Abstract
Epidermal growth factor receptor tyrosine kinase (EGFRtk) and endoplasmic reticulum (ER) stress are important factors in cardiovascular complications. Understanding whether enhanced EGFRtk activity and ER stress induction are involved in cardiac damage, and microvascular dysfunction in type 1 diabetes mellitus is an important question that has remained unanswered. Cardiac fibrosis and microvascular function were determined in C57BL/6J mice injected with streptozotocin only or in combination with EGFRtk inhibitor (AG1478), ER stress inhibitor (Tudca), or insulin for 2 weeks. In diabetic mice, we observed an increase in EGFRtk phosphorylation and ER stress marker expression (CHOP, ATF4, ATF6, and phosphorylated-eIF2α) in heart and mesenteric resistance arteries, which were reduced with AG1478, Tudca, and insulin. Cardiac fibrosis, enhanced collagen type I, and plasminogen activator inhibitor 1 were decreased with AG1478, Tudca, and insulin treatments. The impaired endothelium-dependent relaxation and -independent relaxation responses were also restored after treatments. The inhibition of NO synthesis reduced endothelium-dependent relaxation in control and treated streptozotocin mice, whereas the inhibition of NADPH oxidase improved endothelium-dependent relaxation only in streptozotocin mice. Moreover, in mesenteric resistance arteries, the mRNA levels of Nox2 and Nox4 and the NADPH oxidase activity were augmented in streptozotocin mice and reduced with treatments. This study unveiled novel roles for enhanced EGFRtk phosphorylation and its downstream ER stress in cardiac fibrosis and microvascular endothelial dysfunction in type 1 diabetes mellitus.
Keywords: EGFRtk, ER stress, type 1 diabetes, Tudca, cardiac fibrosis, resistance arteries, endothelial function
Diabetes mellitus is a major cause of morbidity and mortality worldwide and is a threat to human health.1,2 Increasing evidence from experimental and clinical studies indicates a higher prevalence of cardiac damage and microvascular complications in diabetic patients.3–7 Epidermal growth factor receptor (EGFR) is a glycoprotein containing a single transmembrane domain with intracellular portion harboring the tyrosine kinase domain. The EGFRtk is regulated by glucose through EGFR-N-glycosylation,8 and although there is a plethora of information on the growth-promoting effects of EGFR, its role in cardiovascular complications in type 1 diabetes mellitus remains unknown. We and others have demonstrated that increased EGFRtk phosphorylation contributes to resistance artery dysfunction in type 2 diabetes mellitus.9,10 In addition, it has been reported that the inhibition of EGFR activity promotes vasodilatation and reduces elevated arterial blood pressure in hypertensive animal models with or without insulin resistance.11,12
Recently, several studies in different cancer cell lines and human tissues have reported a relationship between aberrant EGFRtk expression-activation and endoplasmic reticulum (ER) stress-related proteins.13,14 ER stress plays a critical role in the pathogenesis of diabetes mellitus and associated cardiovascular complications.15,16 Various cellular stresses, including ischemia, hypoxia, gene mutation, oxidative stress, and protein synthesis overload, lead to impairment of ER function and create a state termed “ER stress” that leads to the activation of a complex signaling network called the unfolded protein response.17,18 The unfolded protein response is regulated in the cell by 3 ER membrane-associated proteins that act as sensors of ER homeostasis. The 3 membrane-bound protein are protein kinase-like ER eukaryotic initiation factor 2α kinase, inositol requiring ER-to-nucleus signaling protein-1α, and activating transcription factor 6 (ATF6). The involvement of ER stress in the development of diseases, such as obesity, stroke, myocardial ischemia, and type 2 diabetes mellitus, has been widely demonstrated and is considered a key element in pancreatic β-cell dysfunction and peripheral insulin resistance.18–23
The significance and role of exacerbated EGFRtk and ER stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus are important questions that have remained unanswered. Thus, the aim of this study was to determine the role of increased EGFRtk activity and its downstream ER stress as important factors in cardiac damage and microvascular dysfunction in the type 1 diabetic mouse model.
Materials and Methods
See the online-only Data Supplement.
