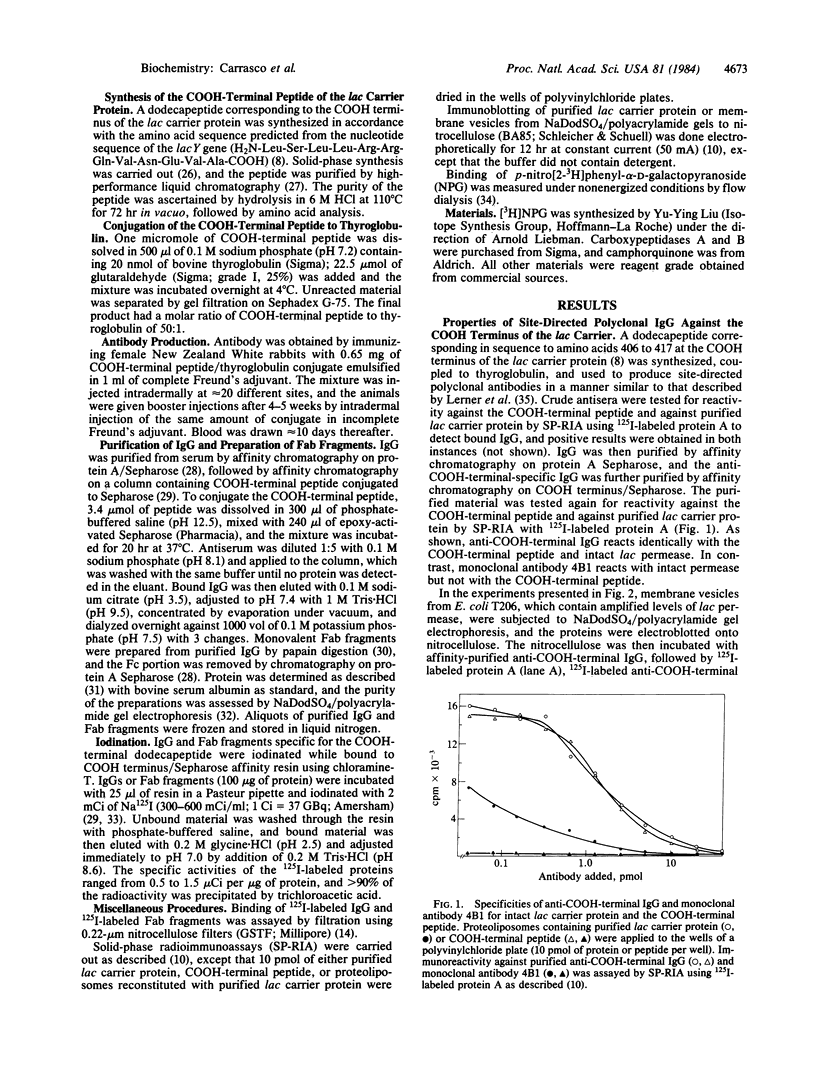
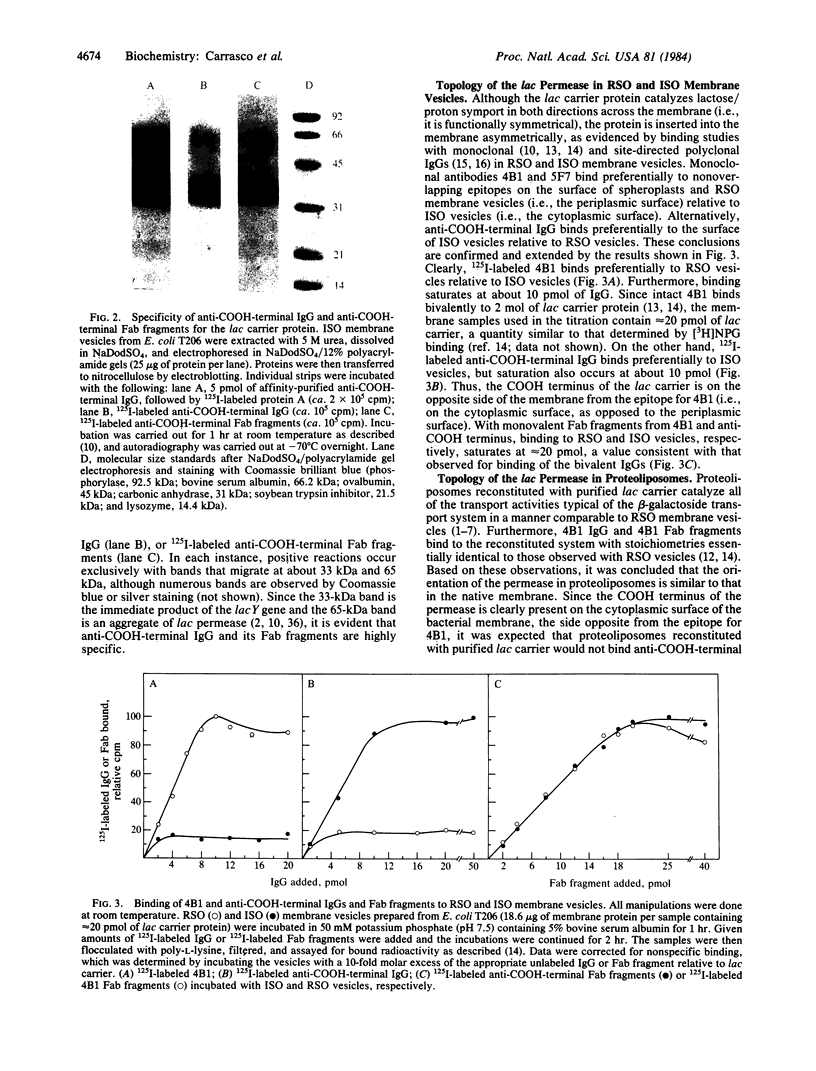
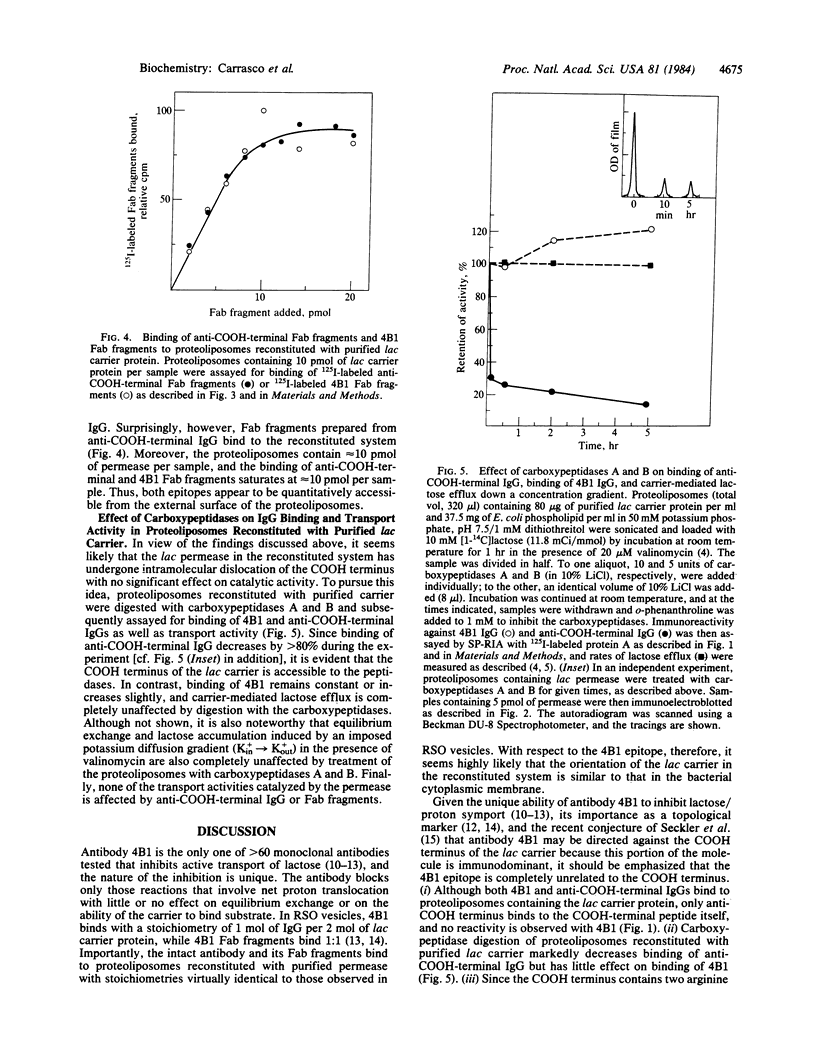
Abstract
A dodecapeptide corresponding to the carboxyl terminus of the lac carrier of Escherichia coli was synthesized, coupled to thyroglobulin, and the conjugate was used to generate site-directed polyclonal antibodies. The antibodies react with the carboxyl-terminal peptide and with the lac carrier protein, while monoclonal antibody 4B1 reacts with intact lac carrier protein, but not with the carboxyl-terminal peptide. Antibody 4B1 binds preferentially to right-side-out membrane vesicles relative to inside-out vesicles, confirming the presence of the 4B1 epitope on the periplasmic surface of the membrane. Alternatively, anti-carboxyl-terminal antibody binds preferentially to inside-out vesicles, demonstrating that the carboxyl terminus of the lac carrier protein is on the cytoplasmic surface. Surprisingly, both antibodies bind to proteoliposomes reconstituted with purified lac carrier protein, and quantitative binding assays indicate that the epitopes are equally accessible. When proteoliposomes containing purified lac carrier protein are digested with carboxypeptidases A and B, binding of anti-carboxyl-terminal antibodies decreases by greater than 80%, while binding of antibody 4B1 and various transport activities remain essentially