Abstract
Prostate cancer remains one of the most common malignancies in men. Besides surgical resection, treatments for prostate cancer include hormone therapy, chemotherapy and radiation therapy. Advancement of prostate cancer to androgen-independent state limits the potential of conventional therapeutic approaches. Bortezomib, an FDA approved proteosomal inhibitor for the treatment of myeloid leukemia, has been shown to have a positive effect on the inhibition of prostate cancer growth. Unfortunately, bortezomib has a very narrow therapeutic window which can lead to severe side effects. Elastin-like polypeptide (ELP) is a genetically engineered, thermally responsive macromolecular carrier that enables a targeted delivery of the bound molecule due to its soluble property under normal physiologic conditions. Additionally, ELP aggregates in response to mild hyperthermia. Using ELP as a carrier, it is possible to improve pharmacological properties of the therapeutic drug as well as reduce toxicity in normal tissues. In this work, we have investigated the combination treatment of androgen-independent prostate cancer cells with bortezomib and C-terminal part of the p21Cip1/Waf1 protein bound to the ELP carrier. We have found that combination treatment with bortezomib and ELP-bound p21Cip1/Waf1 protein leads to increased cell cycle arrest as well as apoptosis with regards to single treatments. We believe that this approach represents a promising direction for the treatment of androgen-independent prostate cancer.
Keywords: Novel drug delivery systems, Proteosomal inhibition, CDK inhibitors, Elastin-like polypeptides, Thermal targeting
Introduction
Prostate cancer is the most common non-skin malignancy and the second leading cause of cancer death in men [1]. In the early stage, prostate cancer is usually androgen-dependent, and the initial treatment is surgical resection followed by androgen deprivation therapy [2]. However, the vast majority of prostate cancer patients eventually develop progressive androgen-independent prostate cancer also known as castrate resistant prostate cancer (CRPC). As indicated by the name, this type of prostate cancer no longer needs androgens for survival, and its proliferation cannot be halted by androgen deprivation therapy. Radiation and chemotherapy remain the major treatment option for CRPC; however, resistance to both treatments severely limits the potential to further inhibit tumor progression and metastasis and improve lives of patients. Consequently, the progression from androgen-dependent to androgen-independent state, resulting in the development of advanced metastatic prostate cancer, represents the primary cause of mortality in prostate cancer patients [3]. Therapeutic strategies that enhance the chemotherapy efficacy and target androgen-independent prostate cancer are urgently required.
Bortezomib, an FDA approved proteasome inhibitor for the treatment of patients with relapsed or refractory multiple myeloma, has been shown to have a positive effect on the inhibition of proliferation of CRPC [4], breast [5], lung [6], and colon [7] cancer cell lines. In addition, in a Phase I study conducted at the M.D. Anderson Cancer Center at the University of Texas, bortezomib showed biological activity against CRPC [8]. The exact mechanism by which bortezomib inhibits tumor cell proliferation is not completely understood, but it is believed that its effect is achieved mainly through inhibition of NF- κB [9].
Therapeutic peptides (TP) represent a new and promising, target specific modality of anticancer drugs [10,11]. However, peptides are often degraded in circulation, poorly deposited in tumor tissue and inefficiently internalized by tumor cells [11]. Several years ago our group reported that a peptide delivery vector consisting of a cell penetrating peptide (CPP) and the thermally responsive elastin-like polypeptide (ELP) is able to deliver bioactive peptides into the cells [12-15]. ELP is a macromolecule soluble in aqueous solutions up to a certain temperature, above which it reversibly aggregates. This property can be used to enhance the concentration of ELP-bound molecules at the tumor site by focusing mild hyperthermia at the tumor region [16,17].
In the present study, we have investigated the effect of bortezomib combined with the p21-mimetic fused to an ELP carrier on the proliferation of two CRPC cell lines. The p21-mimetic peptide encompassed amino acids 139-164 of the p21WAF1/CIP1 protein. The ELP polypeptide was additionally enhanced by the addition of the cell penetrating peptide (CPP) Bac from the bactenecin family. Cell penetrating peptides have been long known as a transport vector tools for the intracellular delivery of a large variety of cargo including peptides [18]. We report here that combinatory treatment with bortezomib and thermally targeted p21-ELP-Bac polypeptide led to increased inhibition of the proliferation with respect to single agents.