Results
Effect of EGFRtk Inhibition on Glucose Level, Body Weight, Cardiac Fibrosis, and ER Stress Markers in the Heart
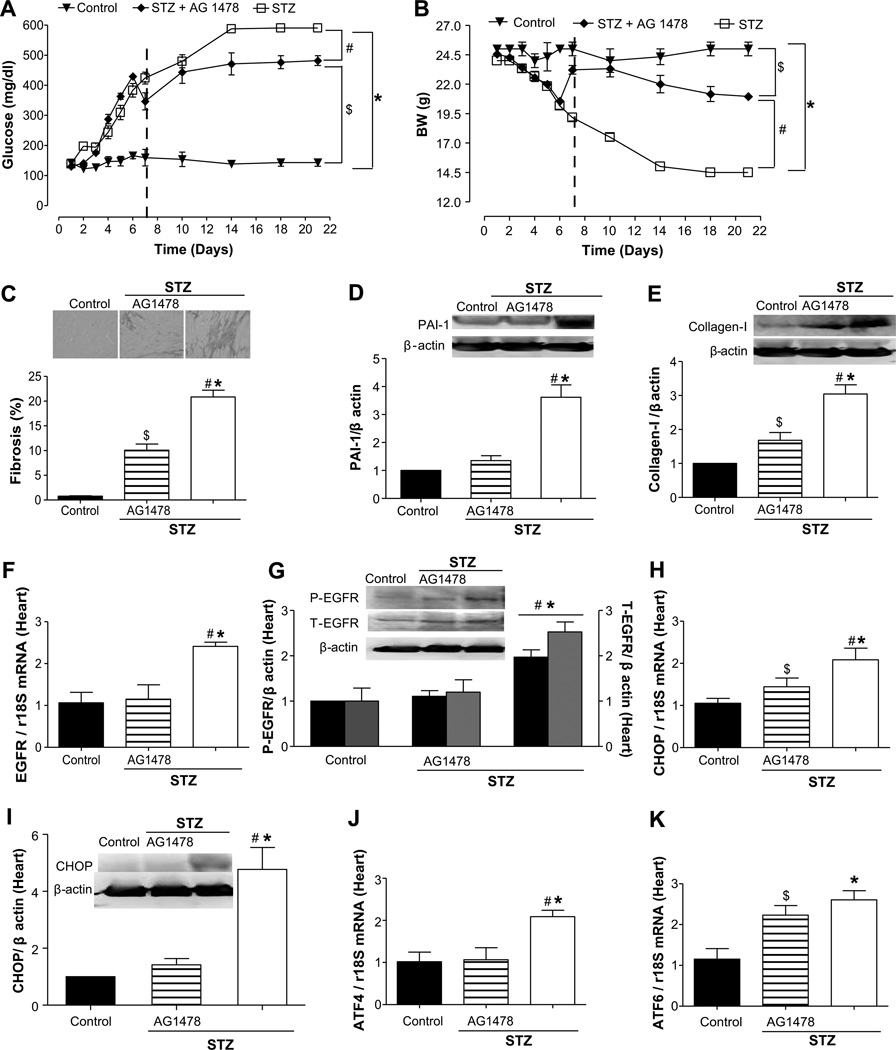
The induction of type 1 diabetes mellitus with streptozotocin (STZ) injection increased blood glucose levels to 408% compared with control mice (100%; Figure 1A), decreased the body weight to 40% (Figure 1B), and induced cardiac fibrosis associated with increased plasminogen activator inhibitor (PAI) 1 (3.5-fold) and collagen type 1 expression (3-fold; Figure 1C through 1E). In STZ mice, the inhibition of EGFRtk reduced blood glucose level to 337% and increased the body weight to 84% (Figure 1A and 1B). Fibrosis was reduced by 50%, and collagen type 1 expression was reduced to 2-fold compared with STZ mice, whereas PAI-1 was blunted (Figure 1C through 1E). Interestingly, the mRNA levels and phosphorylation of EGFRtk were augmented by 2.5- and 2.0-fold, respectively, in cardiac tissue from STZ mice and were significantly reduced after EGFRtk inhibition (Figure 1F and 1G). ER stress marker expression assessed by real-time RT-PCR revealed an increase by 2.0- and 2.5-fold for CHOP, ATF4, and ATF6, respectively, in cardiac tissue from STZ mice and were reduced after EGFRtk inhibition, with the exception of ATF6 (Figure 1H, 1J, and 1K). The protein expression of CHOP was increased by 4-fold in STZ mice and blunted after EGFRtk inhibition (Figure 1I).
Figure 1.
Effect of epidermal growth factor receptor (EGFR) tyrosine kinase (EGFRtk) inhibition on plasma glucose, body weight, fibrosis, EGFRtk, and endoplasmic reticulum (ER) stress markers in heart from streptozotocin (STZ) mice. A and B, Glucose levels (mg/dL) and body weight (BW) were determined in control, STZ, and STZ+AG1478 groups; n=7. The vertical dashed lines denote the start of infusion of AG1478. ▼, control; ◆, STZ+AG1478; □, STZ. C, Representative histological sections from the heart stained with Sirius-red; bars indicate the quantitative data, n=5. D and E, Representative Western blot analysis and quantitative data for plasminogen activator inhibitor (PAI) 1 and collagen I in heart in all groups, n=4 to 5. F and G, EGFR mRNA levels, n=5 and representative Western blot analysis and quantitative data for phosphorylated EGFR and total EGFR in all groups; n=5. H and I, CHOP mRNA levels, n=5, and CHOP representative Western blot and quantitative data in all groups, n=3. J and K, ATF-4 and ATF-6 mRNA levels, normalized to 18S rRNA, in all of the groups, n=5. *P<0.05 for STZ vs control; #P<0.05 for STZ vs STZ+AG1478; $P<0.05 for STZ+AG1478 vs control.
