Abstract
The importance of cooperative hydrogen-bonding effects has been demonstrated using novel 3-methylenecyclopropane-1,2-dicarboxylic acid (Feist's acid (FA)) as hydrogen bond donor catalysts for the addition of indole and pyrrole to trans-β-nitrostyrene derivatives. Because of the hydrogen bond donor (HBD) ability, Feist's acid (FA) has been introduced as a new class of hydrogen bond donor catalysts for the activation of nitroolefin towards nucleophilic substitution reaction. It has effectively catalyzed the Michael addition of indoles and pyrrole to β-nitroolefins under optimum reaction condition to furnish the corresponding Michael adducts in good to excellent yields (up to 98%). The method is general, atom-economical, convenient, and eco-friendly and could provide excellent yields and regioselectivities. Some newly synthesized compounds were for examined in vitro antimicrobial activity and their preliminary results are reported.
1. Introduction
In the field of advanced organic synthesis, Michael addition reaction is one of the most powerful tools for carbon-carbon bond construction reactions [1–6]. Nitroolefins are very attractive among the many Michael acceptors because of their strong electron-withdrawing nitro group which could be easily transformed into a wide variety of different functionalities [7–9] that may lead to synthesizing important biologically active building blocks and products [10]. Until now a numerous number of catalysts have been reported in the literature, mainly for the Michael addition reactions of heteroarenes to carbonyls [11, 12]. Moreover a remarkable number of catalysts have been used to catalyze the Michael addition [13–21] reaction of indoles and pyrrole to trans-β-nitroolefins; however, most of them were Lewis acids [22–25]. Recently some small organic molecules with anion recognition abilities have provided a great deal of inspiration for the development of hydrogen-bonding catalysis [26–28]; in this context, hydrogen bond donor (HBD) catalysis has received some achievement in exploiting the anion recognition abilities offered by urea, thiourea, and guanidinium functionalities [29–31], silanediols [32, 33], silica gel [34], 2-aminopyridinium ions [35–38], sulfamic acid (SA) [9], dipicolinic acid [26], which were found to be highly potent in catalyzing the Michael addition reaction through hydrogen bonding catalysis [26, 39–47]. Yet such small organic molecules with hydrogen bond donor (HBD) ability received little attention in the context of noncovalent catalysis [48]. Excited by the potential of such hydrogen bond donor small molecule, we have initiated a program in our laboratory dedicated toward pioneering the development of Feist's acid (FA) [49, 50] as a new class of catalysts that operate through hydrogen bonding interactions.
Indole and its analogs constitute the active class of compounds possessing wide spectrum of biological activities. A variety of indole derivatives have emerged that possess a range of bioactivities including potent anticancer, antiviral, anti-inflammatory, anti-hypertensive, anti-asthmatic and anti-tubercular properties. Some of these compounds are also known to possess anti-inflammatory and analgesic properties [51–53]. Pyrrole heterocyclic derivatives were reported as having important synthetic and biological activities such as COX-1/COX-2 inhibitors and cytotoxic activity against a variety of marine and human tumor models [54–59].
In this communication, we report initial successes with FA catalysis and its application toward the activation of nitroolefin via hydrogen-bonding mechanistic pathway, which effectively catalyze Michael addition of indoles and pyrrole to nitroolefins under optimum reaction condition to afford the corresponding Michael adducts in good to excellent yields. Some newly synthesized compounds were subjected to in vitro antimicrobial activity.
2. Experimental
2.1. General Information
Glassware was oven-dried overnight at 120°C before use. Reactions were performed under an inert atmosphere using an argon filled glove box and standard Schlenk-line techniques. All the reactions were monitored by TLC analysis using Merck Silica Gel 60 F-254 thin layer plates. Column chromatography was performed on silica gel 100–200 mesh.
2.1.1. Materials
Petroleum ether (PE), hexane, and ethyl acetate for column chromatography were distilled prior to use. CH2Cl2 and EtOH were distilled from P2O5 and Mg, respectively, and stored on 4 Å molecular sieves. Tetrahydrofuran, benzene, and toluene were distilled from sodium benzophenone ketyl. Acetonitrile and dimethylformamide were dried by distillation over calcium hydride. Nitroolefins 2(a–i) were prepared according to procedures reported in literature [60].
2.1.2. Instrumentation
NMR spectra were recorded with a Jeol spectrometer at 400 MHz (1H-NMR) and 100 MHz (13C-NMR.). The chemical shifts (δ in ppm) were reported down field from tetramethylsilane (TMS, δ scale) with the deuterated solvent resonance referenced as internal standard. Elemental analyses were performed on a Perkin Elmer 2400 Elemental Analyzer. IR spectra were obtained using FTIR-800 Model. Mass spectrometric analysis was conducted by using ESI mode on AGILENT Technologies 6410-triple quad LC/MS instrument.
2.2. General Procedure for the Michael Addition Reaction of Indole with β-Nitroolefins Catalyzed by Feist's Acid (GP1)
Indole 4 (50 mg, 0.425 mmol), β-nitroolefins 2(a–i) (0.425 mmol), and a catalytic amount of Feist's acid (1) (6 mg, 0.084 mmol, 10 mol%) in dry ethanol (3 mL) were charged into a Schlenk tube under an argon atmosphere. The reaction was then stirred at 50°C for 48–72 hours. The reaction mixture was monitored by TLC until starting material was completely consumed. Then the solvent was removed under vacuum. The crude products were isolated by column chromatography to afford pure Michael adducts (7a–i). The analytical data of the known compounds were in accordance with reported literature [58].
2.2.1. 3-(2-Nitro-1-phenylethyl)-1H-indole (Table 2, Entry 1, 7a)
Table 2.
Michael addition of indole (4) to nitroolefins 2(a–i) catalyzed by Feist's acid.
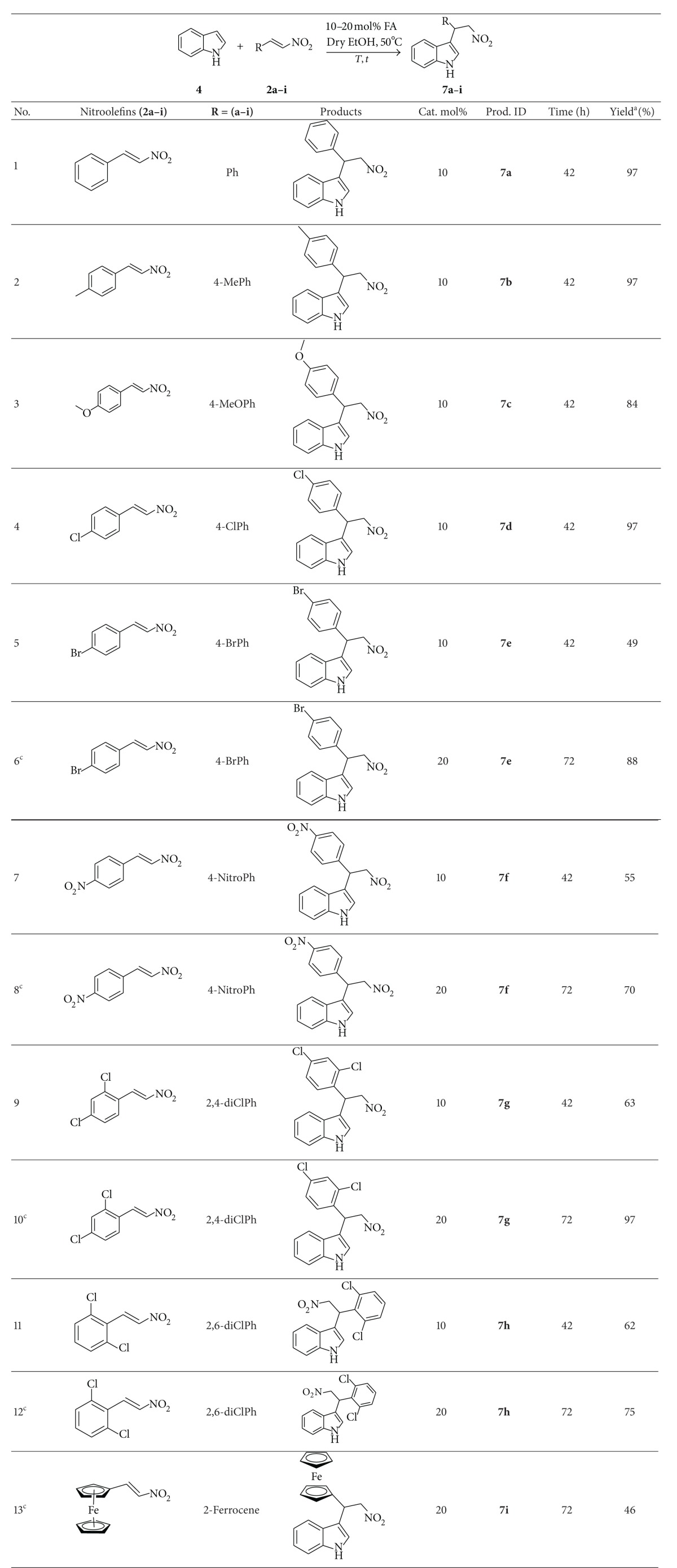
|
aThe reactions were performed on 0.425 mmol scale; bthe isolated yield after column purification; c20 mol% catalyst was used and reactions were run for 72 hours.
