Abstract
Tobacco smoke contains multiple classes of established carcinogens including benzo(a)pyrenes, polycyclic aromatic hydrocarbons, and tobacco specific nitrosamines. Most of these compounds exert their genotoxic effects by forming DNA adducts and generation of reactive oxygen species, causing mutations in vital genes like K-Ras and p53. In addition, tobacco specific nitrosamines can activate nicotinic acetylcholine receptors (nAChRs) and to a certain extent β-Adrenergic receptors (β-ARs), promoting cell proliferation. Further, it has been demonstrated that nicotine, the major addictive component of tobacco smoke, can induce cell cycle progression, angiogenesis, and metastasis of lung and pancreatic cancers. These effects occur mainly through the α7-nAChRs, with possible contribution from the β-ARs and/or epidermal growth factor receptors (EGFRs). This review article will discuss the molecular mechanisms by which nicotine and its oncogenic derivatives such as NNK (4-methylnitrosamino)-1-(3-pyridyl)-1-butanone) and NNN (N-nitrosonornicotine) induce cell cycle progression and promote tumor growth. A variety of signaling cascades are induced by nicotine through nAChRs, including the MAPK/ERK pathway, PI3K/AKT pathway and JAK/STAT signaling. In addition, studies have shown that nAChR activation induces Src kinase in a β-arrestin-1 dependent manner, leading to the inactivation of Rb protein and resulting in the expression of E2F1-regulated proliferative genes. Such nAChR-mediated signaling events enhance the proliferation of cells and render them resistant to apoptosis induced by various agents. These observations highlight the role of nAChRs in promoting the growth and metastasis of tumors and raise the possibility of targeting them for cancer therapy.
Introduction
Lung cancer is the leading cause of cancer related deaths worldwide for both men and women, exceeding that of breast, prostate, and colon cancer combined (1). Smoking is by far the greatest and most preventable risk factor for lung cancer, accounting for approximately 70% of non-small cell lung cancer (NSCLC) cases and 90% of small cell lung cancer (SCLC) cases (2), although there is a subset of patients who develop lung cancer without a history of smoking (3). Tobacco smoke contains multiple classes of carcinogens such as polycyclic aromatic hydrocarbons, tobacco specific nitrosamines, and aldehydes which are capable of initiating tumorigenesis (2, 4–6), primarily through the formation of DNA adducts resulting in mutations of vital genes such as KRAS, p53, and Rb (7). Nicotine, which is the addictive component of tobacco smoke, is unable to initiate tumorigenesis in humans and rodents; at the same time, nicotine has been shown to promote tumor growth and metastasis by inducing cell cycle progression, epithelial-to-mesenchymal transition (EMT), migration, invasion, angiogenesis, and evasion of apoptosis in a variety of systems (8–13). In addition, nicotine has been shown to induce secretion of growth factors and cytokines altering the physiology of multiple organ systems (8–13). These observations suggest that nicotine likely contributes to the progression and metastasis of tumors that are initiated by tobacco carcinogens.
Nicotine is thought to promote tumor progression through the binding to and activation of cell surface receptors, especially nicotinic acetylcholine receptors (nAChRs), and to a certain extent β-adrenergic receptors (β-ARs) (14–16). In addition to nicotine, its oncogenic derivatives NNK (4-methylnitrosamino)-1-(3-pyridyl)-1-butanone) and NNN (N-nitrosonornicotine) present in tobacco smoke can bind to and activate nAChRs, stimulating multiple cancer-promoting signaling cascades (16, 17). The mutagenic effects of tobacco-specific nitrosamines are mainly mediated by diffusion through the cell membrane in a receptor-independent fashion (18); at the same time, the signaling events induced by these agents through nAChRs are also thought to contribute significantly to the oncogenic process. Further, while acetylcholine (Ach) is the physiological ligand for nAChRs, nicotine, NNK and NNN can bind these receptors with greater affinity than Ach and can displace Ach, thus altering their normal function (19).
nAChR function in non-neuronal cells
nAChRs are widely expressed on neuromuscular junctions and in the central and peripheral nervous systems where they function as classical ligand-gated ion channels that facilitate calcium influx, resulting in release of neurotransmitters such as γ-aminobutyric acid (GABA), dopamine and serotonin responsible for nicotine addiction (20). More recently, these receptors have also been shown to be expressed on non-neuronal cells of epithelial and endothelial origin, including lung cancer cells, where they mediate the synthesis and release of growth factors, pro-angiogenic factors as well as neurotrophic factors (15, 16, 21, 22). nAChRs are comprised of five subunits which form hetero- or homomeric pentamer channels composed of either five identical α subunits (α 7,α8 or α9), or combinations of α and β subunits (α2–α6, or α10 subunits together with β2–β4 subunits) (17, 22, 23). To date, nine different types of α subunits (α2–α10) and three types of β subunits (β2–β4) have been cloned and characterized (20); they preferentially bind (−)nicotine than (+)nicotine with about 40 fold more affinity. While multiple nAChR subunits are expressed on non-neuronal cells, the homomeric α7 nAChR has been implicated as the primary receptor facilitating nicotine and NNK mediated cell proliferation. Interestingly, the expression of the α7 receptor itself has been shown to increase in response to nicotine stimulation. The proliferative effects of nicotine are reversed by the α7 antagonist α-bungarotoxin (α-BT) or α cobratoxin (α-CBT), suggesting that the α7 nAChR might present a potential target for cancer therapy (11, 16, 21). In contrast to α7 nAChR, the heteromeric α4β2 receptor regulates growth inhibitory responses including the release of GABA, which typically acts as a tumor suppressor in a number of cancers including lung cancers (17). Both nAChR types are upregulated when exposed to chronic nitrosamine or nicotine stimulation; however, the inhibitory α4β2 nAChRs are desensitized due to constant high affinity stimulation, while the tumor promoting α7 nAChRs are able to remain active to exert its biological function. This is likely due to its lower affinity for these ligands and thus lesser chance of desensitization compared to the α4β2 receptors (24). This may also be one of the reasons that nicotine exerts its cancer promoting effects primarily through the α7 receptors.
Early studies demonstrated that the stimulation with nicotine or tobacco specific nitrosamines such as NNK could induce proliferation in multiple in vitro cell culture models, and this effect could be abrogated by nAChR antagonists such hexamethonium bromide (HEX), mecamylamine, and α-BT (11, 25). The concentration of nicotine used in studies investigating the effects of these tobacco components have ranged from 10nM to 10µM, which is similar to the concentration observed in the bloodstream of smokers; the average steady state level in serum is typically 200nM and can spike to 10µM or more immediately after smoking (26, 27). Similar results to those obtained in vitro have also been demonstrated in in vivo mouse models of lung cancer, where nicotine significantly increased the size and number of tumors forming in the lung, and enhanced metastasis (11). Comparable results have been reported in the case of pancreatic cancer as well (10, 28, 29).