Effect of ER Stress Inhibition on Glucose Level, Body Weight, Insulin, Cardiac Fibrosis, and ER Stress Markers in the Heart
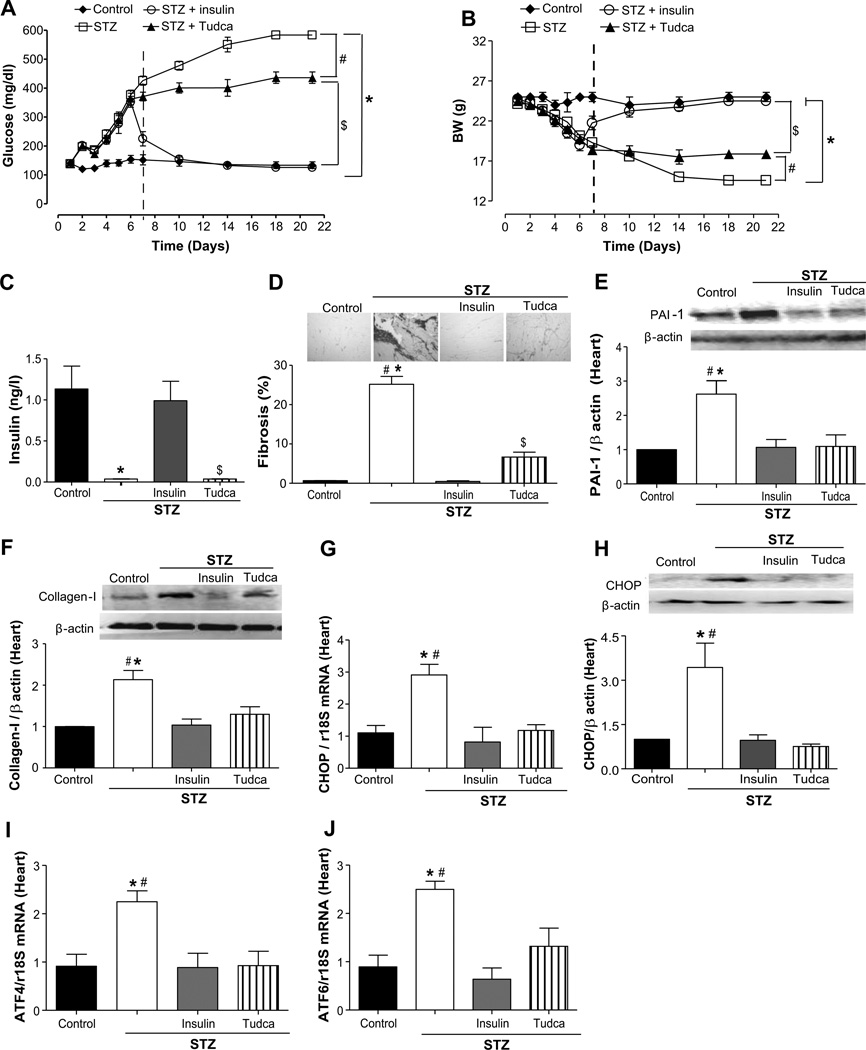
The injection of STZ significantly increased blood glucose concentration to 436% compared with control mice (100%) and was significantly reduced to 326% after ER stress inhibition and normalized with insulin injection (94%; Figure 2A). The ER stress inhibition increased body weight from 58% in STZ mice to 72%, whereas the treatment with insulin completely restored the body weight to control mice value (Figure 2B). Insulin level was not detectable in STZ mice treated with or without Tudca (0.036±0.0 and 0.035±0.0 ng/L, respectively) but was restored with insulin treatment (0.99±0.2 ng/L; Figure 2C).
Figure 2.
Effect of endoplasmic reticulum (ER) stress inhibition on plasma glucose, body weight, plasma insulin, cardiac fibrosis, and ER stress markers expression in heart from streptozotocin (STZ) mice. A and B, Glucose levels (mg/dL) and body weight (BW) were determined in control, STZ, STZ+insulin, and STZ+Tudca groups; n=10. The vertical dashed lines denote the start of the injection of insulin or Tudca. ◆, control; ○, STZ+insulin; □, STZ; ▲, STZ+Tudca. C, Plasma insulin levels (ng/dL) in all groups, n=10. D, Representative histological sections from the heart stained with Sirius-red, bars indicate the quantitative data, n=5. E and F, Representative Western blot analysis and quantitative data for plasminogen activator inhibitor (PAI) 1 and collagen I in heart from all of the groups, n=4 to 5. G and H, CHOP mRNA levels, n=5, and CHOP representative Western blot and quantitative data in all groups, n=3. I and J, ATF-4 and ATF-6 mRNA levels, normalized to 18S rRNA, in all groups, n=5 to 6. *P<0.05 for STZ vs control or STZ+insulin, #P<0.05 for STZ vs STZ+Tudca, $P<0.05 for STZ+Tudca vs control.
We observed cardiac fibrosis induction in STZ mice evidenced by an increase in collagen type I deposition and an increase by 2.5- and 2.0-fold in PAI-1 and collagen type 1 expression, respectively, which were blunted with Tudca and insulin treatments (Figure 2D through 2F). The cardiac fibrosis in STZ mice was associated with ER stress evidenced by augmented mRNA levels of ATF4 (2.5-fold), CHOP (3.0-fold), and ATF6 (2.5-fold), which were reduced with Tudca and insulin treatment to control levels (Figure 2G, 2I, and 2J). The expression of CHOP was increased by 3.5-fold in STZ mice and was blunted after insulin treatment and ER stress inhibition (Figure 2H). In addition, Western blot analysis in heart showed no changes in EGFRtk phosphorylation in the STZ group compared with the STZ+Tudca group (Figure S1A).
