
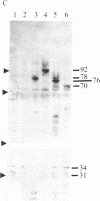
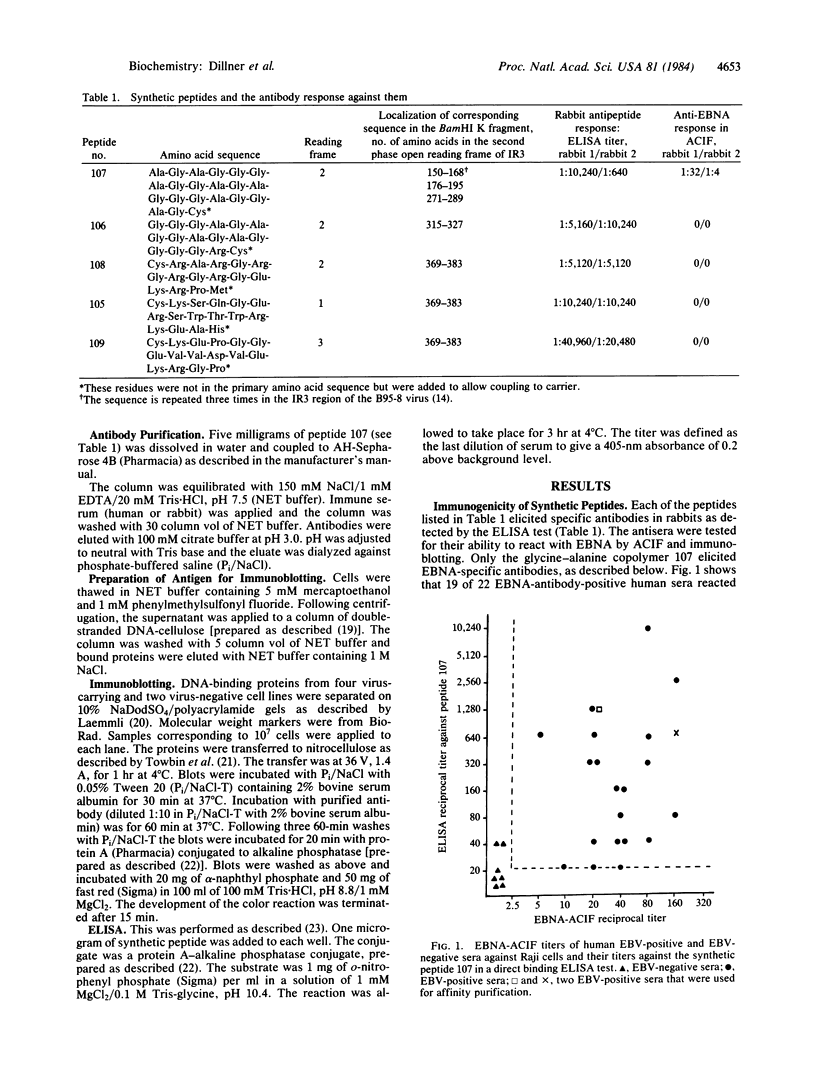
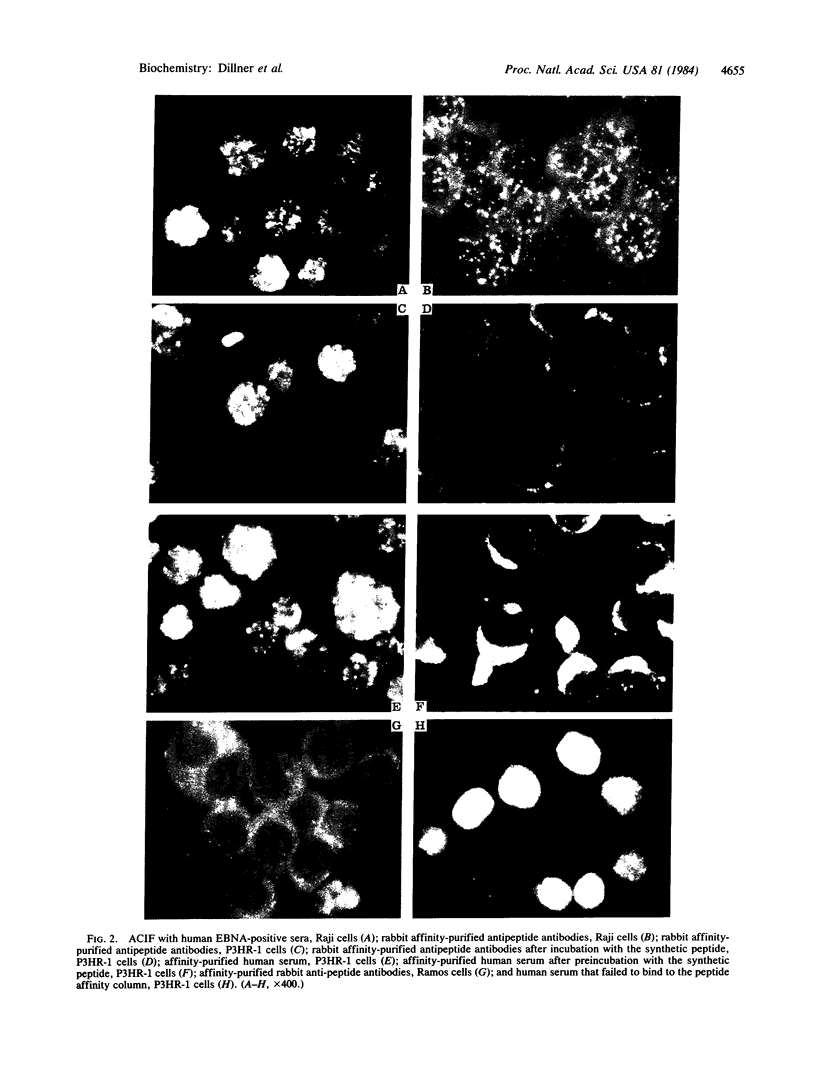
Five peptides corresponding to amino acid sequences predicted from all three reading frames of the nucleotide sequence of the third internal repeat array (IR3) of the Epstein-Barr virus (EBV) genome were synthesized chemically. All five peptides elicited antipeptide antibodies in rabbits. The antiserum raised against a 14-residue copolymer of glycine and alanine gave brilliant EBV-specific nuclear staining in the anticomplement immunofluorescence (ACIF) assay, in line with the original definition of the EBV-determined nuclear antigen (EBNA) [Reedman, B. M. & Klein, G. (1973) Int. J. Cancer 11, 499-520]. Eight EBNA and EBV DNA-carrying lines showed nuclear staining with the antipeptide antibody, whereas five EBV DNA negative lines failed to stain. The staining pattern was more discretely punctate than the finely dispersed diffuse EBNA staining obtained with human antisera. Human EBV antibody-positive but not EBV-negative sera reacted with the synthetic peptide in an ELISA test. The peptide-specific antibodies were purified from the sera of healthy EBV-seropositive persons by affinity chromatography with the peptide. They gave an EBV-specific, brilliant punctate nuclear ACIF staining similar to that of the rabbit antipeptide antibodies. It was concluded that the glycine-alanine structure encoded by the IR3 region contains a native determinant of EBNA, detected by the ACIF test. Immunoblotting with the rabbit and human peptide-specific antibodies identified poly-peptides that varied between 70 and 92 kilodaltons in size in different EBV-positive cell lines, corresponding closely to a previously identified variation pattern in the size of EBNA. In addition, rabbit antipeptide antibodies identified two cellular polypeptides, 44 and 49 kilodaltons in size.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron D., Strominger J. L. Partial purification and properties of the Epstein-Barr virus-associated nuclear antigen. J Biol Chem. 1978 Apr 25;253(8):2875–2881. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Heller M., Henderson A., Kieff E. Repeat array in Epstein-Barr virus DNA is related to cell DNA sequences interspersed on human chromosomes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5916–5920. doi: 10.1073/pnas.79.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., van Santen V., Kieff E. Simple repeat sequence in Epstein-Barr virus DNA is transcribed in latent and productive infections. J Virol. 