Additional support for the correlation between nAChRs, smoking, and cancer was obtained from genome-wide association studies (GWAS), which identified a lung cancer susceptibility locus on chromosome 15q24-25. Polymorphisms in this region correlated with increased risk for lung cancer development, nicotine dependence, and number of cigarettes smoked per day (30). 15q24-25 contains CHRNA3, CHRNA5, and CHRNB4 genes that encode for the α3, α5, and β4 subunits of nAChRs (31, 32). The α5 subunit has been implicated as the primary receptor involved in smoking addiction, and has more recently been associated with smoking behavioral patterns (31). Further, a non-synonymous variation in CHRNA5 (D398N) is strongly associated with increased lung cancer risk (31).
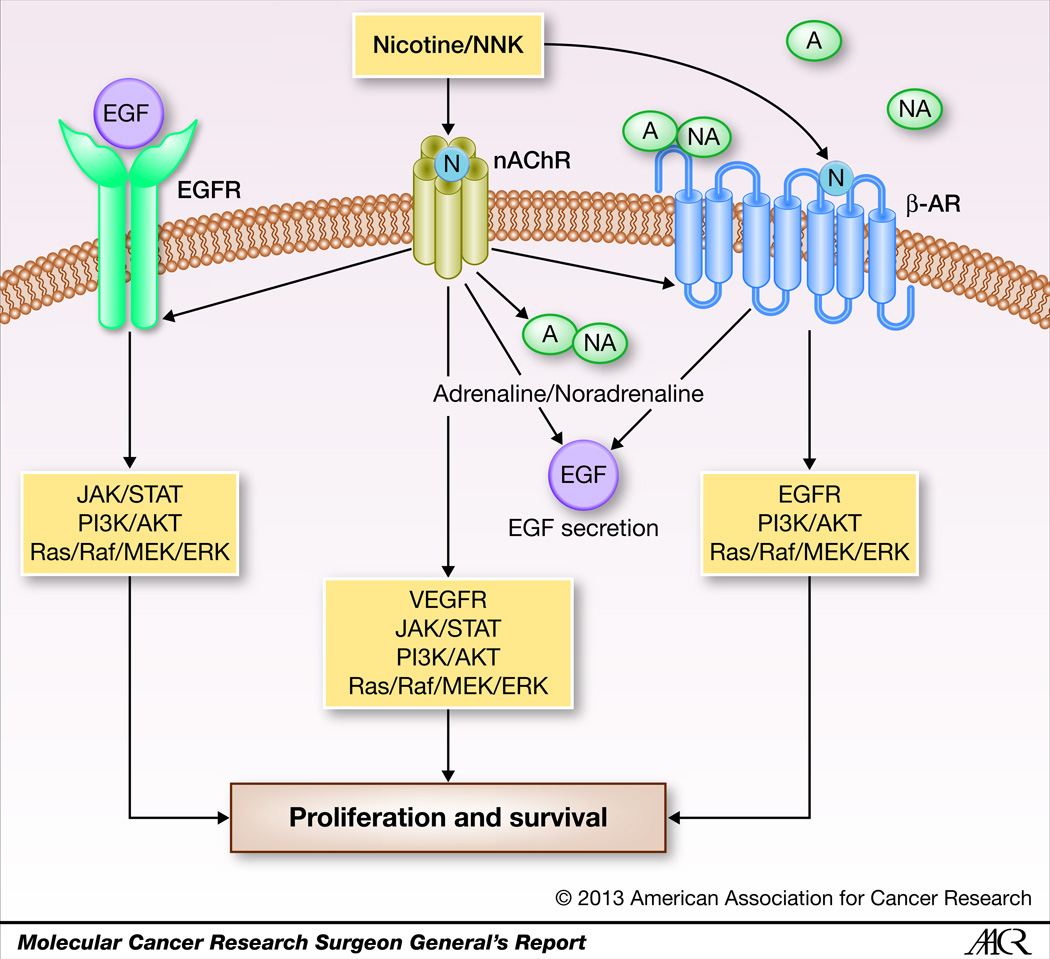
While nAChRs are the primary receptors through which nicotine exerts its effects, β-ARs and EGFRs are also thought to play a role (17, 33, 34). It has been suggested that nAChRs might functionally network with β-AR and EGFR, and all three receptors frequently co-exist on human lung cancer cells, airway endothelial cells, and airway epithelial cells (35). Nicotine binds to α7 nAChRs inducing the secretion of growth factors such as EGF, neurotransmitters such as adrenaline and noradrenaline, and angiogenic factors such as VEGF (34). The nicotine mediated secretion of EGF via nAChRs results in the transactivation of EGFR, leading to the activation of mitogenic and antiapoptotic pathways (16, 36). Similarly, the nicotine mediated release of adrenaline and noradrenaline, which are the physiological ligands for β-AR, leads to the binding to and activation of β-AR, which in turn activates proliferative pathways and the release of EGF, VEGF, and arachidonic acid, which contribute to the development and progression of lung cancer (37). Interestingly, nicotine and NNK have been shown to bind to β-ARs as well, stimulating multiple oncogenic and mitogenic signaling cascades (17, 22, 37). This cooperative interplay between these receptor types can be expected to have significant mitogenic effects on cell cycle machinery, contributing to the oncogenic process. The interplay between the different receptors that respond to nicotine and its derivatives is shown in Figure 1.
Figure 1.
Schematic representation of cooperative receptor crosstalk upon nicotine stimulation. Components of tobacco smoke induce nAChR signaling, which in turn activates additional cell surface receptors such as β-AR and EGFR, stimulating tumor promoting signaling cascades. In addition, upon NNK or nicotine binding, nAChRs are activated stimulating the secretion of growth factors such as EGF which activate EGFRs, as well as neurotrophic factors such as adrenaline and noradrenaline which bind to and activate β-ARs. Upon activation, β-ARs further stimulate secretion of EGF to further transactivate EGFRs. This receptor crosstalk suggests a cooperative interaction that facilitates tobacco induced cancer progression.