Materials and methods
Cloning and polypeptide purification
The p21-mimetic peptide (GRKRRQTSMTDFYHSKRRLIFSKRKP) and the scrambled p21 peptide (RTDKIKFRKFLRSRRSQPMYSTKRGH) were inserted between the NdeI and SfiI sites in a pET25+ plasmid (Novagen, Madison, WI). The pET25+ plasmid already contained the Bac CPP (MRRIRPRPPRLPRPRPRPLPFPPRP) between the SfiI and BamHI restriction sites. ELP was subsequently cloned into the SfiI site. All the construct sequences were confirmed prior to their transformation into BLR(DE3) E. coli bacteria (Novagen, Madison, WI) for protein hyperexpression and subsequent purification by thermal cycling [12,13]
Transition temperature determination
The transition temperature (Tt) was determined as previously described [12]. In this research we used two ELP variants: ELP1 with the Tt around 40°C and ELP2 as a control with the Tt > 60°C. ELP1 variant consisted of (VPGXG)150 and ELP2 of (VPGXG)160 where X is V, G, or A in a 5:3:2 ratio for ELP1 and a 1:7:8 for ELP2. Additionally, scrambled p21-ELP1-Bac polypeptide was designed as a functional control for the effect of p21WAF1/CIP1 protein.
Cell lines and culture conditions
PC-3 and DU-145 androgen-independent prostate carcinoma cells were obtained from ATCC and grown in RPMI media (Cellgro, Mediatech Inc, Manassas, VA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 1% Antibiotic-Antimycotic (Cellgro, Mediatech Inc, Manassas, VA) at 37 °C in a 5% CO2 humidified atmosphere. No further authentication was done for any cell line.
Thermal pull-down assay
DU-145 cells from a 80% confluent T75 cell culture flask were lysed in 1 mL of lysis buffer containing 50 mM Tris (Fisher Chemicals, Fairlawn, NJ) pH 7.6, 150 mM NaCl (Fisher Chemicals, Fairlawn, NJ), 2 mM EDTA (Sigma, St Lous, MO) and 1% NP40 (BioRad, Hercules, CA), and supplemented with Complete Mini protease inhibitors (Roche, Hague Rd, IN). Lysate (200 μL) was incubated for 2 h with 100 μM p21-ELP1-Bac or scrambled p21-ELP1-Bac polypeptide at 4 °C under constant agitation. The solution was then briefly warmed to 42 °C to induce polypeptide aggregation and spun down to precipitate the aggregated polypeptide. The supernatant was removed, and the pellets were dissolved in 100 μL lysis buffer. The solutions were then incubated over night at 4 °C under constant agitation to wash non-specifically bound proteins. The following day, the solutions were again briefly warmed up and spun. The precipitated polypeptides were then dissolved in 40 μL of Laemmli buffer (63 mM Tris (Fisher Chemicals, Fairlawn, NJ) pH 6.8, 0.1 % 2-mercaptoethanol (Sigma, St Louis, MO), 2 % SDS (Sigma, St Louis, MO), 10 % glycerol (Sigma, St Louis, MO), 0.0005 % Bromphenol blue (Sigma, St Louis, MO) and subjected to SDS-PAGE as described below. The membrane was immunoblotted with anti-cyclin E (1:250 dilution, HE12, St Cruz Biotechnology, Santa Cruz, CA) or anti-PCNA (1:250 dilution, PC10, St Cruz Biotechnology, Santa Cruz, CA) antibodies to determine the amount of bound protein to p21-ELP1-Bac or scrambled p21-ELP1-Bac polypeptide.
Conjugation of polypeptides with fluorescent probe
The polypeptides were labeled on their cysteine residues with thiol-reactive probe 5-iodoacetomidofluorescein (Invitrogen, Eugene, OR) according to the previously described protocol [12].
Protein Uptake
The cells were incubated with 10 μM fluorescein isothiocyanate (FITC)-labeled ELP polypeptides at 37 or 42 °C for 1 h and subsequently harvested using Cellstripper™, a non-enzymatic cell dissociation solution (Cellgro, Mediatech Inc, Manassas, VA). The fluorescence intensity of the transduced FITC-labeled polypeptides was measured in channel FL1 using flow cytometer (Gallios, Beckman coulter Inc, Brea, CA).
Cell Proliferation
The cells were incubated with indicated concentrations of ELP polypeptides at 37 or 42 °C for 1 h after which the polypeptide was replaced with fresh media. Cell viability was determined 72 h after treatment using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (MTS, Promega, Madison, WI).