Indole 4 (50 mg, 0.43 mmol) and β-nitrostyrene 2a (64 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (6 mg, 0.084 mmol, 10 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7a as yellow oil [58] (111 mg, 0.42 mmol, 97%). IR (KBr): 3417, 15461375, 742, 700, 587 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.89–4.99 (m, 1H, CHCH 2(a)), 5.02–5.12 (m, 1H, CHCH 2(b)), 5.15–5.23 (m, 1H, CHCH2), 7.01–7.11 (m, 2H, ArH), 7.17–7.24 (m, 1H, ArH), 7.25 (s, 1H, NHCH), 7.26–7.36 (m, 5H, ArH), 7.44 (d, J = 8.0 Hz, 1H. ArH), 8.09 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 41.6, 79.9, 111.4, 114.5, 119.0, 120.0, 121.7, 122.8, 126.7, 127.7, 127.8, 129.0, 136.6, 139.2; [Anal. Calcd. for C16H14N2O2: C, 72.16; H, 5.30; N, 10.52; found: C, 72.37; H, 5.23; N, 10.41]; LC/MS (ESI): M+, found 266.08, C16H14N2O2 requires 266.11.
2.2.2. 3-[1-(4-Methylphenyl)-2-nitroethyl]-1H-indole (Table 2, Entry 2, 7b)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2b (70 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (6 mg, 0.084 mmol, 10 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7b as yellow oil [58] (117 mg, 0.42 mmol, 97.2%). IR (KBr): 3418, 1547, 1377, 739 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 2.31 (s, 1H, CH 3), 4.86–4.98 (m, 1H, CHCH 2(a)), 5.01–5.10 (m, 1H, CHCH 2(b)), 5.10–5.20 (m, 1H, CHCH2), 6.98–7.02 (m, 2H, ArH), 7.06–7.25 (m, 5H, ArH), 7.34 (d, J = 8.0 Hz, 1H, ArH), 7.46 (d, J = 8.0 Hz, 1H. ArH), 8.06 (s, 1H, NH of Indole); 13C-NMR (CDCl3,, 100 MHz): δ 21.1, 41.3, 80.0, 111.4, 114.7, 119.0, 120.0, 121.6, 122.7, 126.2, 127.7, 129.7, 136.2, 136.6, 137.3; [Anal. Calcd. for C17H16N2O2: C, 72.84; H, 5.75; N, 9.99; found: C, 73.03; H, 5.66; N, 10.11]; LC/MS (ESI): M+, found 280.16, C17H16N2O2 requires 280.12.
2.2.3. 3-(1-(4-Methoxyphenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 3, 7c)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2c (77 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (6 mg, 0.084 mmol, 10 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7c as white solid (107 mg, 0.36 mmol, 84%). m.p. 149−151°C [Lit [58] m.p. 148–150°C]; IR (KBr): 3373, 1545, 1509, 1458, 1420, 1374, 1241, 1179, 1027, 742, 608, 548, 525 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 3.76 (s, 1H, OCH 3), 4.84–4.93 (m, 1H, CHCH 2(a)), 5.00–5.08 (CHCH2m, 1H, CHCH 2(b)), 5.08–5.17 (m, 1H,), 6.84 (d, J = 5.2 Hz, 2H. ArH), 7.01 (s, 1H, NHCH), 7.02–7.10 (m, 1H, ArH), 7.19–7.28 (m, 3H, ArH), 7.36 (d, J = 8.0 Hz, 1H. ArH), 7.44 (d, J = 8.0 Hz, 2H. ArH), 8.07 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 40.9, 55.3, 80.0, 111.4, 114.3, 119.1, 120.0, 121.5, 122.8, 126.1, 128.9, 131.2, 136.6, 139.3, 158.9; [Anal. Calcd. for C17H16N2O3: C, 68.91; H, 5.44; N, 9.45; found: C, 79.09; H, 5.53; N, 9.36]; LC/MS (ESI): M+, found 296.18, C17H16N2O2 requires 296.12.
2.2.4. 3-(1-(4-Chlorophenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 4, 7d)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2d (79 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (6 mg, 0.084 mmol, 10 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7d as oily liquid (124 mg, 0.42 mmol, 97.4%). IR (KBr): 3417, 1548, 1376, 1091, 736, 423 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.83–4.96 (m, 1H, CHCH 2(a)), 5.02–5.10 (m, 1H, CHCH 2(b)), 5.10–5.22 (m, 1H, CHCH2), 6.96–7.03 (m, 1H. ArH), 7.04–7.12 (m, 1H, ArH), 7.15–7.34 (m, 4H, ArH), 7.34–7.46 (m, 3H, ArH), 8.12 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 41.0, 79.4, 111.6, 114.0, 118.1, 118.9, 120.2, 121.6, 123.0, 126.0, 129.2, 133.5, 136.6, 137.8; [Anal. Calcd. for C16H13ClN2O2: C, 63.90; H, 4.36; N, 9.31; found: C, 64.11; H, 4.05; N, 9.57]; LC/MS (ESI): M+ & [M+2]+, found 300.01 & 302.05, C16H13ClN2O2 requires 300.07.
2.2.5. 3-(1-(4-Bromophenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 6, 7e)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2e (98 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 20 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7e as yellow oil (130 mg, 0.38 mmol, 88%). IR (KBr): 3405, 1539, 1380, 1006, 743, 594, 533, 424 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.86–4.98 (m, 1H, CHCH 2(a)), 4.99–5.10 (m, 1H, CHCH 2(b)), 5.10–5.18 (m, 1H, CHCH2), 6.95–7.02 (m, 1H. ArH), 7.02–7.14 (m, 1H, ArH), 7.15–7.27 (m, 4H, ArH), 7.29–7.48 (m, 3H, ArH), 8.11 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 41.1, 79.3, 111.8, 113.9, 118.9, 120.2, 121.6, 123.0, 126.0, 129.6, 132.2, 136.6, 138.3, 140.0; [Anal. Calcd. for C16H13BrN2O2: C, 55.67; H, 3.80; N, 8.12; found: C, 55.27; H, 3.73; N, 7.98]; LC/MS (ESI): M+ & [M+2]+, found 344.10 & 346.13, C16H13BrN2O2 requires 344.02.
2.2.6. 3-(1-(4-Nitrophenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 8, 7f)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2f (83 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 20 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7f as yellow oil (94 mg, 0.30 mmol, 70%). IR (KBr): 3418, 1549, 1520, 1341, 713, 424 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 5.00–5.19 (m, 2H, CHCH 2), 5.79–5.96 (m, 1H, CHCH2), 6.96–7.08 (m, 1H. ArH), 7.08–7.61 (m, 7H. ArH), 7.90 (d, J = 8.0 Hz, 1H. ArH), 8.16 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 36.5, 80.1, 111.5, 112.9, 118.6, 120.4, 122.1, 123.1, 125.2, 126.0, 128.7, 130.0, 133.3, 136.4; [Anal. Calcd. for C16H13N3O4: C, 61.73; H, 4.21; N, 13.50; found: C, 68.05; H, 4.42; N, 13.69]; LC/MS (ESI): M+, found 344.08, C16H13N3O4 requires 311.09.
2.2.7. 3-(1-(2,4-Dichlorophenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 10, 7g)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2g (93 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 20 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7g as yellow oil (140 mg, 0.42 mmol, 97.5%). IR (KBr): 3417, 1549, 1464, 1376, 1101, 820, 738 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.88–5.01 (m, 2H, CHCH 2), 5.62–5.75 (m, 1H, CHCH 2), 7.03–7.16 (m, 4H. ArH), 7.18–7.27 (m, 1H, ArH), 7.32–7.42 (m, 2H, ArH), 7.45 (s, 1H, ArH), 8.16 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 37.7, 80.0, 111.5, 112.9, 118.9, 120.3, 122.0, 123.1, 126.0, 127.7, 129.9, 132.2, 134.0, 134.8, 135.3, 136.6; [Anal. Calcd. for C16H12Cl2N2O2: C, 57.33; H, 3.61; N, 8.36; found: C, 57.08; H, 3.43; N, 8.19]; LC/MS (ESI): M+ & [M+2]+, found 334.12 & 336.09, C16H12Cl2N2O2 requires 334.03.
2.2.8. 3-(1-(2,6-Dichlorophenyl)-2-nitroethyl)-1H-indole (Table 2, Entry 12, 7h)
Indole 4 (50 mg, 0.43 mmol) and β-nitroolefin 2h (93 mg, 0.43 mmol) in dry ethanol (3 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 20 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7h yellow oily (108 mg, 0.32 mmol, 75.2%). IR (KBr): 3417, 1549, 1464, 1376, 1101, 820, 738 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 5.28–5.52 (m, 2H, CHCH 2), 6.12–6.34 (m, 1H, CHCH2), 6.95–7.10 (m, 1H. ArH), 7.10–7.24 (m, 3H. ArH), 7.24–7.65 (m, 4H. ArH), 8.14 (s, 1H, NH of Indole); 13C-NMR (CDCl3, 100 MHz): δ 38.0, 78.9, 111.4, 114.0, 115.1, 117.8, 119.0, 120.1, 121.6, 122.5, 126.4, 129.4, 134.2, 136.1; [Anal. Calcd. for C16H12Cl2N2O2: C, 57.33; H, 3.61; N, 8.36; found: C, 57.08; H, 3.43; N, 8.19]; LC/MS (ESI): M+ & [M+2]+; found 334.09 & 336.07, C16H12Cl2N2O2 requires 334.03.