Induction of ERK/MAPK signaling cascade by nAChRs
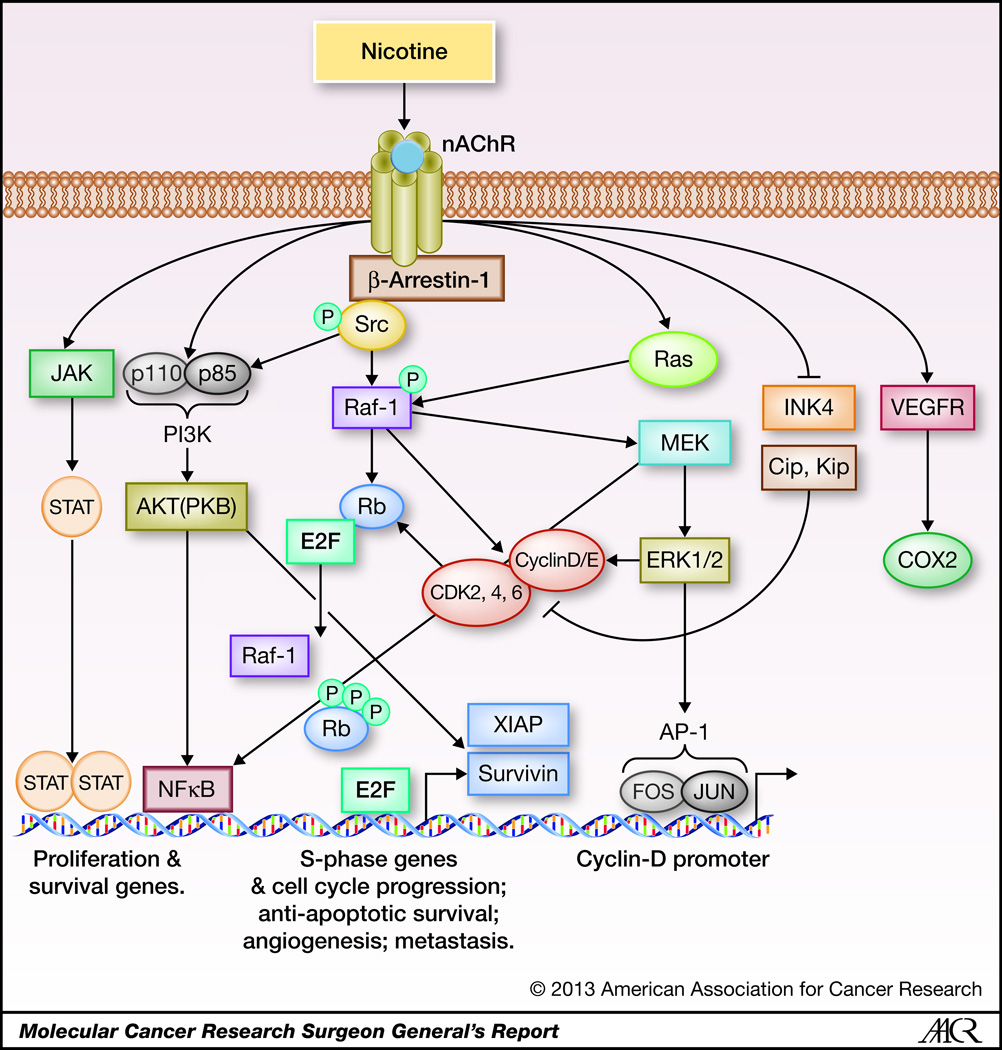
A number of studies have been conducted to elucidate the molecular mechanisms involved in nicotine and nitrosamine mediated cell proliferation, and these have revealed the involvement of multiple signaling cascades downstream of the nAChRs; the major pathways are depicted in Figure 2. One signaling cascade that is induced by nAChRs across various cell types is the MAPK cascade, which is known to facilitate cell proliferation in a wide variety of tumor types. For example, stimulation of SCLC cells with nicotine was found to induce the MAPK cascade in a dose-dependent fashion; this was diminished upon treatment with the nAChR inhibitor mecamylamine (38). Similar results were reported in another study on SCLC and NSCLC cells, where proliferation was increased and MAPK signaling was activated in response to nAChR stimulation; interestingly, this study demonstrated that the ERK2 isoform is specifically activated in response to nicotine. This activation of ERK2 occurs concomitantly, albeit separately, to the activation of PKC, as seen by a combination of western blotting experiments and MAP kinase assays (39). Multiple studies done on human and murine lung cancer cells have further demonstrated that PKC activity was increased in response to nicotine, and was activated independent of the Ras pathway (12, 40–42). Additionally, it was demonstrated that nicotine had no apparent effect of the levels of the MAPK family members p38 or JNK, or on the MAPK phosphatases PAC1 and MKP-1, indicating that these had lesser roles in nicotine mediated activation of MAPK signaling (39). Comparable to this finding on nicotine stimulation, it was found that stimulation of SCLC cells or neuroendocrine cells with the nitrosamine NNK resulted in increased DNA synthesis and proliferation, as well as activation of Raf-1 kinase; this activation could be blocked by inhibition of PKC or by the α7 nAChR antagonist α-BT (43). These results indicate that the observed effects were mediated through the α7 receptor, and that PKC acts upstream of Raf-1 in this signaling sequence (43). Further, it was demonstrated that NNK stimulation increased the expression of c-Myc protein, suggesting that c-Myc is another target upregulated by NNK, probably through the activation of the MAPK cascade (43). A number of additional studies have validated these results, demonstrating that nicotine and NNK activate the MAPK cascade to induce cellular proliferation, and this can be inhibited by α-BT indicating that this chain of events is mediated specifically by the α7 nAChR. This is true for both cancer cells as well as normal cells (15, 36, 44).
Figure 2.
A schematic representing nAChR signaling cascades in lung cancer. NNK and nicotine found in tobacco smoke bind to nAChRs with high affinity and induce multiple signaling cascades resulting in cycle progression, proliferation, and survival. Upon activation of nAChRs by ligand binding, β-arrestin-1 is recruited to the receptor and is necessary for further recruitment of Src kinase, which in turn initiates Raf-1 and PI3K/AKT signaling cascades. NNK and nicotine mediated nAChR activation have also been shown to induce signaling cascades such as JAK/STAT and Ras/Raf/MAPK, and decrease the levels of cell cycle inhibitors like p16INK4 and Cip/Kip proteins.
nAChR-mediated activation of Src and the Rb-E2F pathway
The above studies collectively demonstrate the ability of nicotine and NNK to induce the MAPK signaling cascade through the activation of nAChRs; however, unlike growth factor receptors, nAChRs do not possess intrinsic kinase activity (45, 46). A study designed to understand how α7-nAChRs induce MAPK cascade and promote cell proliferation demonstrated that the scaffolding protein β-arrestin-1 is recruited to the receptor upon nicotine stimulation and this recruitment was necessary for the further recruitment and activation of Src Kinase (47). Src family kinases are known to facilitate signaling from cell surface receptors that lack intrinsic kinase activity and inhibition or depletion of Src could prevent nAChR-mediated cell proliferation (47). This study further demonstrated that the activation of Src upon nAChR stimulation leads to the direct binding of the Raf-1 kinase to the Rb tumor suppressor protein, preceding the cyclin-cdk mediated inactivation of Rb. In vitro kinase assays showed an increase in the activity of kinases associated with cyclins D and E when cells were stimulated with 1µM nicotine; further, ChIP assays showed that Rb dissociated from proliferative promoters like Cdc6 and Cdc25A upon nicotine stimulation, leading to their expression (47). The binding of Raf-1 to Rb is thought to facilitate the inactivation of Rb by cyclins and cdks, and Raf-1-Rb interaction was found to be elevated in NSCLC tumor samples compared to adjacent normal tissue, indicating a potential role for this interaction in tumorigenesis (47). Indeed, a disruptor of the Rb-Raf-1 interaction was found to prevent the nicotine-mediated growth and metastasis of lung and pancreatic tumors in mouse xenograft models (48–50).