Effect of EGFRtk Inhibition on Vascular Relaxation and ER Stress Markers in Mesenteric Resistance Arteries
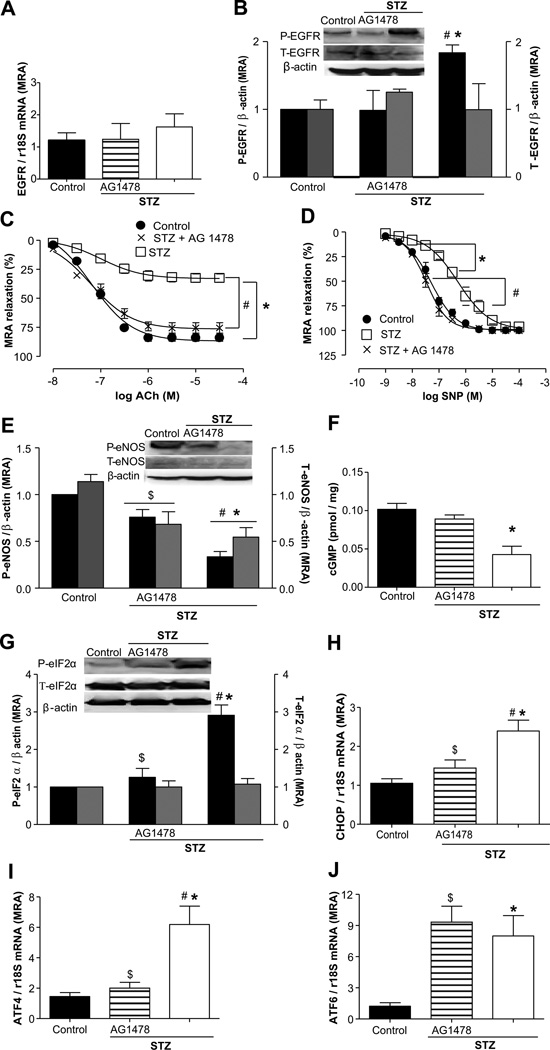
The EGFRtk expression in mesenteric resistance arteries (MRAs) assessed by Western blot and real-time RT-PCR revealed no changes in all of the groups of mice (Figure 3A and 3B), whereas phosphorylated EGFRtk was 2-fold increased in MRA from STZ mice compared with control and diabetic mice treated with the EGFRtk inhibitor (Figure 3B).
Figure 3.
Effect of epidermal growth factor receptor (EGFR) kinase inhibition on EGFR expression, endothelial function, and endoplasmic reticulum (ER) stress markers in mesenteric resistance arteries (MRAs) from streptozotocin (STZ) mice. A, EGFR mRNA levels in all groups, n=5. B, Representative Western blot analysis and quantitative data for phosphorylated EGFR and total EGFR in all groups, n=3. C, Endothelium-dependent relaxation in response to acetylcholine (ACh) in MRA from control, STZ+AG1478, and STZ groups, n=5. D, Endothelium-independent relaxation in response to single nucleotide polymorphism in MRAs from all groups, n=5. ●, control; ×, STZ+AG1478; □, STZ. E, Representative Western blot analysis and quantitative data showing the phosphorylated endothelial NO synthase eNOS (P-eNOS) and total eNOS (T-eNOS), normalized to β-actin, in MRAs from all groups, n=4. F, Cyclic GMP levels in all the groups, n=4. G through J, Representative Western blot analysis showing the expression of phosphorylated eIF2-α, normalized to β-actin; total eIF2-α, n=4; and ATF-4, CHOP, and ATF-6 mRNA levels, normalized to 18S rRNA, in all groups, n=5. *P<0.05 for STZ vs control, #P<0.05 for STZ vs STZ+AG1478, and $P<0.05 for STZ+AG1478 vs control.
To determine the role of EGFRtk in microvascular dysfunction in type 1 diabetes mellitus, we examined the endothelium-dependent relaxation (EDR) response in MRA from STZ mice infused with and without EGFRtk inhibitor. EDR was significantly decreased in STZ mice (33.6%) compared with control (84%; Figure 3C) and was associated with reduced endothelial NO synthase (eNOS) expression and phosphorylation and cGMP levels (Figure 3E and 3F). Endothelium-independent relaxation was shifted to the right in STZ mice (EC50=6.3±0.07) compared with control mice (EC50=7.29±0.03; Figure 3D). Importantly, the inhibition of EGFRtk in STZ mice improved EDR (76.6%), restored endothelial independent sensitivity (EC50=7.47±0.04), increased by 2-fold eNOS phosphorylation, and restored cGMP levels to control in MRAs (Figure 3C through 3F).
The phosphorylation of eIF2α and mRNA levels of CHOP, ATF4, and ATF6 were increased by 3.0-, 2.5-, 4.0-, and 7.0-fold, respectively, in MRAs from STZ mice compared with control mice and were reduced after EGFRtk inhibition by 2.5-, 1.6-, and 2.8-fold, respectively, with the exception of the mRNA level of ATF6 (Figure 3F through 3I). The total eIF2α protein expression was similar in all of the groups of mice.