For the combination treatment with bortezomib, the cells were treated with different concentrations of bortezomib (BioVision Inc, Milpitas, CA). 24 h later the cells were incubated with different concentrations of p21-ELP1-Bac polypeptide for 1h at 42 °C. Cell viability was determined 48h later using the MTS assay. Using the method described by Chou and Talalay, we have determined a combination index (CI) of the treatments [19].
Cell Cycle Distribution
The cells were treated with 7.5 nM bortezomib and/or 20 μM ELP polypeptide as described above. 24 h after the treatment, the cells were pulsed for 1 h with 10 μM 5-bromo-2’-deoxyuridine (BrdU, Sigma, St Lous, MO) in the dark. The incorporated BrdU was detected using AlexaFluor® 488 labeled anti-BrdU antibody (clone MoBU-1, Invitrogen, Eugene, OR) according to the previously described protocol [20]. The cells were analyzed by flow cytometry, and the obtained results were analyzed using FlowJo software version 7.2.5 for Microsoft (TreeStar, Ashland, OR).
Annexin V assay
The cells were treated with 15 nM bortezomib and/or 10 μM ELP polypeptide as described above. 24 h after the protein treatment, both floating and attached cells were collected and stained with Annexin V labeled with AlexaFluor® 488 (Invitrogen, Eugene, OR) and propidium-iodide (Sigma, St Lous, MO) according to the manufacturer’s recommendations. The cells were analyzed by flow cytometry and the obtained results were analyzed using FlowJo software.
Western Blot Analysis
The cells were treated with 15 nM bortezomib and 10 μM p21-ELP1-Bac as described above. 24 h after the protein treatment, both floating and attached cells were collected, washed in PBS (Cellgro, Mediatech Inc, Manassas, VA) and resuspended in lysis buffer used for the pull down assay described above supplemented with protease inhibitors. Total proteins were measured using Bradford Protein Assay reagent (BioRad, Hercules, CA) and 25 μg of the total protein in Laemmli buffer was resolved by SDS-PAGE and transferred to 0.2 μm PVDF membrane (BioRad, Hercules, CA). The membrane was probed with anti-p21 antibody raised against amino acids 139-164 (1:250 dilution F8, St Cruz Biotechnology, Santa Cruz, CA) mouse primary antibodies and goat anti-mouse HRP conjugated secondary antibodies (Cell Signaling, Boston, MA). SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific, Waltham, MA) was used to detect the chemiluminescence. Equal loading was confirmed using mouse anti-GAPDH (1:250 dilution, A3, St Cruz Biotechnology, Santa Cruz, CA) antibody. Images were acquired using ChemiDocTM MP System (BioRad, Hercules, CA). Precision Plus ProteinTM Dual Color Standards (Bio-Rad, Hercules, CA) was used as a molecular weight marker.
Statistical analysis
All the experiments were repeated at least three times; and, where appropriate, the data was analyzed using one way ANOVA with Bonferroni multiple comparisons to analyze the statistical differences between the treatment groups and the untreated control.
Results
Design and the thermal properties of the polypeptides
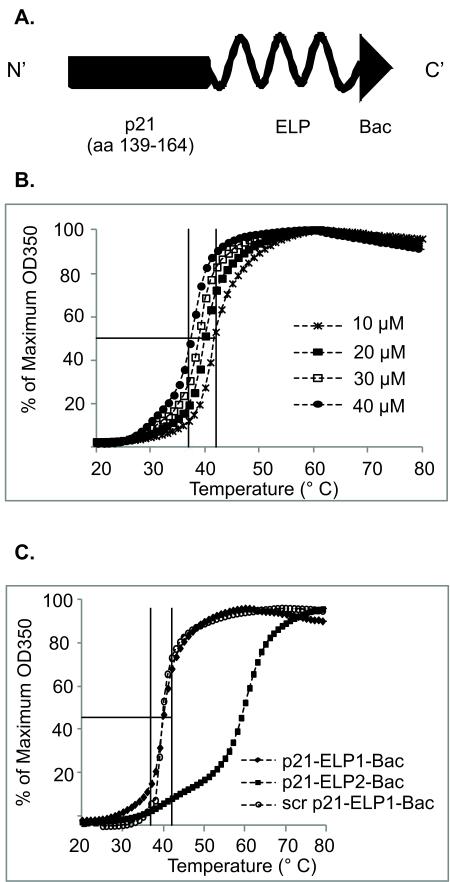
The previously described Bac-ELP-p21 polypeptide was modified in order to obtain higher yields of the purified ELP protein (Fig. 1A) [20]. Modifications of the ELP polypeptide often lead to changes in its Tt; therefore, the Tt was determined for each newly designed ELP polypeptide by measuring the turbidity of the polypeptide at different concentrations as a function of temperature (Fig. 1B, C). The Tt of 20 μM p21-ELP1-Bac as well as the scrambled p21-ELP1-Bac polypeptide was found to be around 40 °C. The Tt of the thermally unresponsive control polypeptide p21-ELP2-Bac was around 60 °C and was used as a control for determining the non-specific effects of thermal targeting.