Interestingly, a subsequent study showed that stable depletion of β-arrestin-1 using shRNA resulted in decreased cell proliferation with a corresponding decrease in levels of phosphorylated Src kinase and ERK1/2 upon nicotine stimulation in comparison to control cells, indicating a predominant role for β-arrestin-1 in nicotine mediated activation of these signaling events (51). The same study investigated the sequence of signaling events induced by nicotine, and it was found that phospho-Src levels were increased within 5 minutes of nicotine stimulation, while phospho-AKT1 and phospho-ERK1/2 levels increased after 15 minutes, suggesting that the latter two kinases are activated subsequent to Src activation (51). It was additionally found that a subset of β-arrestin-1 molecules translocate into the nucleus upon nicotine stimulation where it physically associates with E2F1; β-arrestin-1 recruited the histone acetyl transferase p300 to E2F-regulated proliferative and pro-survival promoters, facilitating histone H3 acetylation and gene expression (51). The potential role for β-arrestin-1 in lung tumorigenesis was further suggested by the detection of elevated levels of β-arrestin-1 on proliferative promoters in human tumor samples compared to adjacent normal tissues. These observations reveal that activation of the Rb-E2F cascade is a major consequence of the nAChR mediated signaling events and that β-arrestin-1 contributes to this process significantly. The involvement of β-arrestin-1 also suggests an additional functional link between nAChRs and G-protein coupled receptors like β-ARs and opioid receptors upon exposure to nicotine, which might have a significant impact on the growth of lung cancer in smokers.
Induction of the JAK-STAT pathway by nAChR signaling
In addition to activation of the MAPK and Rb-E2F pathways, it has been observed that nicotine stimulation results in the activation of the JAK-STAT signaling pathway and its target genes, which are altered in multiple cancer types (29, 52, 53). In a study conducted on oral keratinocytes, stimulation of cells with physiologically relevant concentrations of either nicotine or sidestream cigarette smoke resulted in increased STAT3 mRNA and protein levels; these levels were diminished if the cells were pretreated with the α7 nAChR specific inhibitor α-BT, or if α7 nAChR was depleted using siRNA (54). Further experiments utilizing specific pathway inhibitors revealed that the α7 dependent upregulation of STAT3 occurred through activation of the Ras-Raf-1-MEK-ERK pathway, as well as through the activation of JAK2, indicating the involvement of two complementary pathways (54). In α7 null mouse cells, nicotine was unable to induce Ras mediated activation of STAT3, further demonstrating that this signaling event is mediated through the α7 nAChR (54). In a separate study done on NSCLC, stimulation with physiologically relevant concentrations of nicotine or NNK resulted in increased phospho-STAT3 levels, leading to an induction of the STAT3 target IKBKE (53). Src kinase is known to activate the JAK-STAT pathway in multiple systems, and nicotine was shown to activate Src kinase which in turn activated the JAK/STAT pathway signaling in cooperation with MEK/ERK1/2 pathway resulting in increased proliferation of pancreatic cancer cells (29). In the same study, nicotine stimulation resulted in increased expression of phospho-JAK2 and enhanced activation of STAT3 through phosphorylation at Y705 in a dose dependent manner, while total STAT3 levels remained unchanged. These effects were abrogated by treatment with the antagonist α-BT, indicating that the signaling was mediated by α7 nAChR (29). It was further demonstrated that nicotine stimulation resulted in an ERK1/2 mediated increase in STAT3 phosphorylation at S727, and this could be reversed by treatment with an ERK inhibitor (29). Immunohistochemical staining of tumors from pancreatic mouse xenograft studies supported these findings, showing increased phosphorylation of STAT3 at Y705 in mice exposed to cigarette smoke (29). Nicotine stimulation was shown to activate STAT3 via nAChRs as well as β-ARs in bladder cancer cells, leading to overexpression of cyclin D1 driving cell cycle progression, resulting in reduced sensitivity to chemotherapy which could be restored by depletion of STAT3 (55). These studies clearly suggest a role for the JAK-STAT pathway in mediating the proliferative functions of nAChRs.
Upregulation of cell cycle regulatory molecules by nAChRs
In addition to inducing multiple mitogenic signaling events, nicotine and nitrosamines have been shown to target various components of the cell cycle machinery itself. Numerous studies have shown that the expression of cyclins as well as proteins involved in cell cycle checkpoints are impacted by nicotine and other components of tobacco smoke. In this context, increased expression of cyclin D1 in response to nicotine stimulation has been reported frequently in the literature (47, 56, 57). Initial studies conducted on mouse lung epithelial cells demonstrated the ability of nicotine to induce cyclin D1 promoter as well as protein expression through nAChR-mediated induction of Ras signaling and activation of its downstream effectors Raf/MAPK (58). It was further demonstrated that Ras activation of c-Jun, which is part of the AP-1 transcription factor complex, binds to the cyclin D1 promoter and is necessary for its nicotine mediated induction (58). The upregulation of cyclin D1 in response to nicotine was shown to occur concomitantly with an increase in Rb phosphorylation and E2F transcriptional activity (58), promoting the cell cycle progression of lung epithelial cells. nAChR-mediated proliferation via upregulation of cyclin D1 has additionally been reported in mouse pre-osteoblasts (59). In normal human lung epithelial cells, nicotine and NNK have been shown to upregulate cyclin D1 through the ERK1/2 mediated activation of the NFκB, and this signal transduction is facilitated via nAChRs (60). Similar results have been reported in human NSCLC cells, where nAChR activation by nicotine stimulation led to an induction of cyclin D-cdk4 and cyclin E-cdk2 kinase activity and Rb phosphorylation, resulting in dissociation of Rb from E2F1 and the induction of E2F1 regulated proliferative genes, including Cdc25A and Cdc6 (47, 57). In addition, nicotine and NNK facilitate cell cycle progression via nAChR and β-AR mediated induction of COX2 and prostaglandin E2 (PGE2), with an associated increase in cyclin D1 and cdk4/6 expression and Rb phosphorylation (61).