Effect of ER Stress Inhibition on Microvascular Function in MRAs
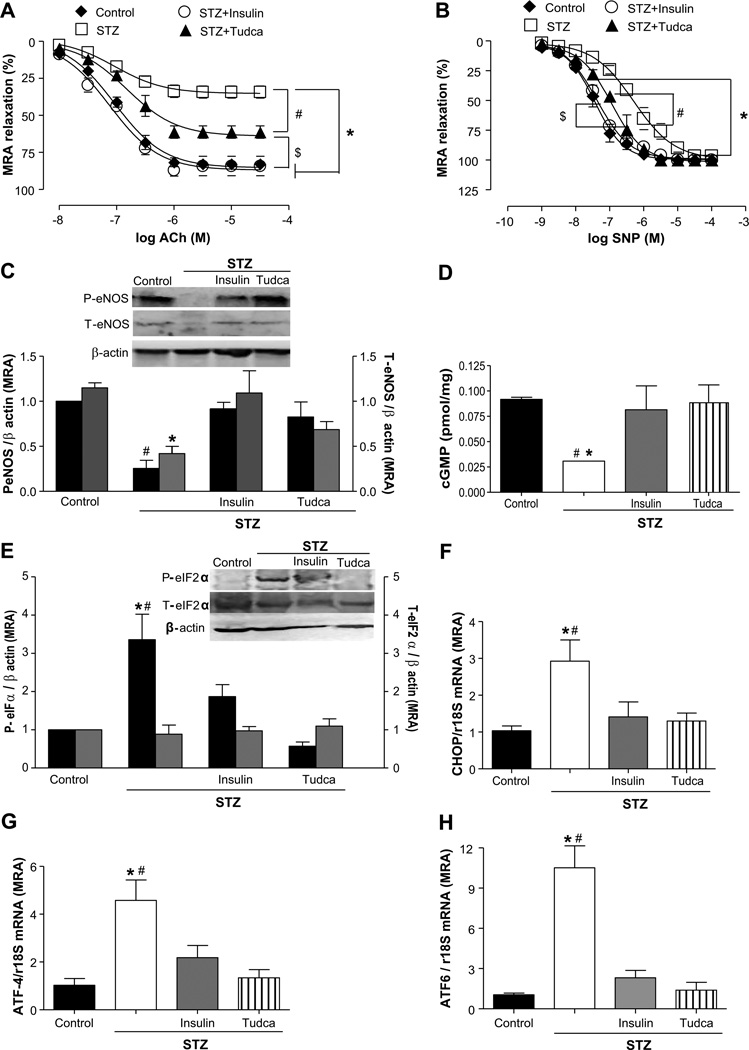
To delineate the role of ER stress in microvascular dysfunction in type 1 diabetes mellitus, we first examined EDR response in MRAs from STZ mice with or without Tudca or insulin. EDR was significantly improved in STZ mice treated with Tudca or insulin (61.3% and 84.3%, respectively; Figure 4A). Endothelium-independent relaxation was shifted to the right in STZ mice (EC50=6.27±0.05) and was normalized after ER stress inhibition (EC50=6.94±0.05) or insulin injection (EC50=7.43±0.06; Figure 4B). These results were supported with the measurements of eNOS phosphorylation and expression and cGMP level, which were decreased by 4.0-, 3.0-, and 2.5-fold, respectively, in STZ mice and were normalized with Tudca and insulin treatments (Figure 4C and 4D). Microvascular endothelial dysfunction in STZ mice was associated with ER stress induction, as evidenced by enhanced phosphorylated eIF2α (4-fold) and the increase in the mRNA levels of ATF4 (4.5-fold), CHOP (3.0-fold), and ATF6 (9.0-fold; Figure 4E through 4H). Interestingly, insulin and ER stress inhibition (Tudca) were able to significantly reduce ER stress marker expression in MRAs from STZ mice (Figure 4E through 4H). The total eIF2α protein expression was similar in all of the groups of mice. In addition, EGFRtk phosphorylation in MRAs was similar in the STZ and STZ+Tudca groups (Figure S1B). The inhibition of NO-synthesis (NG-nitro-l-arginine methyl ester; 100 µmol/L) reduced EDR by 17.3% in STZ mice, whereas a great reduction was observed in control and STZ mice treated with AG1478 (40%), Tudca (40%), or insulin (64%; Figure 5A through 5E).
Figure 4.
Effect of endoplasmic reticulum (ER) stress inhibition on endothelial function and ER stress markers expression in mesenteric resistance arteries (MRAs) from streptozotocin (STZ) mice. A and B, Endothelium-dependent and independent relaxation in response to acetylcholine (ACh) and single nucleotide polymorphism (SNP), respectively, in MRAs from control, STZ, STZ+insulin, and STZ+Tudca groups, n=5. ◆, control; □, STZ; ○, STZ+insulin; ▲, STZ+Tudca. C, Representative Western blot analysis and quantitative data showing the phosphorylated eNOS (P-eNOS) and total eNOS (T-eNOS), normalized to β-actin, in all groups, n=4. D, Cyclic GMP levels in all the groups, n=4. E through H, Representative Western blot and quantitative data showing the expression of phosphorylated eIF2-α, normalized to β-actin, and total eIF2-α protein in all groups, n=4, and CHOP, ATF-4, and ATF-6 mRNA levels, normalized to 18S rRNA, in all groups, n=5 to 6. *P<0.05 for STZ vs control, STZ+insulin; $P<0.05 for STZ+Tudca vs STZ+insulin or control; #P<0.05 for STZ vs STZ+Tudca.
Figure 5.