Figure 1. Design and thermal properties of polypeptides.
(A) Schematic representation of the modified synthesized ELP-based polypeptide (p21-ELP-Bac). The C-terminus of the p21WAF1/CIP1 (amino acids 139-146) protein was cloned at the N-terminus of the ELP polypeptide, while Bac CPP was cloned to the C-terminus of the ELP carrier. (B) The concentration dependence of p21-ELP1-Bac Tt was determined by monitoring the solution turbidity of various concentrations of polypeptide in cell culture media while heating at 1°C/min. (C) The turbidity profiles of 20 μM p21-ELP1-Bac, scr p21-ELP1-Bac and p21-ELP2-Bac polypeptides in cell culture media while heating at 1°C/min. The absorbance data was converted to the percentage of the maximum absorbance in order to view all the curves on the same plot.
p21-ELP1-Bac specifically binds to cyclin E and PCNA
The thermally responsive ELP1 polypeptide enables simple peptide purification by inverse transition cycling, due to its property to reversibly aggregate and precipitate between 39 and 42 °C [12]. The C-terminal part of the p21WAF1/CIP1 protein has been shown to interact with both cyclin E and PCNA to regulate the cell cycle transition [21]. The ELP1 property to reversibly aggregate was utilized for the thermal pull-down assay to test the interactions between the ELP1-bound C-terminal part of the p21WAF1/CIP1 protein and cyclin E and PCNA. We found that, when incubated with DU-145 cellular lysates, p21-ELP1-Bac precipitated both cyclin E and PCNA (Fig. 2). Under the same conditions, the control scrambled p21-ELP1-Bac bound some of the target protein, but at much lesser extent, which can be attributed to non-specific binding. These data demonstrate that the p21 peptide fused to ELP can directly interact with its molecular targets.
Figure 2. Pull down assay.
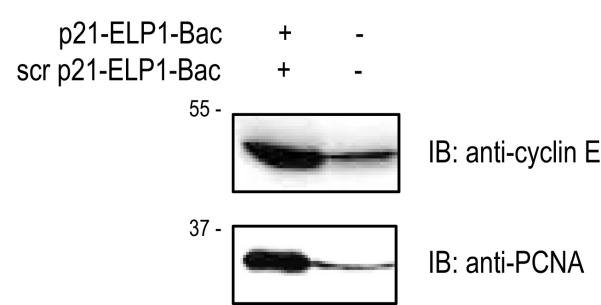
For the pull-down assay, 200 μL of DU-145 cellular lysate was incubated with 100 μM p21-ELP1-Bac or scrambled p21-ELP1-Bac polypeptide and subjected to thermall pull down assay and analyzed by SDS-PAGE using anti-cyclin E and anti-PCNA antibodies.