Suppression of cell cycle inhibitors by nicotine and NNK
It has been established that components of tobacco smoke can also repress negative regulators of cell cycle progression, such as cdk inhibitors (CDKis) (56, 62). CDKis act to regulate cdk activity and are divided into two classes: the INK4 inhibitors including p16, p15, p19, and p18; and the Cip/Kip inhibitors including p21 and p27 (56, 62). Both CDKi types are capable of arresting cells in G1 phase by inhibition of cdks, preventing their phosphorylation of Rb and thus halting cell cycle progression (56, 62). In human gastric cancer cells, p53, p21, and p27 expression levels are significantly reduced in response to nicotine or NNK stimulation (61). In NSCLC cell lines, nicotine has been shown to induce the transcriptional repressor, ID1, in a Src dependent manner through the α7-nAChR (34). ID1 is known to induce proliferation by inhibiting the transcription of CDKis p16, p21, and p27, preventing their normal growth suppressive effect (63). This data implies that tobacco smoke not only activates components driving cell cycle progression, but also inhibits components that arrest cell cycle.
In addition, it has also been shown in murine and human lung epithelial cells that nicotine acts to compromise activation of normal DNA damage checkpoint response at G1/S, but not the G2/M checkpoint (64). DNA damage check point is activated after exposure to γ-radiation or the tobacco carcinogen benzopyrene (BP), which induces DNA double strand breaks; however, when exposed to nicotine, growth restriction was attenuated due to an increase of cyclins D and A, and a decrease in the phosphorylation of the checkpoint kinase 2 (Chk2) (64). Typically ATM and ATR protein kinases are activated in response to double or single stranded DNA breaks respectively, and they stimulate downstream effectors involved in DNA damage checkpoint response including p53, Chk1, Chk2, and H2AX (62). Activation of these proteins results in cell cycle arrest and DNA repair, or if the damage is beyond repair apoptosis will be induced (62). Once activated, Chk2 phosphorylates Cdc25A phosphatase, preventing it from dephosphorylating and thus activating its target cdk2, resulting in cell cycle arrest (62). The decrease in Chk2 in lung epithelial cells exposed to nicotine suggests that nicotine may be capable of overriding DNA damage checkpoint activation, disrupting genetic surveillance and increasing the risk of oncogenesis (62, 64). These studies show that nicotine as well as tobacco smoke components can modulate multiple components of the cell cycle machinery, facilitating cell cycle progression.
Regulation of survival pathways by nAChRs – anti-apoptotic effects of nicotine
While tobacco smoke carcinogens initiate tumors, the current literature raises the possibility that nicotine additionally confers a survival advantage to already initiated tumors (28, 57, 65, 66) by promoting cell cycle progression and by preventing apoptosis (66, 67). This is of particular concern in the context of tobacco smoke components conferring resistance to chemotherapeutic drugs (66, 67) as well as radiation (68). Multiple studies have reported that patients who smoke demonstrate poor response to cancer chemotherapy, and have worse prognosis than their non-smoking counterparts (65, 69, 70). In this context, many attempts have been made to elucidate the pro-survival and anti-apoptotic effects of nAChR signaling.
The PI3K/AKT pathway is a major cancer-associated signaling network that is activated by exposure to nicotine and tobacco carcinogens (71–75). The serine/threonine kinase AKT is a known regulator of key cellular processes such as cell cycle progression, as well as cell survival (71, 75). Both nicotine and NNK were found to induce AKT phosphorylation at S473 and T308 in a time and dose dependent manner in normal human bronchial epithelial cells and small airway epithelial cells (71). This effect was evident as early as 5 minutes post nicotine stimulation and at a range of physiologically relevant concentrations, from 10nM to 10µM; however, response to NNK was not apparent for 15 minutes but was seen at concentrations as low as 1nM (71). Similarly, downstream substrates of AKT involved in cell cycle progression including GSK-3α, FKHR, and 4EBP-1 were all induced by nicotine, while GSK3-α and 4EBP-1 were induced by NNK (71). Further, this response could be abrogated by the PI3K inhibitor and by the α3/α4 specific nAChR antagonist DHβE, but not by the α7 specific antagonist α-BT, indicating the AKT was being activated through PI3K and this was being facilitated through α3 and α4 containing nAChRs (71). Interestingly, phosphorylated AKT was detected in airway epithelial cells and lung tumors from mice treated with NNK, as well as in human lung cancer samples derived from smokers (71); similar results were observed in a study conducted on NSCLC and SCLC cells (75). In the latter study, stimulation of cells with physiological concentrations of nicotine and NNK induced activation of AKT as well as its downstream substrates GSK-3, FKHR, tuberin, ASK1, 4EBP-1and S6K1; nicotine additionally induced mTOR and MDM2 (75). This study further demonstrated nicotine and NNK could induce proliferation of wild type cells, but not cells transduced with a mutant AKT, indicating that the proliferation seen in response to these tobacco components is AKT dependent (75).
In this context, a direct link between activation of PI3K/AKT pathway by nicotine and induction of chemoresistance has been proposed. Exposure of multiple NSCLC cells to 1 µM nicotine conferred resistance to apoptosis induced by cisplatin, gemcitabine and taxol (57). It was found that exposure to nicotine led to an increase in the levels of inhibitor of apoptosis proteins (IAPs) XIAP and survivin in a α3β4 nAChR dependent manner, downstream of AKT signaling (57, 66); interestingly, nicotine did not induce cIAP1 or cIAP2 (57). AKT mediated phosphorylation is known to prevent the ubiquitination and degradation of XIAP (76); reduced ubiquitination and stabilization of XIAP was observed upon nicotine stimulation, correlating with reduced apoptosis (77). Further, induction of survivin by nicotine occurred at the transcriptional level in an E2F1-dependent fashion, suggesting that exposure to nicotine can confer resistance to apoptosis through multiple mechanisms (57).
NFκB transcription factor is induced by nicotine and nitrosamines, promoting cell survival. Nicotine has been shown to induce NFκB via the MAPK pathway, promoting anti-apoptotic functions in mesothelioma cells, mesothelial cells, normal bronchial airway cells, and small airway epithelial cells (44, 60). Similarly, nicotine was shown to confer a survival advantage to NSCLC and SCLC cells treated with paclitaxel, and this occurred with a concomitant increase in NFκB levels. This study also showed that the ability of nicotine to promote survival was dependent on NFκB, which was activated downstream of AKT (75). In normal human bronchial epithelial cells, NNK was found to induce nuclear translocation of NFκB and increase its DNA binding activity within 5 to 10 minutes of stimulation, reaching maximal activation within 30 minutes (60). It was further demonstrated that the activation of NFκB occurred through the degradation of its negative regulator IκBα, mediated through ERK1/2 phosphorylation of IκBα (60). In colon cancer cells, NNK has been shown to induce NFκB in association with proliferation, specifically the p65/RelA subunit, with a concomitant decrease in IκB, which is known to have inverse correlation to NFκB levels (78). These results strongly suggest that nAChR stimulation enhances NFκB activity by targeting IκBα for degradation.