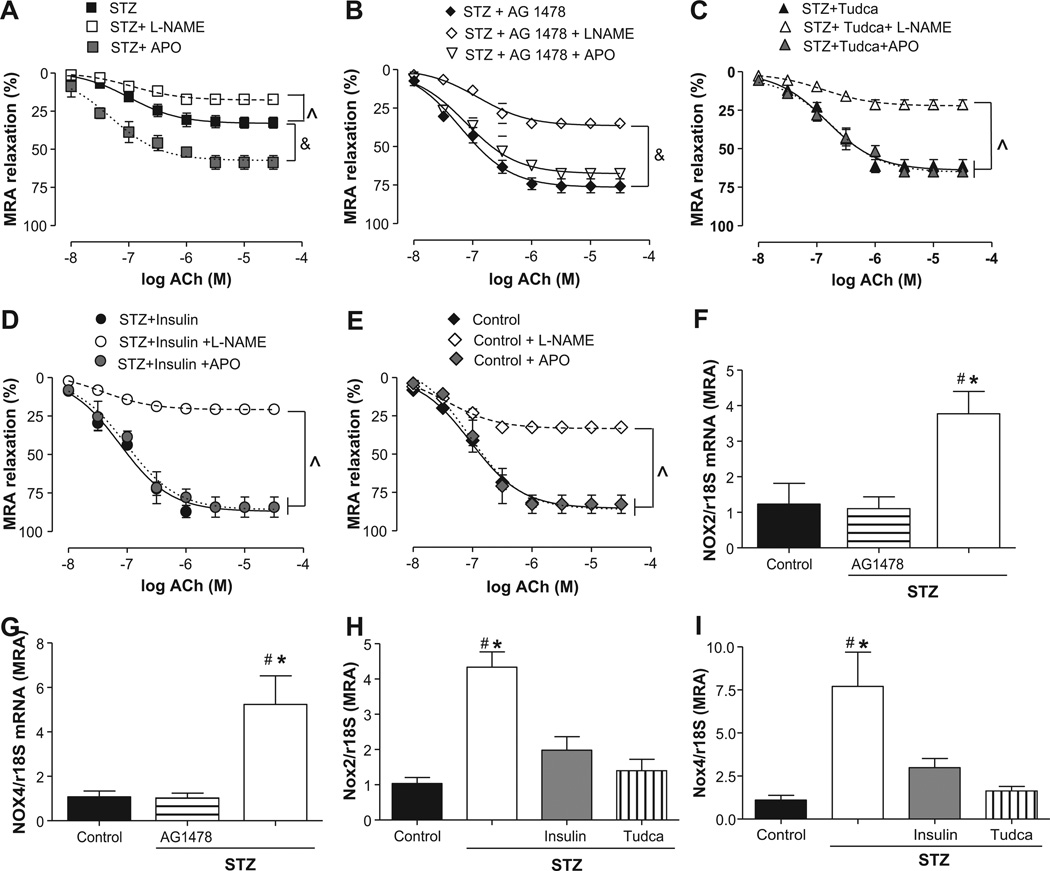
Endothelial function in mesenteric resistance arteries (MRAs) incubated with apocynin (APO) or NG-nitro-l-arginine methyl ester (l-NAME) from streptozotocin (STZ) mice treated with or without insulin or Tudca and Nox2/4 mRNA levels. A, Endothelium-dependent relaxation in response to acetylcholine (ACh) with and without apocynin (APO) or l-NAME treatments in STZ group, n=5. ■, STZ; □, STZ+l-NAME;  , STZ+APO. B, Endothelium-dependent relaxation in response to ACh in the presence of apocynin or l-NAME in MRAs from STZ+AG1478 group, n=5. ◆, STZ+AG1478; ◊, STZ+AG1478+l-NAME; ◊ STZ+AG1478+APO. C, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME in STZ+Tudca group, n=5. ▲, STZ+Tudca; △, STZ+Tudca+l-NAME; grey triangle, STZ+Tudca+APO. D, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME in the STZ+insulin group, n=5. ●, STZ+insulin; ○, STZ+insulin+l-NAME; grey circle, STZ+insulin+APO. E, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME treatment in the control group, n=5. ◆, control; ◊, control+l-NAME; grey diamond, control+APO. F through I, Nox2 and Nox4 mRNA levels, normalized to 18S rRNA, in all groups, n=4 to 6. &P<0.05 for STZ+APO versus STZ. P̂<0.05 for STZ, STZ+AG1478, STZ+Tudca, STZ+insulin, and control vs STZ+l-NAME, STZ+AG1478+l-NAME, STZ+Tudca+l-NAME, STZ+insulin+l-NAME and control+l-NAME. *P<0.05 for STZ vs control or STZ+insulin. #P<0.05 for STZ vs STZ+Tudca or STZ+insulin.
, STZ+APO. B, Endothelium-dependent relaxation in response to ACh in the presence of apocynin or l-NAME in MRAs from STZ+AG1478 group, n=5. ◆, STZ+AG1478; ◊, STZ+AG1478+l-NAME; ◊ STZ+AG1478+APO. C, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME in STZ+Tudca group, n=5. ▲, STZ+Tudca; △, STZ+Tudca+l-NAME; grey triangle, STZ+Tudca+APO. D, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME in the STZ+insulin group, n=5. ●, STZ+insulin; ○, STZ+insulin+l-NAME; grey circle, STZ+insulin+APO. E, Endothelium-dependent relaxation in response to ACh with and without apocynin or l-NAME treatment in the control group, n=5. ◆, control; ◊, control+l-NAME; grey diamond, control+APO. F through I, Nox2 and Nox4 mRNA levels, normalized to 18S rRNA, in all groups, n=4 to 6. &P<0.05 for STZ+APO versus STZ. P̂<0.05 for STZ, STZ+AG1478, STZ+Tudca, STZ+insulin, and control vs STZ+l-NAME, STZ+AG1478+l-NAME, STZ+Tudca+l-NAME, STZ+insulin+l-NAME and control+l-NAME. *P<0.05 for STZ vs control or STZ+insulin. #P<0.05 for STZ vs STZ+Tudca or STZ+insulin.
To determine the link between ER stress and reactive oxygen species in microvascular endothelial dysfunction, we incubated MRAs with apocynin (100 µmol/L). The results revealed that apocynin significantly improved EDR in STZ mice (25%), whereas no effect was observed in control or STZ mice treated with AG1478, Tudca, or insulin (Figure 5A through 5E). The enhanced mRNA levels of Nox2 and Nox4 isoforms by 4- and 6-fold, respectively, in MRA from STZ mice supported these findings, which were blunted after inhibition of EGFRtk, ER stress, or insulin injection (Figure 5F through 5I). Moreover, the NADPH oxidase activity, determined in heart and MRA lysates, was increased in STZ mice by 3- and 4-fold, respectively, compared with control mice. The inhibition of EGFRtk and ER stress significantly decreased this activity (Figure S1C and S1D).