1982 Oct;44(1):311–320. doi: 10.1128/jvi.44.1.311-320.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hennessy K., Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Giovanella B. C., Lindahl T., Fialkow P. J., Singh S., Stehlin J. S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luka J., Lindahl T., Klein G. Purification of the Epstein-Barr virus-determined nuclear antigen from Epstein-Barr virus-transformed human lymphoid cell lines. J Virol. 1978 Sep;27(3):604–611. doi: 10.1128/jvi.27.3.604-611.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Hibi N., Nishi S., Hirai H., Osato T. Studies on Epstein-Barr virus-related antigens. III. Purification of the virus-determined nuclear antigen (EBNA) from non-producer Raji cells. Int J Cancer. 1978 Dec;22(6):747–752. doi: 10.1002/ijc.2910220618. [DOI] [PubMed] [Google Scholar]
- Menezes J., Jondal M., Leibold W., Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976 Feb;13(2):303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G., Pope J. H., Walters M. K., Hilgers J., Singh S., Johansson B. Epstein-Barr virus-associated complement-fixing and nuclear antigens in Burkitt lymphoma biopsies. Int J Cancer. 1974 Jun 15;13(6):755–763. doi: 10.1002/ijc.2910130604. [DOI] [PubMed] [Google Scholar]
- Sculley T. B., Kreofsky T., Pearson G. R., Spelsberg T. C. Partial purification of the Epstein-Barr virus nuclear antigen(s). J Biol Chem. 1983 Mar 25;258(6):3974–3982. [PubMed] [Google Scholar]
- Sternås L., Luka J., Kallin B., Rosén A., Henle W., Henle G., Klein G. Enzyme-linked immunosorbent assay for the detection of Epstein-Barr virus-induced antigens and antibodies. J Immunol Methods. 1983 Oct 14;63(2):171–185. doi: 10.1016/0022-1759(83)90422-2. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T. C., Hopkins R. F., 3rd, Neubauer R. H., Rabin H. Identification of an Epstein-Barr virus nuclear antigen by fluoroimmunoelectrophoresis and radioimmunoelectrophoresis. J Virol. 1981 Jun;38(3):996–1004. doi: 10.1128/jvi.38.3.996-1004.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Liu F. T., Niman H. L., Lerner R. A. Chemical synthesis of a polypeptide predicted from nucleotide sequence allows detection of a new retroviral gene product. Nature. 1980 Oct 30;287(5785):801–805. doi: 10.1038/287801a0. [DOI] [PubMed] [Google Scholar]
- Tamura T., Bauer H., Birr C., Pipkorn R. Antibodies against synthetic peptides as a tool for functional analysis of the transforming protein pp60src. Cell. 1983 Sep;34(2):587–596. doi: 10.1016/0092-8674(83)90391-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]