2.2.9. 3-(1-Ferrocenyl)-2-nitroethyl)-1H-indole (Table 1, Entry 12, 7i)
Table 1.
Condition optimization of Feist's acid catalysis in the addition of indolea (4) to β-nitro-styrene (2a).
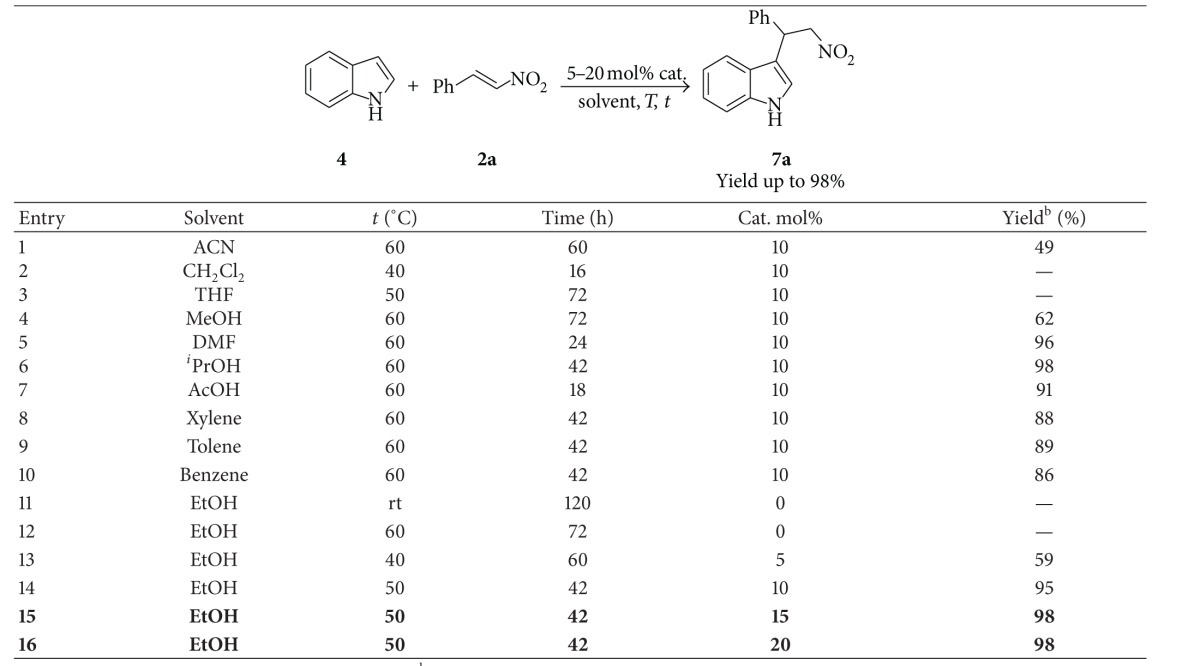
|
aThe reactions were performed on an 0.425 mmol scale; bthe isolated yield after column purification.
Indole 4 (50 mg, 0.43 mmol) and β-nitro styrene 2i (118 mg, 0.43 mmol) in dry ethanol (5 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 20 mol%) according to GP1. The product was purified by column chromatography on silica (EtOAc/hexane 1 : 9) yielded 7i as oily liquid (78 mg, 0.20 mmol, 46.4%). IR (KBr): 3417, 1549, 1464, 1376, 1101, 820, 738 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.09 (s, 5H, protons of Cp), 4.11–4.25 (m, 4H, protons of Cp), 4.83–5.89 (m, 1H, CpCHCH2), 4.93–5.65 (m, 2H, CpCHCH 2), 7.01 (s, 1H. ArH), 7.11 (t, J = 5.2 Hz, 1H. ArH), 7.18 (t, J = 5.2 Hz, 1H. ArH), 7.34 (d, J = 8.0 Hz, 1H. ArH), 7.57 (d, J = 8.0 Hz, 1H. ArH), 8.06 (s, 1H, NH of Indole); 13C-NMR (CDCL3, 100 MHz): δ 36.7, 66.8, 67.7, 68.2, 69.0, 80.6, 111.5, 113.9, 119.1, 119.9, 121.8, 122.5, 126, 129.3; [Anal. Calcd. for C20H18FeN2O2: C, 64.19; H, 4.84; N, 7.48; found: C, 63.97; H, 4.71; N, 7.59]; LC/MS (ESI): M+, found 374.22, C20H18FeN2O2 requires 374.19.
2.3. General Procedure for the Michael Addition Reaction of Indole with Pyrrole Catalyzed by Feist's Acid (GP2)
Pyrrole 5 (100 mg, 0.86 mmol), β-nitrostyrene 2(a–i) (0.86 mmol), and a catalytic amount of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) in dry isopropanol (7 mL) were charged into a Schlenk tube under an argon atmosphere. The reaction was then stirred at 50°C for 24–48 hours. The reaction mixture was monitored by TLC until starting material was completely consumed. Then the solvent was removed under vacuum. The crude products were isolated by column chromatography to afford pure Michael adducts 8(a–i) as major region-isomer, 9(a–d) and 9h as minor region-isomer.
2.3.1. 2-(2-Nitro-1-phenylethyl)-1H-pyrrole (Table 3, Entry 1, 8a) and 2,5-Bis(2-nitro-1-phenylethyl)-1H-pyrrole (Table 3, Entry 1, 9a)
Table 3.
Feist's acid catalysis substrate scopea, pyrrole 5.
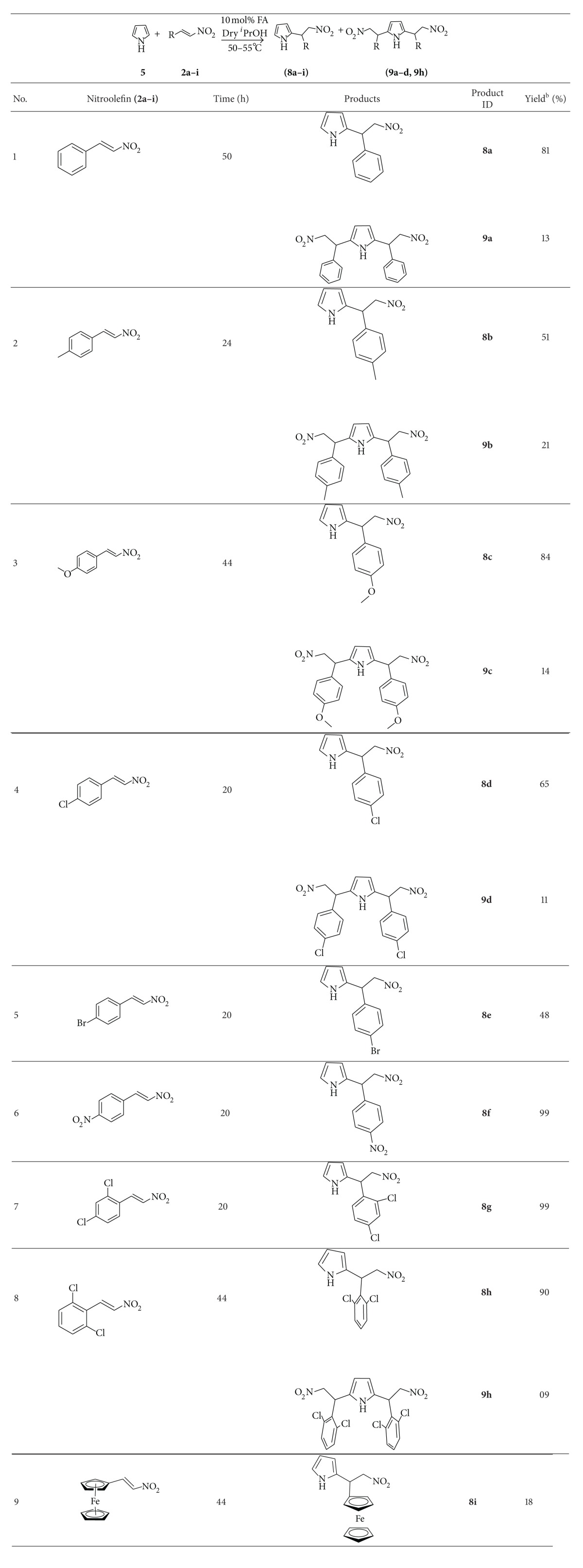
|
aThe reactions were performed on 0.86 mmol scale; bthe isolated yield after column purification.