Effect of ER Stress Induction by Tunicamycin on Phosphorylated EGFR Expression in Heart and MRAs
The EGFRtk phosphorylation in heart and MRAs was similar in control and mice injected with Tunicamycin with and without Tudca (Figure S2A and S2B). In addition, the phosphorylated EGFRtk expression, in MRAs, remained unchanged in all of the groups (Figure S2C).
Discussion
In the present study, we found that EGFRtk phosphorylation and expression were upregulated in heart and microvessels of diabetic mice and were associated with ER stress induction, cardiac fibrosis, and microvascular endothelial dysfunction. Our results are supported by previous studies reporting that EGFRtk inhibition improved microvascular function in type 2 diabetes mellitus.9,10 Interestingly, the inhibition of EGFRtk improved glucose levels, body weight, and microvascular function and reduced cardiac fibrosis and ER stress markers, with the exception of ATF6. These results suggest that exacerbated EGFRtk phosphorylation regulates cardiovascular dysfunction and metabolic alteration in type 1 diabetes mellitus, likely through phosphorylated protein kinase–like ER eukaryotic initiation factor 2α kinase-ATF4-derived ER stress branch but independent of the ATF6 branch.
Emerging evidence from experimental and clinical studies indicate that ER stress plays an important role in cardiovascular diseases17 and diabetes mellitus, as evidenced by peripheral insulin resistance and pancreatic β-cell dysfunction22,23 related to ER stress.15,19 In addition, ER stress has been demonstrated to be involved in the development of diabetes mellitus affecting different organs like liver, kidney, and skeletal muscle in several models of diabetic animals15,24,25; however, the role and mechanisms of ER stress in cardiac fibrosis and microvascular endothelial dysfunction in type 1 diabetes mellitus remain unclear. Previous studies have shown that ER stress is associated with heart failure and cardiomyopathy in nondiabetic and diabetic animals supporting the potential role of ER stress in cardiac damage.26–28 In the present work, we found that ER stress induction and cardiac fibrosis were associated with enhanced collagen type 1 and PAI-1 expression in diabetic mice. The inhibition of EGFRtk and ER stress reduced ER stress markers, suggesting that EGFRtk is upstream to ER stress activation. These data are supported by a recent publication showing that overexpression of aberrant EGFRtk in several cancers induces the expression of CHOP.14 In addition, chemical inhibition of EGFRtk and ER stress reduced cardiac fibrosis, collagen type 1, and PAI-1. Although the reduction on myocardial fibrosis appears pronounced in these animals, the effect of AG1487 and Tudca on blood glucose levels is modest, indicating that these drugs may act by mechanisms independent of their hypoglycemic effects. These results suggest that cardiac fibrosis in type 1 diabetes mellitus is regulated by an ER stress-dependent mechanism. However, it is unclear how ER stress controls collagen type 1 turnover, and additional studies are needed to delineate the mechanism.
It is well established that diabetes mellitus impairs microvascular function.29 It is known that hyperglycemia causes an enhancement in advanced glycation end products, oxidative stress levels, and increases EGFR tyrosine kinase activity leading to microvascular endothelial dysfunction, in part through the loss of NO bioavailability.9,30–32 The impaired EDR was also associated with a decrease in eNOS phosphorylation and expression in diabetic mice. These results are supported by previous studies showing a decrease in eNOS mRNA levels in KKAy mice33 and reduced eNOS phosphorylation in diabetic mice.9 Importantly, inhibition of EGFRtk and ER stress restored eNOS phosphorylation and expression in MRAs of diabetic mice indicating that eNOS expression and activity are regulated by EGFRtk and ER stress-dependent mechanisms. Although ER stress inhibition restored eNOS phosphorylation and expression to the control levels, EDR was partially improved, suggesting that other factors contribute to EDR impairment in MRAs.
Previous reports provided evidence that ER stress increases oxidative stress levels, which represent another mechanism regulating eNOS activity and NO bioavailability.34,35 NADPH oxidase seems to be the main source of oxidative stress in animal models of diabetes mellitus.36 Our data demonstrated that microvascular EDR was improved after the inhibition of NADPH oxidase activity in diabetic mice, whereas no effect was observed in control and diabetic mice treated with EGFRtk and ER stress inhibitors. These data indicate that EGFRtk and ER stress-dependent mechanisms regulate NADPH oxidase activity. These results are supported by the reduction in NADPH oxidase activity and Nox-2 and Nox-4 expression in diabetic mice after EGFRtk and ER stress inhibition. In addition, Nox2 and Nox4 have been shown to be predominantly located in the perinuclear and/or ER membranes, suggesting a relationship between reactive oxygen species generation and ER stress in diabetes mellitus.37
In conclusion, we demonstrated that, in type 1 diabetes mellitus, the exacerbation in EGFRtk signaling contributes to ER stress induction as a mechanism in part responsible for cardiac fibrosis and microvascular dysfunction. Thus, EGFRtk and ER stress could be potential targets for novel therapeutic strategies to improve cardiovascular function in diabetes mellitus.