Additional survival pathways have also been reported to be induced by nicotine. For example, nicotine stimulation could modulate the levels of Bcl-2 family of anti-apoptotic proteins (79). In SCLC cells, nicotine exerts a protective effect against apoptosis induced by cisplatin by inducing the activation of Bcl-2 (66); additionally, nicotine exposure results in the phosphorylation and inactivation of the proapoptotic proteins Bax and Bad, suppressing cell death (57, 79). This inactivation is mediated by the induction of PKC, PKA, MEK, and P13K signaling cascades (9).
In addition, components of the TGF-β signaling cascade are modulated by nicotine to promote survival. It was found that exposure of NSCLC cells and immortalized bronchial epithelial cells to nicotine decreased the expression of SMAD3, resulting in reduced TGF-β mediated growth inhibition (80). Simultaneously, levels of anti-apoptotic Bcl-2 were increased, resulting in increased cell viability and reduced apoptosis. Interestingly, SMAD3 levels were lower in tumor samples from current smokers compared to never-smokers, and withdrawal of smoking reduced SMAD3 levels (80). Another study conducted on NSCLC cells showed that long-term exposure to nicotine resulted in increased half-maximal inhibitory concentration (IC50) of carboplatin (81). This increase in IC50 was associated with elevated Bcl-2 expression and decreased SMAD3 levels (81); the sensitivity to carboplatin was dependent upon this reciprocal relationship (81). These studies clearly show that exposure to nicotine and activation of nAChR signaling not only promotes the proliferative capacity of lung cancer cells, but also renders them resistant to various apoptotic signals and chemotherapeutic agents.
Additional tumor-promoting functions of nAChRs
In addition to inducing proliferative and survival pathways, nicotine and nAChRs have been found to affect subsequent steps in the growth and metastasis of cancers. nAChRs were found to promote the invasion and migration of cells derived from tumors of the breast, pancreas or the lungs (10). Nicotine has been found to be a strong inducer of epithelial-mesenchymal transition, by suppressing the expression of E-cadherin as well as membranous β-catenin; it could also facilitate the transcriptional induction of mesenchymal genes like fibronectin and vimentin in multiple cell lines (10). To a certain extent, these events are regulated through ID-1 (34), while β-arrestin-1 might also play a part in this process. In a similar fashion, nicotine has been found to promote metastasis by upregulating the expression of various matrix metalloproteinases, including MMP9, MMP14 and MMP15; this induction occurred in an E2F1-dependent manner (50).
It has also been proposed that nicotine and nAChRs can contribute to stem-like functions of tumor initiating cells, by inducing embryonic stem cell transcription factors including Oct4 and Nanog (82, 83). Nicotine treatment also enhanced the expression of CD44 and BMI-1, and promoted the sphere formation capacity of squamous cell carcinoma cells (83). Similar results have been reported in oral epithelial cells as well, supporting the contention that exposure to nicotine can promote EMT as well as stemness (82). Ongoing studies in our laboratory have also demonstrated the ability of nicotine to promote the self-renewal of stem-like side-population cells from NSCLC; this occurred through the transcriptional induction of stem cell factor (c-Kit ligand) by nicotine (Perumal et al., manuscript in preparation). While the above studies indicate additional roles for nicotine in promoting EMT and stemness, further studies in this direction would be needed to obtain a complete picture of these events.
nAChRs as potential therapeutic targets for cancer therapy
Given the multifaceted roles of nAChRs in promoting cell proliferation, tumor growth and metastasis, attempts have been made to target nAChRs to combat smoking induced lung cancers (84, 85). As can be imagined, one major concern regarding the use of nAChR antagonists is the potential deleterious effect on the neuromuscular system. Further, many nAChR antagonists are known toxins; these include α-cobratoxin and α-bungarotoxin present in snake venom. Interestingly, attempts have been made in mouse models to use these toxins at low doses to combat xenografted tumors. In one such study, mice which were administered α-CbT intravenously at low doses showed no signs of toxic or lethal effects. Further, there were no histological alterations in the brain tissues examined, and there were no observed differences in neurological responses as measured by testing of the autonomic, convulsive, excitability, neuromuscular, sensorimotor, and general motor activity domains of the brain (85). This study also examined the effect of α-CbT and cisplatin on tumors established in mice; the mice which received cisplatin had 16% improved survival compared to the control mice whereas mice which received 0.12ng/kg of α-CbT had a 90% improved survival compared to the controls, probably due to increased apoptosis of tumor cells (85). In contrast to these observations, a study on five lung cancer cell lines reported conflicting results (86). This study measured tumor growth in in vivo orthotopic mouse models using the same protocols and doses as the prior mentioned study, yet found no significant reduction of tumor growth or survival of mice treated with 0.12ng/kg of either long or short chain α-CbT as compared to controls; and in the case of the short chain α-CbT there was an increase in tumor growth which did not reach statistical significance (86). Interestingly, in the study which found no response to α-CbT, all of the mice in had to be sacrificed at day 28 due to tumor burden, while in the initial study which reported a response to α-CbT the mice were followed up to 170 days. It is not clear why the two studies reported conflicting results; however, it should be noted that a number of additional studies have reported decreased tumor growth in response to α-CbT, although the experimental conditions varied in each study (87–90). These results raise the intriguing possibility that targeting nAChRs, specifically α7 nAChR, might be a viable strategy to combat NSCLC, but a significant amount of additional studies would be needed to pursue this further. Given the potential toxicity of α7 antagonists, and given the neuronal function of these receptors, targeting the downstream effectors of nAChRs might be an alternate approach that is worth considering.
Summary and conclusions
Tobacco smoke is the single greatest preventable risk factor for cancer (2), yet is still responsible for an estimated 160,848 cancer related deaths each year in the United States, let alone globally. The current literature has shed light upon the multiple molecular mechanisms by which components of tobacco smoke can initiate tumor formation, impact cell cycle progression and proliferation, and promote tumor progression in multiple cancer types. In a review of 10 studies, it was found that people who continue to smoke after diagnosis of early stage lung cancer nearly double their risk of dying (91), and cessation of smoking after diagnosis improves multiple aspects of lung cancer including decreased risk of second tumors, increased overall well-being, improved immune response, and increased response to chemotherapeutic agents (69). There are a number of smoking cessation aids available, including varenicline, bupropion as well as various nicotine replacement therapy (NRT) agents.
Given that nicotine has tumor promoting properties, concerns have been raised regarding the deleterious effects of NRT on cancer therapy and survival. There have been limited efforts made to examine how exposure to nicotine through NRT products affects tumor growth or normal physiology including immune function in human subjects. The advantage of NRT is that while tobacco smoke contains literally hundreds of carcinogens, these are absent in NRT products, and hence NRT products are unable to initiate tumors. Supporting this contention, a model was developed to estimate mortality patterns associated with NRT use and success in smoking cessation; this compared the risks and benefits of NRT population-wide taking into account cancer-related safety of these products (92). This study found that the benefits from successful cessation through the use of NRT outweighed the risks in terms of mortality and survival (92). Further, although a significant amount of additional studies need to be conducted to assess whether NRT affects the response to cancer therapy in general, it is reasonable to conclude that nicotine replacement therapy is significantly less harmful than smoking; nevertheless, it might be prudent to reduce the exposure to nicotine in any form for prolonged periods of time.