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitrostyrene 2a (128 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The products were isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded as yellow solid 8a (major region-isomer) (165 mg, 0.74 mmol, 86.0%) and 9a (minor region-isomer) as yellow oil (40 mg, 0.11 mmol, 12.7%). (Major region-isomer 8a): IR (KBr): 3423, 1548, 1376, 723, 703, 524 (cm−1); 1H-NMR (CDCl3, 400 MHz) δ 4.69–4.85 (m, 1H, CHCH 2(a)), 4.85–4.93 (m, 1H, CHCH 2(b)), 4.93–5.02 (m, 1H, CHCH2), 6.03–6.10 (m, 1H, ArH), 6.14–6.17 (m, 1H, ArH), 6.64–6.72 (m, 1H. ArH), 7.17–7.26 (m, 2H, ArH), 7.26–7.40 (m, 3H, ArH), 7.84 (s, 1H,NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 43.0 (CHCH2), 79.6 (CHCH2), 105.9 (PyC 2), 108.8 (PyC 3), 118.3 (PyC 4), 128.0 (2C, PhC 2), 128.24 (PhC 4), 129.0 (PyC 1), 129.3 (2C, PhC 3), 138.0 (PhC 1); [Anal. Calcd. for C12H12N2O2: C, 66.65; H, 5.59; N, 12.96; found: C, 66.45; H, 5.51; N, 13.11]; LC/MS (ESI): M+, found 216.11, C12H12N2O2 requires 216.09; (minor region-isomer 9a): IR (KBr): 3407, 1548, 1376, 703, 524 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.68–4.86 (m, 4H, CHCH 2), 4.86–4.98 (m, 2H, CHCH2), 5.98 (d, J = 2.2 Hz, 2H, pyrrole), 7.05–7.18 (m, 4H, ArH), 7.18–7.39 (m, 6H, ArH), 7.54 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 42.9 (CHCH2), 79.2 (CHCH2), 106.2 (PyC 2), 106.6 (PyC 2), 127.85 (PhC 2), 127.92 (PhC 4), 128.24 (PhC 3), 129.30 (PyC 1), 138.1 (PhC 1); [Anal. Calcd. for C20H19N3O4: C, 65.74; H, 5.24; N, 11.50; found: C, 66.03; H, 5.37; N, 11.69]; LC/MS (ESI): M+, found 365.13, C12H12N2O2 requires 365.14.
2.3.2. 2-(2-Nitro-1-(p-tolyl)ethyl)-1H-pyrrole (Table 3, Entry 2, 8b) and 2,5-Bis(2-nitro-1-(p-tolyl)ethyl)-1H-pyrrole (Table 3, Entry 2, 9b)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2b (140 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The products were isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded as oily liquid 8b (major region-isomer) (100 mg, 0.43 mmol, 51%) and 9b (minor region-isomer) as yellow oil (70 mg, 0.18 mmol, 21%). (Minor region-isomer 8b): IR (KBr): 3388, 1542, 1378, 716, 584 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 2.25 (s, 1H, CH 3), 4.67–4.73 (m, 1H, CHCH 2(a)), 4.73–4.81 (m, 1H, CHCH 2(b)), 4.82–4.93 (m, 1H, CHCH2), 5.99 (s, 1H, ArH), 6.07–6.13 (m, 1H, ArH), 6.58–6.59 (m, 1H. ArH), 7.02–7.05 (m, 2H, ArH), 7.05–7.08 (m, 2H, ArH), 7.73 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 21.2 (CH3), 42.7 (CHCH2), 79.4 (CHCH2), 105.7 (PyC 2), 108.7 (PyC 3), 118.2 (PyC 4), 127.9 (2C, PhC 2), 129.2 (PyC 1), 130 (2C, PhC 3), 134.9 (PhC 4), 138.0 (PhC 1); [Anal. Calcd. for C13H14N2O2: C, 67.81; H, 6.13; N, 12.17; found: C, 68.07; H, 5.95; N, 11.91]; LC/MS (ESI): M+, found 230.06, C13H14N2O2 requires 230.11; (minor region-isomer 9b): IR (KBr): 3388, 1514, 1377, 717, 519 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 2.25 (s, 1H, CH 3), 4.54–4.79 (m, 4H, CHCH 2), 4.79–4.82 (m, 2H, CHCH2), 5.86–5.94 (m, 2H, ArH), 6.89–6.98 (m, 4H, ArH), 6.98–7.09 (m, 4H, ArH), 7.45 (s, 1H,NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 21.1 (CH3), 42.6 (CHCH2), 79.3 (CHCH2), 106.0 (PyC 2), 106.4 (PyC 2), 127.7 (PhC 2), 129.9 (PyC 1), 129.9 (PhC 3), 134.7 (PhC 4), 137.9 (PhC 1); [Anal. Calcd. for C22H23N3O4: C, 67.16; H, 5.89; N, 10.68; found: C, 66.86; H, 6.13; N, 10.94]; LC/MS (ESI): M+, found 393.19, C22H23N3O4 requires 393.17.
2.3.3. 2-(1-(4-Methoxyphenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 3, 8c) and 2,5-Bis(1-(4-methoxy-phenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 3, 9c)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2c (154 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The products were isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded as oily liquid 8c (major region-isomer) (177 mg, 0.72 mmol, 84%) and 9c (minor region-isomer) as yellow oil (50 mg, 0.12 mmol, 14%). Major region-isomer 8c: IR (KBr): 3388, 1542, 1378, 716, 584 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 3.78 (s, 1H, OCH 3), 4.69–4.80 (m, 1H, CHCH 2(a)), 4.80–4.89 (m, 1H, CHCH 2(b)), 4.90–5.01 (m, 1H, CHCH2), 6.06 (s, 1H, ArH), 6.15–6.22 (m, 1H, ArH), 6.67 (s, 1H. ArH), 6.86 (d, 2H, J = 8.8 Hz, ArH), 7.13 (d, 2H, J = 8.0 Hz, ArH), 7.87 (s, 1H,NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 42.3 (CHCH2), 55.4 (OCH3), 79.5 (CHCH2), 105.6 (PyC 2), 108.7 (PyC 3), 114.6 (2C, PhC 3), 118.2 (PyC 4), 129.1 (2C, PhC 2), 129.4 (PyC 1), 130 (PhC 4), 159.4 (PhC 1); [Anal. Calcd. for C13H14N2O3: C, 63.40; H, 5.73; N, 11.38; found: C, 63.62; H, 5.58; N, 11.19]; LC/MS (ESI): M+, found 246.14, C13H14N2O3 requires 246.10; minor region-isomer 9c: IR (KBr): 3388, 1514, 1377, 717, 519 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 3.76 (s, 1H, CH 3), 4.60–4.78 (m, 4H, CHCH 2), 4.78–4.93 (m, 2H, CHCH2), 5.92–6.01 (m, 2H, ArH), 6.80–6.84 (m, 4H, ArH), 6.01–7.12 (m, 4H, ArH), 7.54 (s, 1H,NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 41.2 (CHCH2), 55.4 (CH3), 79.4 (CHCH2), 105.9 (PyC 2), 106.3 (PyC 2), 114.6 (PhC 3), 128.96 (PhC 2), 129.02 (PyC 1), 129.8 (PhC 1), 159.4 (PhC 4); [Anal. Calcd. for C22H23N3O6: C, 62.11; H, 5.45; N, 9.88; found: C, 62.27; H, 5.39; N, 10.13]; LC/MS (ESI): M+, found 425.13, C22H23N3O6 requires 425.16.
2.3.4. 2-(1-(4-Chlorophenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 4, 8d) and 2,5-Bis(1-(4-chlorophenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 4, 9d)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2d (157 mg, 0.86 mmol) in dry ethanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The products were isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded white solid 8d (major region-isomer) (140 mg, 0.56 mmol, 65%) and 9d (minor region-isomer) as yellow oil (40 mg, 0.09 mmol, 11%). Major region-isomer 8d: IR (KBr): 3379, 1740, 1545, 1377, 1092, 725, 512, 452 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.66–4.83 (m, 2H, CHCH 2(a)), 4.83–4.91 (m, 2H, CHCH 2(b)), 4.91–5.06 (m, 1H, CHCH2), 6.07 (s, 1H, ArH), 6.12–6.21 (m, 1H, ArH), 6.70 (s, 1H. ArH), 7.17 (d, 2H, J = 6.84 Hz, ArH), 7.33 (d, 2H, J = 6.84, Hz, ArH), 7.84 (s, 1H,NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 42.4 (CHCH2), 79.0 (CHCH2), 106.1 (PyC 2), 108.9 (PyC 3), 118.6 (PyC 4), 128.4 (PhC 4), 129.4 (2C, PhC 2), 129.5 (2C, PhC 3), 134.2 (PyC 1), 136.6 (PhC 1); [Anal. Calcd. for C12H11ClN2O2: C, 57.49; H, 4.42; N, 11.17; found: C, 57.36; H, 4.53; N, 11.01]; LC/MS (ESI): M+ & [M+2]+, found 250.08 & 252.03, C12H11ClN2O2 requires 250.05; minor region-isomer 9d: IR (KBr): 3413, 1549, 1490, 1375, 1091, 726, 512 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.61–4.82 (m, 4H, CHCH 2), 4.82–5.03 (m, 2H, CHCH2), 5.93–6.02 (m, 2H, ArH), 7.01–7.20 (m, 4H, ArH), 7.20–7.39 (m, 4H, ArH), 7.53 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 42.2 (CHCH2), 78.9 (CHCH2), 106.5 (PyC 2), 106.9 (PyC 2), 129.2 (PhC 3), 129.3 (PhC 2), 129.52 (PyC 1), 134.3 (PhC 4), 136.2 (PhC 1); [Anal. Calcd. for C20H17Cl2N3O4: C, 55.31; H, 3.95; N, 9.68; found: C, 55.19; H, 4.14; N, 9.43]; LC/MS (ESI): M+ & [M+2]+, found 433.11 & 435.15, C20H17Cl2N3O4 requires 433.05.