unchanged. It is suggested that during reconstitution, the lac carrier protein undergoes intramolecular dislocation of the carboxyl terminus with no significant effect on its catalytic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Foster D. L., Garcia M. L., Newman M. J., Patel L., Kaback H. R. Lactose-proton symport by purified lac carrier protein. Biochemistry. 1982 Oct 26;21(22):5634–5638. doi: 10.1021/bi00265a038. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Viitanen P., Foster D. L., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983 May 10;22(10):2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Hinkle P. C. Oxidative phosphorylation and proton translocation in membrane vesicles prepared from Escherichia coli. Biochem Biophys Res Commun. 1974 May 7;58(1):178–184. doi: 10.1016/0006-291x(74)90908-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Sutcliffe J. G., Shinnick T. M. Antibodies to chemically synthesized peptides predicted from DNA sequences as probes of gene expression. Cell. 1981 Feb;23(2):309–310. doi: 10.1016/0092-8674(81)90126-4. [DOI] [PubMed] [Google Scholar]
- Marglin A., Merrifield R. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Patel L., Gennis R. B., Kaback H. R. Reconstitution of active transport in proteoliposomes containing cytochrome o oxidase and lac carrier protein purified from Escherichia coli. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4889–4893. doi: 10.1073/pnas.80.16.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande C. S., Pelzig M., Glass J. D. Camphorquinone-10-sulfonic acid and derivatives: convenient reagents for reversible modification of arginine residues. Proc Natl Acad Sci U S A. 1980 Feb;77(2):895–899. doi: 10.1073/pnas.77.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra W. W., Patel L., Rottenberg H., Kaback H. R. Electrochemical proton gradient in inverted membrane vesicles from Escherichia coli. Biochemistry. 1980 Jan 8;19(1):1–9. doi: 10.1021/bi00542a001. [DOI] [PubMed] [Google Scholar]
- Rosen B. P., McClees J. S. Active transport of calcium in inverted membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5042–5046. doi: 10.1073/pnas.71.12.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G., Schildiner S., Kaback H. R. Equilibrium between two forms of the lac carrier protein in energized and nonenergized membrane vesicles from Escherichia coli. Biochemistry. 1976 Nov 16;15(23):5126–5131. doi: 10.1021/bi00668a028. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Foster D. L., Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983 May 10;22(10):2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Garcia M. L., Kaback H. R. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]