Perspectives
Diabetes mellitus is a metabolic disease associated with cardiovascular complications, including cardiac damage and impaired microvascular EDR. Most of clinical studies indicate that diabetic patients are at high risk for cardiovascular diseases. Despite the fact that treatments have progressed, the development of novel effective treatments for diabetic patients with vascular complications remains a major research goal. Therefore, there is a significant medical need to develop novel therapies to restore microvascular endothelial function in these patients. Our results indicate that exacerbated EGFRtk activity and ER stress play key roles in heart damage and vascular dysfunction in type 1 diabetic mice. Interestingly, inhibition of EGFRtk activity decreases ER stress markers, suggesting that ER stress is downstream of the EGFRtk pathway. The inhibition of EGFRtk and ER stress reduces cardiac fibrosis and improves microvascular function associated with enhance in eNOS phosphorylation, cGMP levels, and reduction in NADPH oxidase activity. Therefore, EGFRtk and ER stress could be potential targets for novel therapeutic strategies to improve cardiovascular function in diabetes mellitus.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first study to demonstrate that the exacerbation in EGFRtk signaling contributes to ER stress induction as a mechanism responsible for cardiac fibrosis and microvascular dysfunction in type 1 diabetes mellitus.
What Is Relevant?
This study unveils novel roles for enhanced EGFRtk phosphorylation and its downstream ER stress in cardiac fibrosis and microvascular endothelial dysfunction in type 1 diabetes mellitus.
Summary
EGFRtk phosphorylation and expression were upregulated in heart and microvessels of diabetic type 1 mice and were associated with ER stress induction, cardiac fibrosis, and microvascular endothelial dysfunction. Interestingly, the inhibition of EGFRtk and ER stress improved body parameters, cardiac fibrosis, and microvascular function.
Acknowledgments
Sources of Funding
We acknowledge grant support from the National Institutes of Health (HL095566 to K.M. and HL097111 to M.T.). Grant P20RR017659-COBRE to Dr LG Navar paid 50% of M.K.’s salary.
Footnotes
M.G. and M.K. researched data and wrote the article; S.-Y.C. and M.P. researched data. M.T. and D.H. contributed to the discussion and reviewed the article. K.M. was the project director, contributed to the discussion, wrote the article, and was the guarantor.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.112.192500/-/DC1.
Disclosures
None.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(suppl 5):S1–S85. [PubMed] [Google Scholar]
- 2.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care. 2009;32:2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 4.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Lucas R, Caldwell R, Yao L, Romero MJ, Caldwell RW. Novel mechanisms of endothelial dysfunction in diabetes. J Cardiovasc Dis Res. 2010;1:59–63. doi: 10.4103/0975-3583.64432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol. 2010;88:241–248. doi: 10.1139/Y10-018. [DOI] [PubMed] [Google Scholar]
- 7.Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens. 2010;4:102–115. doi: 10.1016/j.jash.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem. 2003;278:35049–35056. doi: 10.1074/jbc.M304913200. [DOI] [PubMed] [Google Scholar]
- 9.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benter IF, Yousif MH, Griffiths SM, Benboubetra M, Akhtar S. Epidermal growth factor receptor tyrosine kinase-mediated signalling contributes to diabetes-induced vascular dysfunction in the mesenteric bed. Br J Pharmacol. 2005;145:829–836. doi: 10.1038/sj.bjp.0706238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94:68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 12.Nagareddy PR, MacLeod KM, McNeill JH. GPCR agonist-induced transactivation of the EGFR upregulates MLC II expression and promotes hypertension in insulin-resistant rats. Cardiovasc Res. 2010;87:177–186. doi: 10.1093/cvr/cvq030. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Shang Y, Yu Y. Induced endoplasmic reticulum (ER) stress and binding of over-expressed ER specific chaperone GRP78/BiP with dimerized epidermal growth factor receptor in mammalian cells exposed to low concentration of N-methyl-N′-nitro-N-nitrosoguanidine. Mutat Res. 2006;596:12–21. doi: 10.1016/j.mrfmmm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Piccione EC, Lieu TJ, Gentile CF, Williams TR, Connolly AJ, Godwin AK, Koong AC, Wong AJ. A novel epidermal growth factor receptor variant lacking multiple domains directly activates transcription and is overexpressed in tumors. [Accessed May 23, 2012];Oncogene. 2011 Oct 10; doi: 10.1038/onc.2011.465. http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2011465a.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 18.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 19.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 20.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhoták S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 21.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic β-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic βcells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales JC, Gentile CL, Pfaffenbach KT, Wei Y, Wang D, Pagliassotti MJ. Chemical induction of the unfolded protein response in the liver increases glucose production and is activated during insulin-induced hypoglycaemia in rats. Diabetologia. 2008;51:1920–1929. doi: 10.1007/s00125-008-1094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raciti GA, Iadicicco C, Ulianich L, Vind BF, Gaster M, Andreozzi F, Longo M, Teperino R, Ungaro P, Di Jeso B, Formisano P, Beguinot F, Miele C. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 2010;53:955–965. doi: 10.1007/s00125-010-1676-1. [DOI] [PubMed] [Google Scholar]
- 26.Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med. 2009;13:1499–1512. doi: 10.1111/j.1582-4934.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Fontaine J, Harkless LB, Davis CE, Allen MA, Shireman PK. Current concepts in diabetic microvascular dysfunction. J Am Podiatr Med Assoc. 2006;96:245–252. doi: 10.7547/0960245. [DOI] [PubMed] [Google Scholar]
- 30.Kagota S, Yamaguchi Y, Nakamura K, Kunitomo M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen Pharmacol. 2000;34:201–209. doi: 10.1016/s0306-3623(00)00061-6. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia. 2004;47:1727–1734. doi: 10.1007/s00125-004-1525-1. [DOI] [PubMed] [Google Scholar]
- 32.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Sakamoto K, Nakatani Y, Ohtoshi K, Hayaishi-Okano R, Kosugi K, Hori M, Yamasaki Y. Decreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complications. Diabetes Care. 2005;28:2716–2721. doi: 10.2337/diacare.28.11.2716. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 36.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.