2.3.5. 2-(1-(4-Bromophenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 5, 8e)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2e (195 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The product was isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded as white solid 8e (120 mg, 0.41 mmol, 47%). IR (KBr): 3376, 1543, 1377, 1007, 725, 510 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.71–4.81 (m, 1H, CHCH 2(a)), 4.81–4.90 (m, 1H, CHCH 2(b)), 4.90–5.02 (m, 2H, CHCH2), 6.07 (s, 1H, ArH), 6.12–6.22 (m, 1H, ArH), 6.70 (s, 1H. ArH), 7.11 (d, 2H, J = 8.8 Hz, ArH), 7.48 (d, 2H, J = 8.8, Hz, ArH), 7.86 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 42.5 (CHCH2), 79.0 (CHCH2), 106.1 (PyC 2), 108.9 (PyC 3), 118.6 (PyC 4), 122.3 (PhC 4), 128.3 (PyC 1), 129.7 (2C, PhC 2), 132.4 (PhC 3), 137.1 (PhC 1); [Anal. Calcd. for C12H11BrN2O2: C, 48.84; H, 3.76; N, 9.49; found: C, 49.07; H, 3.89; N, 9.35]; LC/MS (ESI): M+ & [M+2]+, found 294.01 & 296.05, C12H11BrN2O2 requires 294.0.
2.3.6. 2-(2-Nitro-1-(4-nitrophenyl) ethyl)-1H-pyrrole (Table 3, Entry 6, 8f)
Pyrrole 5 (100 mg, 0.8 mmol) and β-nitroolefin 2f (167 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The product was isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded as oily liquid 8f (224 mg, 0.86 mmol, 99.8%). IR (KBr): 3421, 1551, 1520, 1373, 1349, 784, 717 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.93–5.05 (m, 2H, CHCH 2), 5.51–5.57 (m, 1H, CHCH2), 6.12–6.19 (m, 2H, ArH), 6.73 (s, 1H. ArH), 7.31–7.48 (m, 2H, ArH), 7.48–7.61 (m, 1H, ArH), 7.81–7.93 (m, 1H, ArH), 8.45 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 36.9 (CHCH2), 79.8 (CHCH2), 105.6 (PyC 2), 108.8 (PyC 3), 118.9 (PyC 4), 127.8 (PyC 3), 128.8 (2C, PhC 2), 129.4 (PyC 1), 133.7 (PhC 4), 149.6 (PhC 1); [Anal. Calcd. for C12H11N3O4: C, 55.17; H, 4.24; N, 16.09; found: C, 55.36; H, 4.19; N, 15.93]; LC/MS (ESI): M+, found 261.15, C12H11N3O4 requires 261.07.
2.3.7. 2-(1-(2,4-Dichlorophenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 7, 8g)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2g (187 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The product was isolated by column chromatography on silica (EtOAc/hexane 1 : 9) yielded as oily liquid 8g (245 mg, 0.86 mmol, 99.9%). IR (KBr): 3417, 1550, 1469, 1376, 1099, 797, 726 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.71–4.98 (m, 2H, CHCH 2), 5.32–5.49 (m, 1H, CHCH2), 6.06–6.23 (m, 2H, ArH), 6.71 (s, 1H, ArH), 6.94–7.10 (m, 1H, ArH), 7.10–7.29 (m, 1H, ArH), 7.31–7.58 (m, 1H, ArH), 8.02 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 39.0 (CHCH2), 79.9 (CHCH2), 106.6 (PyC 2), 108.9 (PyC 3), 118.7 (PyC 4), 127.4 (PyC 1), 128.0 (PhC 6), 130.0 (PhC 5), 130.1 (PhC 3), 134.3–134.6 (PhC 1, PhC 2 & PhC 4); [Anal. Calcd. for C12H10Cl2N2O2: C, 50.55; H, 3.54; N, 9.82; found: C, 50.33; H, 3.69; N, 10.07]; LC/MS (ESI): M+, found 284.05, C12H11N3O4 requires 284.01.
2.3.8. 2-(1-(2,6-Dichlorophenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 8, 8h) and 2,5-Bis(1-(2,6-dichloro-phenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 8, 9h)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitrostyrene 2h (187 mg, 0.86 mmol) in isopropanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The products were isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) yielded yellow oil 8h (major region-isomer) (220 mg, 0.77 mmol, 90%) and 9h (minor region-isomer) as yellow oil (40 mg, 0.09 mmol, 9%). Major region-isomer 8h: IR (KBr): 3439, 1550, 1430, 1374, 775, 720, 536 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 5.21–5.30 (m, 1H, CHCH 2(a)), 5.30–4.42 (m, 1H, CHCH 2(b)), 5.90–6.01 (m, 1H, CHCH2), 6.09 (s, 1H, ArH), 6.12–6.28 (m, 1H, ArH), 6.66–6.75 (m, 1H. ArH), 7.12–7.24 (m, 1H, ArH), 7.28–7.48 (m, 2H, ArH), 8.06 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 39.0 (CHCH2), 79.5 (CHCH2), 106.8 (PyC 2), 108.7 (PyC 3), 118.1 (PyC 4), 124.4 (2C, PhC 3), 126.8 (PhC 4), 129.8 (2C, PhC 2), 133.8 (PyC 1), 142.8 (PhC 1); [Anal. Calcd. for C12H10Cl2N2O2: C, 50.55; H, 3.54; N, 9.82; found: C, 50.45; H, 3.43; N, 9.74]; LC/MS (ESI): M+ & [M+2]+, found 284.05 & 286.11, C12H10Cl2N2O2 requires 284.01; minor region-isomer 9h: IR (KBr): 3437, 1741, 1551, 1431, 1372, 1210, 773, 532 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 5.14–5.32 (m, 4H, CHCH 2), 5.83 (t, J = 6.6 Hz, 2H, CHCH2), 5.93–6.02 (m, 2H, ArH), 7.12–7.23 (m, 2 h, ArH), 7.26–7.42 (m, 4 h, ArH), 8.16 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3,100 MHz): δ 42.2 (CHCH2), 78.9 (CHCH2), 106.5 (PyC 2), 106.9 (PyC 2), 129.2 (PhC 3), 129.3 (PhC 2), 129.52 (PyC 1), 134.3 (PhC 4), 136.2 (PhC 1 ); [Anal. Calcd. for C20H15Cl4N3O4: C, 47.74; H, 3.0; N, 8.35; found: C, 48.09; H, 3.11; N, 8.51]; LC/MS (ESI): M+ & [M+2]+, found 501.03 and 503.06, C20H15Cl4N3O4 requires 500.98.
2.3.9. 2-(1-Ferrocenyl)-2-nitroethyl)-1H-pyrrole (Table 3, Entry 9, 8i)
Pyrrole 5 (100 mg, 0.86 mmol) and β-nitroolefin 2g (167 mg, 0.86 mmol) in dry ethanol (7 mL) were reacted in the presence of Feist's acid (1) (12 mg, 0.168 mmol, 10 mol%) according to GP2. The product was isolated by column chromatography on silica (EtOAc/hexane 0.5 : 9.5) dark red solid 8g (51 mg, 18%). IR (KBr): 3358, 1740, 1551, 1410, 1251, 1219, 1044, 728, 488 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 4.13 (s, 1H, protons of Cp), 4.23 (s, 5H, protons of Cp), 4.30–4.39 (m, 2H, protons of Cp), 4.64 (s, 2H, m, 2H, protons of Cp), 5.10–5.21 (m, 1H, CHCH2), 5.21–5.40 (m, 2H, CHCH 2), 6.26–6.36 (m, 1H, ArH), 6.81–6.90 (m, 1H, ArH), 7.18 (m, 1H, ArH), 7.79 (s, 1H, NH of pyrrole); 13C-NMR (CDCl3, 100 MHz): δ 29.8, 69.1, 70.0, 70.6, 72.3, 84.0, 111.6, 123.0, 125.2, 126.6; [Anal. Calcd. for C16H16FeN2O2: C, 59.28; H, 4.98; N, 8.64; found: C, 59.17; H, 5.07; N, 8.49]; LC/MS (ESI): M+, found 324.12, C16H16FeN2O2 requires 324.16.
3. Result and Discussion
At the outset, synthetic strategies adopted in our work, for the preparation of Feist's acid (FA) (1) as hydrogen bond donor catalyst, has been outlined in Scheme 1. Feist's acid is commercially available but highly expensive therefore, it was prepared in our laboratory from a very cheap and readily available material ethyl acetoacetate with an overall yield of 19% in three steps, using the well-known method reported by Al-Majid et al. [50].
Scheme 1.