While smoking cessation is the most effective method to reduce the risk of lung cancer, the elucidation of the signaling mechanisms as well as genetic alterations that facilitate smoking-induced lung cancers has opened up new opportunities to develop novel therapeutic agents that target these pathways. While this process could be increasingly complex and painstakingly long, such efforts could hold promise for the treatment of tobacco-related cancers in the future.
ACKNOWLEDGEMENTS
Studies in the Chellappan lab are supported by the grants CA127725 and CA139612 from the NCI. We thank Smitha Pillai and other lab members for critical comments. Our apologies to the authors whose work could not be cited due to space limitations.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 3.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 5.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 6.Furrukh M. Tobacco Smoking and Lung Cancer: Perception-changing facts. Sultan Qaboos University medical journal. 2013;13:345–358. doi: 10.12816/0003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuller HM, McGavin MD, Orloff M, Riechert A, Porter B. Simultaneous exposure to nicotine and hyperoxia causes tumors in hamsters. Laboratory investigation; a journal of technical methods and pathology. 1995;73:448–456. [PubMed] [Google Scholar]
- 9.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D, et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 13.Sopori M. Effects of cigarette smoke on the immune system. Nature reviews Immunology. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 14.Schuller HM. Beta-adrenergic signaling, a novel target for cancer therapy? Oncotarget. 2010;1:466–469. doi: 10.18632/oncotarget.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2007;20:629–641. doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 18.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 19.Schuller HM. Nitrosamines as nicotinic receptor ligands. Life Sci. 2007;80:2274–2280. doi: 10.1016/j.lfs.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itier V, Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS letters. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Wu CH, Ho YS. From smoking to cancers: novel targets to neuronal nicotinic acetylcholine receptors. J Oncol. 2011;2011:693424. doi: 10.1155/2011/693424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CH, Lee CH, Ho YS. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin Cancer Res. 2011;17:3533–3541. doi: 10.1158/1078-0432.CCR-10-2434. [DOI] [PubMed] [Google Scholar]
- 24.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochemical pharmacology. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 25.Cattaneo MG, Codignola A, Vicentini LM, Clementi F, Sher E. Nicotine stimulates a serotonergic autocrine loop in human small-cell lung carcinoma. Cancer Res. 1993;53:5566–5568. [PubMed] [Google Scholar]
- 26.Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. British medical journal. 1980;280:972–976. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. British medical journal. 1975;4:313–316. doi: 10.1136/bmj.4.5992.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevino JG, Pillai S, Kunigal S, Singh S, Fulp WJ, Centeno BA, et al. Nicotine induces inhibitor of differentiation-1 in a Src-dependent pathway promoting metastasis and chemoresistance in pancreatic adenocarcinoma. Neoplasia. 2012;14:1102–1114. doi: 10.1593/neo.121044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Momi N, Ponnusamy MP, Kaur S, Rachagani S, Kunigal SS, Chellappan S, et al. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene. 2013;32:1384–1395. doi: 10.1038/onc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 32.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature genetics. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ, et al. ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol Cell Biol. 2011;31:3052–3067. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung cancer. 2013 doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Schuller HM. Neurotransmitter receptor-mediated signaling pathways as modulators of carcinogenesis. Prog Exp Tumor Res. 2007;39:45–63. doi: 10.1159/000100045. [DOI] [PubMed] [Google Scholar]
- 37.Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- 38.Cattaneo MG, D'Atri F, Vicentini LM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. The Biochemical journal. 1997;328(2):499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 40.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. The Journal of pharmacology and experimental therapeutics. 1998;287:435–439. [PubMed] [Google Scholar]
- 41.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell growth & differentiation: the molecular biology journal of the American Association for Cancer Research. 1994;5:1033–1040. [PubMed] [Google Scholar]
- 42.Guo J, Chu M, Abbeyquaye T, Chen CY. Persistent nicotine treatment potentiates amplification of the dihydrofolate reductase gene in rat lung epithelial cells as a consequence of Ras activation. J Biol Chem. 2005;280:30422–30431. doi: 10.1074/jbc.M504688200. [DOI] [PubMed] [Google Scholar]
- 43.Jull BA, Plummer HK, 3rd, Schuller HM. Nicotinic receptor-mediated activation by the tobacco-specific nitrosamine NNK of a Raf-1/MAP kinase pathway, resulting in phosphorylation of c-myc in human small cell lung carcinoma cells and pulmonary neuroendocrine cells. Journal of cancer research and clinical oncology. 2001;127:707–717. doi: 10.1007/s004320100289. [DOI] [PubMed] [Google Scholar]
- 44.Trombino S, Cesario A, Margaritora S, Granone P, Motta G, Falugi C, et al. Alpha7-nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res. 2004;64:135–145. doi: 10.1158/0008-5472.can-03-1672. [DOI] [PubMed] [Google Scholar]
- 45.Sharma G, Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 46.Sharma G, Vijayaraghavan S. Nicotinic Receptors: Role in Addiction and Other Disorders of the Brain. Subst Abuse. 2008;2008:81. [PMC free article] [PubMed] [Google Scholar]
- 47.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinkade R, Dasgupta P, Carie A, Pernazza D, Carless M, Pillai S, et al. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–3818. doi: 10.1158/0008-5472.CAN-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trevino JG, Verma M, Singh S, Pillai S, Zhang D, Pernazza D, et al. Selective Disruption of Rb-Raf-1 Kinase Interaction Inhibits Pancreatic Adenocarcinoma Growth Irrespective of Gemcitabine Sensitivity. Molecular cancer therapeutics. 2013 doi: 10.1158/1535-7163.MCT-12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson JL, Pillai S, Pernazza D, Sebti SM, Lawrence NJ, Chellappan SP. Regulation of matrix metalloproteinase genes by E2F transcription factors: Rb-Raf-1 interaction as a novel target for metastatic disease. Cancer Res. 2012;72:516–526. doi: 10.1158/0008-5472.CAN-11-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasgupta P, Rizwani W, Pillai S, Davis R, Banerjee S, Hug K, et al. ARRB1-mediated regulation of E2F target genes in nicotine-induced growth of lung tumors. J Natl Cancer Inst. 2011;103:317–333. doi: 10.1093/jnci/djq541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. Journal of cell science. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 53.Guo J, Kim D, Gao J, Kurtyka C, Chen H, Yu C, et al. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non-small cell lung cancer. Oncogene. 2013;32:151–159. doi: 10.1038/onc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 55.Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldi A, De Luca A, Esposito V, Campioni M, Spugnini EP, Citro G. Tumor suppressors and cell-cycle proteins in lung cancer. Patholog Res Int. 2011;2011:605042. doi: 10.4061/2011/605042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu M, Guo J, Chen CY. Long-term exposure to nicotine, via ras pathway, induces cyclin D1 to stimulate G1 cell cycle transition. J Biol Chem. 2005;280:6369–6379. doi: 10.1074/jbc.M408947200. [DOI] [PubMed] [Google Scholar]