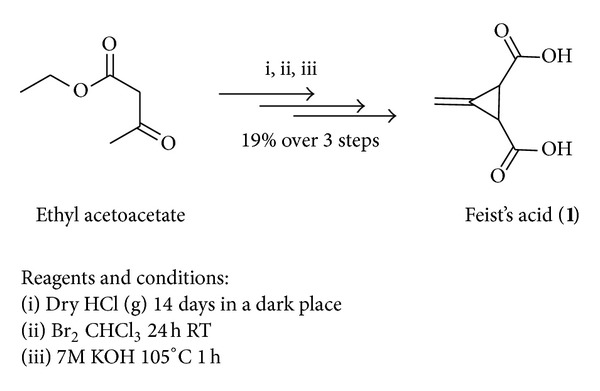
Feist's acid synthesis.
Initially, to evaluate the catalytic activity of Feist's acid for the Michel addition reaction, indole 4 and β-nitrostyrene 2a have been chosen as model substrates. 5–20 mol% of FA (1) were used, aiming at screening the optimal conditions, and the results are summarized in Table 1.
By screening different solvents such as methanol, ethanol, isopropanol (iPrOH), toluene, benzene, xylene, acetonitrile, tetrahydrofuran (THF), and dichloromethane (CH2Cl2), using 10 mol% of Feist's acid, we found that most of the solvents produced good to excellent yields (86–96%) for the Michael addition reaction of indole 4 to β-nitrostyrene 2a, affording the corresponding product 7a at 50–60°C temperature within 24–42 h, (Table 1, entries 5–10, 14), while in case of ACN and MeOH, moderate yields were observed (49% and 62%), respectively (Table 1, entries 1, 4). But in case of other solvents like CH2Cl2 and THF, no products were formed (Table 1, entries 2, 3). Without using Feist's acid as catalyst, the reaction was carried out in ethanol at room temperature as well as at 60°C for 72 h; no product formation was observed at all (Table 1, entries 11, 12). But with the use 5 mol% catalyst in ethanol at 50°C for 60 h, 59% yield was observed (Table 1, entry 13). The yields of product were improved remarkably from 59% to 98% by increasing the loading of catalyst from 5 to 20 mol% in ethanol (Table 1, entries 13, 14). It is noteworthy to mention that solvents like DMF and AcOH with 10% catalyst produced very good results in 42 h and 18 h accordingly (Table 1, entries 5, 7). But due to the work-up problem these solvents cause they have been discarded from further reaction optimization. Xylene, toluene, and benzene also have been discarded for their high boiling point as compared to ethanol. The optimized procedure for Michael addition of indole to nitroolefin was found to be as follows: the mixture of indole 4 (0.43 mmol), β-nitrostyrene 2a (0.43 mmol), and FA (Feist's acid, 10 mol%) was heated at 50°C in ethanol for 42 h according to general procedure GP1.
To illustrate the generality of this Michael addition reaction of indole 4, with various nitroolefins 2(a–i), sixteen examples were carried out catalyzed by Feist's acid with 10 mol% and 20 mol% in ethanol at 50°C temperature and, the results are shown in Table 2.
The exclusive 3-substituted indole derivatives 7a–i were obtained in good to excellent yields (46–97%) with the use of 10 mol% of catalyst at 50°C temperature in 48 hours (Table 2, entries 1–13). The reaction of indole (4) with nitroolefins 2(e–h) bearing 4-bromophenyl, 2,4-dichlorophenyl, 2,6-dichlorophenyl, and 4-nitrophenyl produced the corresponding Michael adducts 7(e–h) with poor to moderate yield (49%, 55%, 63%, and 62%), respectively (Table 2, entries 5, 7, 9, and 11). The poor reactivity of these nitroolefins could be inferred due to their less solubility and bulkier environment. But the chemical yields were increased dramatically to 87%, 70%, 97%, and 75%, respectively, when the loading of catalyst were increased from10–20 mol% and the reactions were run for 72 hours (Table 2, entries 6c, 8c, 10c, 12c). In contrast, the nitroolefin bearing phenyl, tolyl, and 4-chlorophenyl, afforded the corresponding Michael products in excellent yield (97%) (Table 2, entries 1, 2 and 4), while in the case of nitroolefin with 4-methoxyphenyl, the yield was slightly lower (84%) (Table 2, entry 3), which was probably attributed to the steric effect of the 4-methoxyphenyl group.
On the basis of the above results obtained for Michael addition reaction of indole with the derivatives of β-nitrostyrenes, the reaction was extended to pyrrole, and it was found that Feist's acid (FA) can also efficiently catalyze the reaction of pyrrole with different β-nitroolefins in isopropanol, affording 2-substituted pyrrole and in some cases both 2-substituted and 2,4-disubstituted pyrrole derivatives in good to excellent yields; the results are summarized in Table 3.
The Michael addition of pyrrole 5 to nitroolefins 2(a–i) catalyzed by FA (10 mol%) in isopropanol at 50°C for 20–50 h afforded 2-substituted pyrrole 8(a–i) as major region-isomer (Table 3, entries 1–9), with some 2,4-disubstituted pyrrole derivatives 9(a–d) and 9h (Table 3, entries 1–4, 8), which also showed good regioselectivity of pyrrole at the 2-position. The reaction of pyrrole with nitroolefins bearing phenyl, tolyl, 4-methoxyphenyl, 4-chloroophenyl and, 24-dichlorophenyl 2(a–d) and 2h afforded major region-isomer 2-substituted pyrrole 8(a–d) and 8h (81%, 50%, 84%, 65%, and 90%) with some minor region-isomer 2,6-disubstituted pyrrole 9(a–d) and 9h (13%, 21%, 13%, 11%, and 9%) in 50 h, 24 h, 44 h, 20 h, and 44 h, respectively (Table 3, entries 1–4, 8). But 2-substituted Michael adducts 8(e–g) and 8i were formed exclusively with 48%, 99.8%, 99.9%, and 18%, when nitroolefins bearing 4-bromophenyl, 4-nitrophenyl, 2,4-dichlorophenyl, and ferrocene groups reacted with pyrrole in 20 h and 44 h, accordingly (Table 3, entries 5–7, 9). Obviously the best results were found in the case of nitroolefin 2f and 2g producing their corresponding adducts 8f and 8g with excellent yields 99.8 and 99.9%, respectively (Table 3, entries 6, 7). On the other hand, poor yields (8e and 8i with 48% and 18%) were observed corresponding to the nitroolefins 2e and 2i. The reason for this low yield could be attributed to the poor solubility of 2e and 2i.
We reasoned that, the catalytic cycle would begin with activation of an electrophile, such as β-nitrostyrenes (2a–i), with Feist's acid (1) to afford intermediate 3 (Figure 1). Addition of nucleophiles (4 and 5), to the intermediate 3 give rise to another intermediate 6 followed by proton transfer and release of the catalyst (1), would complete the cycle and generate the desired products 7a–i, 8a–i, 9a–d and 9h corresponding to the substrate 4 and 5 (Tables 2 and 3). All the analytical data are available in the Supplementary Material available online at http//dox.doi.org/10.1155/2014/649197.
Figure 1.
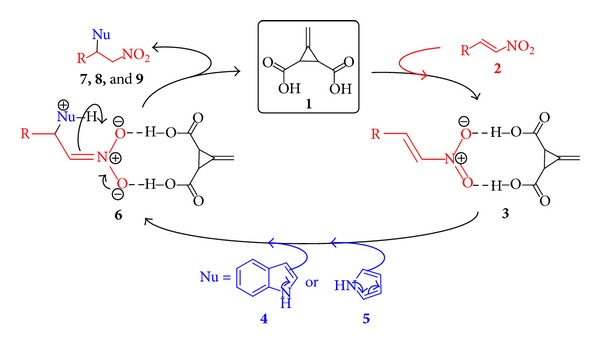
Activation of nitrostyrene via hydrogen bonding mechanism by Feist's acid catalysis.
4. General Procedure for Antimicrobial Activity
Chemical compounds were individually tested against a panel of Gram-positive and Gram-negative bacterial pathogens. Antimicrobial tests were carried out by the agar well diffusion method using 100 mL of suspension containing 1 × 108 CFU/mL of pathological tested bacteria, 1 × 106 CFU/mL of yeast and 1 × 104 spore/mL of fungi spread on nutrient agar (NA), Sabouraud dextrose agar (SDA), and potato dextrose agar (PDA) medium, respectively. After the media had cooled and solidified, wells (10 mm in diameter) were made in the solidified agar and loaded with 100 mL of tested compound solution prepared by dissolving 100 mg of the chemical compound in one mL of dimethyl sulfoxide (DMSO). The inculcated plates were then incubated for 24 h at 37°C for bacteria and 48 h at 28°C for fungi. Negative controls were prepared using DMSO employed for dissolving the tested compound. Ciprofloxacin (50 mg/mL) and Ketoconazole (50 mg/mL) were used as standard for antibacterial and antifungal activity, respectively. After incubation time, antimicrobial activity was evaluated by measuring the zone of inhibition against the test organisms and compared with that of the standard. The observed zone of inhibition is presented in Table 1. Antimicrobial activities were expressed as inhibition diameter zones in millimeters (mm) as follows: N.A. (no activity) ≤ 4 mm; + (weak) = 5–9 mm; ++ (moderate) = 10–15 mm; +++ (strong) = 16–20 mm, and ++++ (very strong) ≥ 21 mm. The experiment was carried out in triplicate and the average zone of inhibition was calculated.
4.1. Antimicrobial Activity
A sample of some synthesized compounds (7b, 7d, 7e, 7f and 8e, 8f, 8g) has been subjected to antimicrobial activity studies including Gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus), Gram-negative bacteria (Pseudomonas Aeruginosa and Escherichia coli), and fungi (Candida albicans). Antimicrobial tests were carried out by the agar well diffusion method. When compared to the standard drug Ciprofloxacin, it was seen that compounds 7b, 7d, 7e, and 7f with frame structure of indole moiety showed an inhibition effect against Bacillus subtilis ATCC 10400 (Table 4). On the other hand, 8e, 8g with frame structure of pyrrole moiety showed effect against Bacillus subtilis ATCC 10400. Not worthy to mention that 8f showed potent inhibition against the same Gram-positive bacteria Bacillus subtilis ATCC 10400 compared with standard Ciprofloxacin (Table 4). Interestingly, 8e, 8g with frame structure of pyrrole moiety showed antifungal activity against Candida albicans ATTCC-10231, while 8f showed potent inhibition against the same fungi Candida albicans ATTCC-1023 compared to standard drug Ketoconazole. Nevertheless, 7b, 7d, 7e, 7f, 8e, 8f, and 8g were not active against S. aureus ATCC 29213, E. coli ATCC-35218, and P. aeruginosa ATCC 29336. The results obtained are summarized in Table 4 [59].
Table 4.
Antimicrobial activity of the newly synthesized compounds against the pathological strains based on well diffusion assaya.
| Comp. no. | Gram-positive bacteria | Gram-negative bacteria | Fungi | ||
|---|---|---|---|---|---|
| Staphylococcus aureus ATTCC-29213 | Bacilils subtilis ATTCC-10400 | Escherichia coli ATTCC-35218 | Pseudomonas aeruginosa ATTCC-29336 | Candida albicans ATTCC-10231 | |
| 7b | N.A. | ++ | N.A. | N.A. | N.A. |
| 7d | N.A. | ++ | N.A. | N.A. | N.A. |
| 7e | N.A. | ++ | N.A. | N.A. | N.A. |
| 7f | N.A. | ++ | N.A. | N.A. | N.A. |
| 7g | N.A. | ++ | N.A. | N.A. | N.A. |
| 8e | N.A. | ++ | N.A. | N.A. | +++ |
| 8f | N.A. | ++++ | N.A. | N.A. | ++++ |
| 8g | N.A. | ++ | N.A. | N.A. | ++ |
| Ciprofloxacin | +++ | ++++ | ++++ | ++++ | N.A. |
| Ketoconazole | N.A. | N.A. | N.A. | N.A. | ++++ |
aAntimicrobial activities were expressed as inhibition diameter zones in millimeters (mm) as follows: N.A. (no activity) ≤ 4 mm; + (weak) = 5–9 mm; ++ (moderate) = 10–15 mm; +++ (strong) = 16–20 mm; and ++++ (very strong) ≥ 21 mm. The experiment was carried out in triplicate and the average zone of inhibition was calculated.
5. Conclusion
In summary, Feist's acid has been introduced as a new class of hydrogen bond donor catalysts for the activation of nitroalkene in conjugate addition reactions. This study includes the original report of Feist's acid catalysis of Michel addition of indole and pyrrole to a variety of nitroolefins. All the synthesized indole and pyrrole derivatives have be screened for antimicrobial activity (MIC determination) in our laboratory and the results are reported here in. Investigations surrounding the potential associated with Feist's acid catalysis, including the development of enantioselective variants, as a new tool for organic synthesis are under progress in our laboratory.
Supplementary Material
Scan copies of 1H and 13C spectra of the Michael adducts 7a-i, 8a-I, 9a-d, & 9h
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group Project no. RGP-VPP-044.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Trost BM. On inventing reactions for atom economy. Accounts of Chemical Research. 2002;35(9):695–705. doi: 10.1021/ar010068z. [DOI] [PubMed] [Google Scholar]
- 2.Trost BM. The atom economy—a search for synthetic efficiency. Science. 1991;254(5037):1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 3.Marqués-López E, Diez-Martinez A, Merino P, Herrera RP. The role of the indole in important organocatalytic enantioselective Friedel-Crafts alkylation reactions. Current Organic Chemistry. 2009;13(16):1585–1609. [Google Scholar]
- 4.You S-L, Cai Q, Zeng M. Chiral Brønsted acid catalyzed Friedel-Crafts alkylation reactions. Chemical Society Reviews. 2009;38(8):2190–2210. doi: 10.1039/b817310a. [DOI] [PubMed] [Google Scholar]
- 5.Terrasson V, De Figueiredo RM, Campagne JM. Organocatalyzed asymmetric Friedel-Crafts reactions. European Journal of Organic Chemistry. 2010;2010(14):2635–2655. [Google Scholar]
- 6.Zeng M, You S-L. Asymmetric Friedel-Crafts alkylation of indoles: the control of enantio- and regioselectivity. Synlett. 2010;2010(9):1289–1301. [Google Scholar]
- 7.Ono N. The Nitro Group in Organic Synthesis. New York, NY, USA: John Wiley & Sons; 2001. [Google Scholar]
- 8.Seebach D, Colvin EW, Lehr F, Weller T. Nitroaliphatic compounds-ideal intermediates in organic synthesis. Chimia. 1979;33:1–18. [Google Scholar]
- 9.Calderari G, Seebach D. Asymmetric Michael additions. Stereoselective alkylation of chiral, non-racemic enolates by nitroolefins. Preparation of enantiomerically pure γ-aminobutyric acid and succinic acid derivatives. Helvetica Chimica Acta. 1985;68(6):1592–11604. [Google Scholar]
- 10.Berner OM, Tedeschi L, Enders D. Asymmetric Michael additions to nitroalkenes. European Journal of Organic Chemistry. 2002;2002(12):1877–1894. [Google Scholar]
- 11.Chakrabarty M, Basak R, Ghosh N. Microwave-assisted Michael reactions of 3-(2′-nitrovinyl)indole with indoles on TLC-grade silica gel. A new, facile synthesis of 2,2-bis(3′-indolyl)nitroethanes. Tetrahedron Letters. 2001;42(23):3913–3915. [Google Scholar]
- 12.Wang B. Sulfamic acid: a very useful catalyst. Synlett. 2005;2005(8):1342–1343. [Google Scholar]
- 13.Sulzer-Mossé S, Alexakis A. Chiral amines as organocatalysts for asymmetric conjugate addition to nitroolefins and vinyl sulfones via enamine activation. Chemical Communications. 2007;(30):3123–3135. doi: 10.1039/b701216k. [DOI] [PubMed] [Google Scholar]
- 14.Almaşi D, Alonso DA, Nájera CN. Organocatalytic asymmetric conjugate additions. Tetrahedron: Asymmetry. 2007;18(3):299–365. [Google Scholar]
- 15.Tsogoeva SB. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. European Journal of Organic Chemistry. 2007;2007(11):1701–1716. [Google Scholar]
- 16.Roca-López D, Sadaba D, Delso I, Herrera RP, Tejero T, Merino P. Asymmetric organocatalytic synthesis of γ-nitrocarbonyl compounds through Michael and Domino reactions. Tetrahedron: Asymmetry. 2010;21(21-22):2561–2601. [Google Scholar]
- 17.Herrera RP, Sgarzani V, Bernardi L, Ricci A. Catalytic enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angewandte Chemie. 2005;44(40):6576–6579. doi: 10.1002/anie.200500227. [DOI] [PubMed] [Google Scholar]
- 18.Fleming EM, McCabe T, Connon SJ. Novel axially chiral bis-arylthiourea-based organocatalysts for asymmetric Friedel-Crafts type reactions. Tetrahedron Letters. 2006;47(39):7037–7042. [Google Scholar]
- 19.Ganesh M, Seidel D. Catalytic enantioselective additions of indoles to nitroalkenes. Journal of the American Chemical Society. 2008;130(49):16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 20.Itoh J, Fuchibe K, Akiyama T. Chiral phosphoric acid catalyzed enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes: cooperative effect of 3 Å molecular sieves. Angewandte Chemie. 2008;47(21):4016–4018. doi: 10.1002/anie.200800770. [DOI] [PubMed] [Google Scholar]
- 21.Marqués-López E, Alcaine A, Tejero T, Herrera RP. Enhanced efficiency of thiourea catalysts by external Brønsted acids in the Friedel-Crafts alkylation of indoles. European Journal of Organic Chemistry. 2011;2011(20-21):3700–3705. [Google Scholar]
- 22.Olah GA, Krishnamurty R, Prakash GKS. Friedel-Crafts alkylation. In: Trost BM, Fleming I, editors. Comprehensive Organic Synthesis. 1st edition. Vol. 3. Oxford, UK: Pergamon Press; 1991. p. p. 293. (Comprehensive Organic Synthesis). [Google Scholar]
- 23.Komoto I, Kobayashi S. Lewis acid catalysis in supercritical carbon dioxide. Use of poly(ethylene glycol) derivatives and perfluoroalkylbenzenes as surfactant molecules which enable efficient catalysis in ScCO2 . Journal of Organic Chemistry. 2004;69(3):680–688. doi: 10.1021/jo0353177. [DOI] [PubMed] [Google Scholar]