- 59.Sato T, Abe T, Nakamoto N, Tomaru Y, Koshikiya N, Nojima J, et al. Nicotine induces cell proliferation in association with cyclin D1 up-regulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun. 2008;377:126–130. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 60.Ho YS, Chen CH, Wang YJ, Pestell RG, Albanese C, Chen RJ, et al. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol Appl Pharmacol. 2005;205:133–148. doi: 10.1016/j.taap.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Shin VY, Jin HC, Ng EK, Yu J, Leung WK, Cho CH, et al. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol Appl Pharmacol. 2008;233:254–261. doi: 10.1016/j.taap.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 63.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. Journal of cell science. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 64.Nishioka T, Yamamoto D, Zhu T, Guo J, Kim SH, Chen CY. Nicotine overrides DNA damage-induced G1/S restriction in lung cells. PLoS One. 2011;6:e18619. doi: 10.1371/journal.pone.0018619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen RJ, Ho YS, Guo HR, Wang YJ. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci. 2010;115:118–130. doi: 10.1093/toxsci/kfq028. [DOI] [PubMed] [Google Scholar]
- 66.Zeng F, Li YC, Chen G, Zhang YK, Wang YK, Zhou SQ, et al. Nicotine inhibits cisplatin-induced apoptosis in NCI-H446 cells. Med Oncol. 2012;29:364–373. doi: 10.1007/s12032-010-9792-9. [DOI] [PubMed] [Google Scholar]
- 67.Egleton RD, Brown KC, Dasgupta P. Angiogenic activity of nicotinic acetylcholine receptors: implications in tobacco-related vascular diseases. Pharmacol Ther. 2009;121:205–223. doi: 10.1016/j.pharmthera.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK, et al. Nicotinic modulation of therapeutic response in vitro and in vivo. Int J Cancer. 2012;131:2519–2527. doi: 10.1002/ijc.27556. [DOI] [PubMed] [Google Scholar]
- 69.Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78:289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126:347–351. doi: 10.1378/chest.126.2.347. [DOI] [PubMed] [Google Scholar]
- 71.West KA, Castillo SS, Dennis PA. Activation of the PI3K/AKT pathway and chemotherapeutic resistance. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 72.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid AKT activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3'-kinase/AKT pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 74.Nishioka T, Guo J, Yamamoto D, Chen L, Huppi P, Chen CY. Nicotine, through upregulating pro-survival signaling, cooperates with NNK to promote transformation. J Cell Biochem. 2010;109:152–161. doi: 10.1002/jcb.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ, et al. Tobacco components stimulate AKT-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–1195. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 76.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al. AKT phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 77.Cheng YJ, Jiang HS, Hsu SL, Lin LC, Wu CL, Ghanta VK, et al. XIAP-mediated protection of H460 lung cancer cells against cisplatin. European journal of pharmacology. 2010;627:75–84. doi: 10.1016/j.ejphar.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH. Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway. The Journal of pharmacology and experimental therapeutics. 2004;308:66–72. doi: 10.1124/jpet.103.058321. [DOI] [PubMed] [Google Scholar]
- 79.Wen J, Fu JH, Zhang W, Guo M. Lung carcinoma signaling pathways activated by smoking. Chinese journal of cancer. 2011;30:551–558. doi: 10.5732/cjc.011.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samanta D, Gonzalez AL, Nagathihalli N, Ye F, Carbone DP, Datta PK. Smoking attenuates transforming growth factor-beta-mediated tumor suppression function through downregulation of Smad3 in lung cancer. Cancer Prev Res (Phila) 2012;5:453–463. doi: 10.1158/1940-6207.CAPR-11-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samanta D, Kaufman J, Carbone DP, Datta PK. Long-term smoking mediated down-regulation of Smad3 induces resistance to carboplatin in non-small cell lung cancer. Neoplasia. 2012;14:644–655. doi: 10.1593/neo.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu CC, Chang YC. Enhancement of cancer stem-like and epithelial-mesenchymal transdifferentiation property in oral epithelial cells with long-term nicotine exposure: reversal by targeting SNAIL. Toxicol Appl Pharmacol. 2013;266:459–469. doi: 10.1016/j.taap.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 83.Yu MA, Kiang A, Wang-Rodriguez J, Rahimy E, Haas M, Yu V, et al. Nicotine promotes acquisition of stem cell and epithelial-to-mesenchymal properties in head and neck squamous cell carcinoma. PLoS One. 2012;7:e51967. doi: 10.1371/journal.pone.0051967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 85.Paleari L, Negri E, Catassi A, Cilli M, Servent D, D'Angelillo R, et al. Inhibition of nonneuronal alpha7-nicotinic receptor for lung cancer treatment. Am J Respir Crit Care Med. 2009;179:1141–1150. doi: 10.1164/rccm.200806-908OC. [DOI] [PubMed] [Google Scholar]
- 86.Alama A, Bruzzo C, Cavalieri Z, Forlani A, Utkin Y, Casciano I, et al. Inhibition of the nicotinic acetylcholine receptors by cobra venom alpha-neurotoxins: is there a perspective in lung cancer treatment? PLoS One. 2011;6:e20695. doi: 10.1371/journal.pone.0020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paleari L, Cesario A, Fini M, Russo P. alpha7-Nicotinic receptor antagonists at the beginning of a clinical era for NSCLC and Mesothelioma? Drug discovery today. 2009;14:822–836. doi: 10.1016/j.drudis.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 88.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D, et al. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell proliferation. 2008;41:936–959. doi: 10.1111/j.1365-2184.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Grozio A, Paleari L, Catassi A, Servent D, Cilli M, Piccardi F, et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anti-cancer drug development. Int J Cancer. 2008;122:1911–1915. doi: 10.1002/ijc.23298. [DOI] [PubMed] [Google Scholar]
- 90.Catassi A, Paleari L, Servent D, Sessa F, Dominioni L, Ognio E, et al. Targeting alpha7-nicotinic receptor for the treatment of pleural mesothelioma. European journal of cancer. 2008;44:2296–2311. doi: 10.1016/j.ejca.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 91.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Bmj. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Apelberg BJ, Onicescu G, Avila-Tang E, Samet JM. Estimating the risks and benefits of nicotine replacement therapy for smoking cessation in the United States. American journal of public health. 2010;100:341–348. doi: 10.2105/AJPH.2008.147223. [DOI] [PMC free article] [PubMed] [Google Scholar]