- 24.Zhan Z-P, Yang R-F, Lang K. Samarium triiodide-catalyzed conjugate addition of indoles with electron-deficient olefins. Tetrahedron Letters. 2005;46(22):3859–3862. [Google Scholar]
- 25.Jia Y-X, Zhu S-F, Yang Y, Zhou Q-L. Asymmetric Friedel-Crafts alkylations of indoles with nitroalkenes catalyzed by Zn(II)-bisoxazoline complexes. Journal of Organic Chemistry. 2006;71(1):75–80. doi: 10.1021/jo0516537. [DOI] [PubMed] [Google Scholar]
- 26.Pihko PM. Hydrogen Bonding in Organic Synthesis. Weinheim, Germany: John Wiley & Sons; 2009. [Google Scholar]
- 27.Doyle AG, Jacobsen EN. Small-molecule H-bond donors in asymmetric catalysis. Chemical Reviews. 2007;107(12):5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama T. Stronger Brønsted acids. Chemical Reviews. 2007;107(12):5744–5758. doi: 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]
- 29.Takemoto Y. Recognition and activation by ureas and thioureas: stereoselective reactions using ureas and thioureas as hydrogen-bonding donors. Organic and Biomolecular Chemistry. 2005;3(24):4299–4306. doi: 10.1039/b511216h. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Schreiner PR. (Thio)urea organocatalysis—what can be learnt from anion recognition? Chemical Society Reviews. 2009;38(4):1187–1198. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- 31.Connon SJ. The design of novel, synthetically useful (thio)urea-based organocatalysts. Synlett. 2009;2009(3):354–376. [Google Scholar]
- 32.Tran NT, Wilson SO, Franz AK. Cooperative hydrogen-bonding effects in silanediol catalysis. Organic Letters. 2012;14(1):186–189. doi: 10.1021/ol202971m. [DOI] [PubMed] [Google Scholar]
- 33.Schafer AG, Wieting JM, Mattson AE. Silanediols: a new class of hydrogen bond donor catalysts. Organic Letters. 2011;13(19):5228–5231. doi: 10.1021/ol2021115. [DOI] [PubMed] [Google Scholar]
- 34.Shumaila AMA, Kusurkar RS. Silica gel, an effective catalyst for the reaction of electron-deficient nitro-olefins with nitrogen heterocycles. Synthetic Communications. 2010;40(19):2935–2940. [Google Scholar]
- 35.Schneider JF, Falk FC, Freohlich R, Paradies J. Planar-chiral thioureas as hydrogen-bond catalysts. European Journal of Organic Chemistry. 2010;2010(12):2265–2269. [Google Scholar]
- 36.Ganesh M, Seidel D. Catalytic enantioselective additions of indoles to nitroalkenes. Journal of the American Chemical Society. 2008;130(49):16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 37.Takenaka N, Sarangthem RS, Seerla SK. 2-Aminopyridinium ions activate nitroalkenes through hydrogen bonding. Organic Letters. 2007;9(15):2819–2822. doi: 10.1021/ol071032v. [DOI] [PubMed] [Google Scholar]
- 38.Gu Y, Ogawa C, Kobayashi S. Silica-supported sodium sulfonate with ionic liquid: a neutral catalyst system for Michael reactions of indoles in water. Organic Letters. 2007;9(2):175–178. doi: 10.1021/ol062446b. [DOI] [PubMed] [Google Scholar]
- 39.Dessole G, Herrera RP, Ricci A. H-bonding organocatalysed Friedel-Crafts alkylation of aromatic and heteroaromatic systems with nitroolefins. Synlett. 2004;(13):2374–2378. [Google Scholar]
- 40.Zhuang W, Hazell RG, Jørgensen KA. Enantioselective Friedel-Crafts type addition of indoles to nitro-olefins using a chiral hydrogen-bonding catalyst—synthesis of optically active tetrahydro-β-carbolines. Organic and Biomolecular Chemistry. 2005;3(14):2566–2571. doi: 10.1039/b505220c. [DOI] [PubMed] [Google Scholar]
- 41.Lin C, Hsu J, Sastry MNV, et al. I2-catalyzed Michael addition of indole and pyrrole to nitroolefins. Tetrahedron. 2005;61(49):11751–11757. [Google Scholar]
- 42.Schreiner PR. Metal-free organocatalysis through explicit hydrogen bonding interactions. Chemical Society Reviews. 2003;32(5):289–296. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]
- 43.Connon SJ. Organocatalysis mediated by (thio)urea derivatives. Chemistry European Journal. 2006;12(21):5418–5427. doi: 10.1002/chem.200501076. [DOI] [PubMed] [Google Scholar]
- 44.Taylor MS, Jacobsen EN. Asymmetric catalysis by chiral hydrogen-bond donors. Angewandte Chemie. 2006;45(10):1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Schreiner PR. (Thio)urea organocatalysis—what can be learnt from anion recognition? Chemical Society Reviews. 2009;38(4):1187–1198. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- 46.Zhou W, Xu L-W, Yang L, Zhao P-Q, Xia C-G. Novel Brønsted acid-catalyzed Michael-type Friedel-Crafts reactions of indoles and acetalization of aldehydes. Journal of Molecular Catalysis A. 2006;249(1-2):129–134. [Google Scholar]
- 47.Zhou W, Xu L-W, Li L, Yang L, Xia C-G. Enantioselective Michael-type Friedel-Crafts reactions of indoles to enones catalyzed by a chiral camphor-based Brønsted acid. European Journal of Organic Chemistry. 2006;2006(23):5225–5227. [Google Scholar]
- 48.Kondo S-I, Harada T, Tanaka R, Unno M. Anion recognition by a silanediol-based receptor. Organic Letters. 2006;8(20):4621–4624. doi: 10.1021/ol061822p. [DOI] [PubMed] [Google Scholar]
- 49.Goss FR, Ingold CK, Thorpe JF. XLI.—the chemistry of the glutaconic acids. Part XIV. Three-carbon tautomerism in the cyclopropane series. Journal of the Chemical Society: Transactions. 1923;123:327–361. [Google Scholar]
- 50.Al-Majid AM, Booth BL, Gomes JT. C2-symmetric ligands for asymmetric catalysis based on Feist's acid. Journal of Chemical Research: Part S. 1998;(2):78–79. [Google Scholar]
- 51.Zee-Cheng RKY, Cheng CC. Structure-activity relationship study of anthraquinones: 1, 4-dihydroxy-5, 8-bis[[2-(2-hydroxyethoxy)ethyl]amino]-9, 10-anthracenedione, an analog of an established antineoplastic agent. Journal of Pharmaceutical Science. 1982;71(6):708–709. doi: 10.1002/jps.2600710626. [DOI] [PubMed] [Google Scholar]
- 52.Epifano F, Genovese S, Menghini L, Curini M. Chemistry and pharmacology of oxyprenylated secondary plant metabolites. Phytochemistry. 2007;68(7):939–953. doi: 10.1016/j.phytochem.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: the importance of a 2,4-substituted resorcinol moiety. Bioorganic & Medicinal Chemistry. 2005;13(2):433–441. doi: 10.1016/j.bmc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Almerico AM, Diana P, Barraja P, et al. Glycosidopyrroles. Part 1. Acyclic derivatives: 1-(2-hydroxyethoxy) methylpyrroles as potential anti-viral agents. IL Farmaco. 1998;53(1):33–40. doi: 10.1016/s0014-827x(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 55.Carpio H, Galeazzi E, Greenhouse R, et al. Synthesis of l, 2-dihydro-3H-pyrrolo[1, 2-a]pyrrole-1-carboxylic acids and homologous pyridine and azepine analogues thereof. Canadian Journal of Chemistry. 1982;60(18):2295–2312. [Google Scholar]
- 56.Dannhardt G, Kiefer W, Krämer G, Maehrlein S, Nowe U, Fiebich B. The pyrrole moiety as a template for COX-1/COX-2 inhibitors. European Journal of Medicinal Chemistry. 2000;35(5):499–510. doi: 10.1016/s0223-5234(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 57.Evans MA, Smith DC, Holub JM, et al. Synthesis and cytotoxicity of substituted ethyl 2-phenacyl-3-phenylpyrrole-4-carboxylates. Archiv der Pharmazie. 2003;336(3):181–190. doi: 10.1002/ardp.200390018. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Li X, Wu F, Wan B. A new type of bis(sulfonamide)-diamine ligand for a Cu(OTf)2-catalyzed asymmetric Friedel-Crafts alkylation reaction of indoles with nitroalkenes. Organic Letters. 2011;13(18):4834–4837. doi: 10.1021/ol201914r. [DOI] [PubMed] [Google Scholar]
- 59.Collee JG, Duguid JP, Fraser AG, Marmion BP, Scott AC. Mackie and MacCartney Practical Medical Microbiology. 13th edition. Vol. 2. London, UK: Churchill Livingstone; 1989. Laboratory control of antimicrobial therapy; pp. 161–181. [Google Scholar]
- 60.Lange NA, Hambourger WE. Condensation of aromatic aldehydes with nitromethane in the presence of alcoholic sodium hydroxide. Journal of the American Chemical Society. 1931;53(10):3865–3867. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scan copies of 1H and 13C spectra of the Michael adducts 7a-i, 8a-I, 9a-d, & 9h