The intracellular uptake of p21-ELP1-Bac polypeptide is increased in DU-145 and PC-3 cell lines with thermal targeting
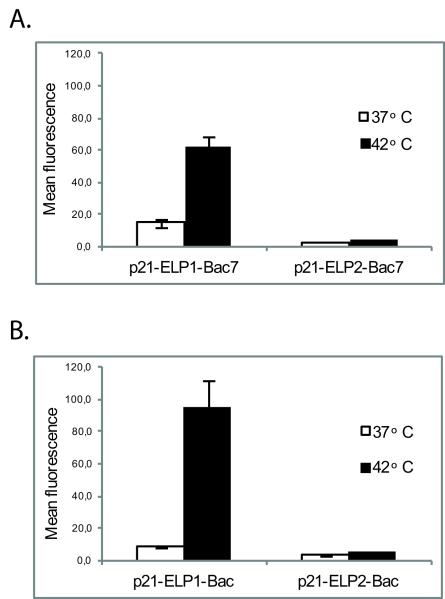
Bac CPP has been previously shown to efficiently cross biological membranes and deliver its cargo into the nucleus [22]. To assess the cellular uptake of ELP-Bac polypeptide and its p21-mimetic cargo, FITC-p21-ELP1-Bac or FITC-p21-ELP2-Bac were incubated with DU-145 and PC-3 cells, and the mean cellular fluorescence was determined by flow cytometry. Immediately after the incubation at 42 °C with the thermally responsive ELP1 polypeptide, the fluorescence intensity increased more than 4-fold in DU-145 cells and more than 10-fold in PC-3 cells with the respect to non-heated cells (p<0.0003 and p<0.0008, respectively, Fig. 3A, 3B). Furthermore, the uptake of p21-ELP1-Bac was 15-fold higher in the DU-145 and 18 fold higher in PC-3 cell line compared to thermally non-responsive FITC-p21-ELP2-Bac after the incubation at 42 °C (p<0.0003 and p<0.0008, respectively). Additionally, at 37 °C, FITC-p21-ELP1-Bac showed increased intracellular localization with respect to the ELP2 construct; however this increase was not statistically significant. Taken together, these results confirm that the p21-ELP1-Bac polypeptide can be delivered in both DU-145 and PC-3 cell lines and the uptake of the polypeptide is a specific result of the hyperthermia-triggered phase transition due to the thermal properties of ELP1.
Figure 3. p21-ELP-Bac polypeptide intracellular uptake.
DU-145 (A) and PC-3 (B) cells were incubated for 1 h at 37 °C or 42 °C with 20 μM FITC-p21-ELP-Bac polypeptides. Immediately after the treatment, cells were harvested and analyzed on flow cytometer. Non-internalized polypeptides bound to the cell surface were quenched by the addition of Trypan blue to the cell suspension prior to the analysis. Forward and side scatter gating was used to eliminate cell debris from the analysis and fluorescence data was corrected for variations in labeling efficiency among the polypeptides. Data represents mean ± SEM of 3 experiments, *p<0.0003, #p<0.0008.
The thermally targeted p21-ELP1-Bac inhibits androgen independent prostate cancer cell proliferation
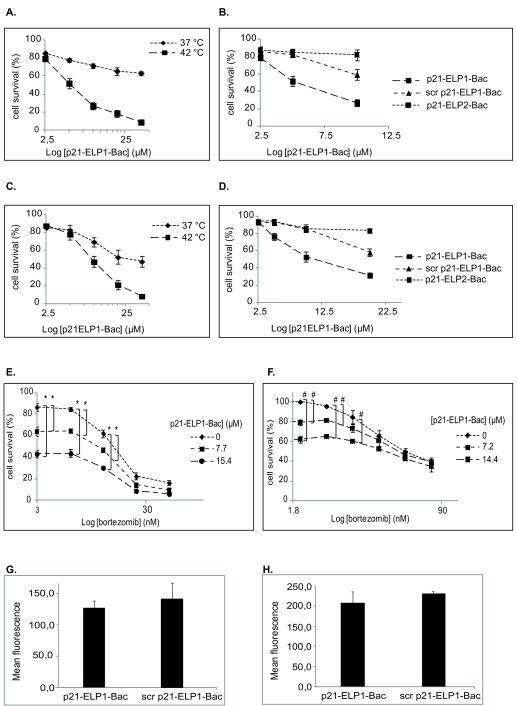
To assess whether the ELP1-bound p21-mimetic peptide was able to inhibit proliferation of CRPC cell lines in thermally dependent manner, DU-145 and PC-3 cells were incubated with increasing concentrations of p21-ELP1-Bac. p21-ELP1-Bac’s antiproliferative effect was significantly enhanced by the hyperthermia treatment in a concentration dependent manner in both cell lines (Fig. 4A, 4B). In addition, the effect of scrambled p21-ELP1-Bac polypeptide was statistically insignificant in comparison to the effect of p21-ELP1-Bac (Fig. 3C, 3D) although its uptake was comparable to that of p21-ELP1-Bac in both cell lines (Fig 3G, 3H). In both cell lines at 42 °C, the thermally non-responsive p21-ELP2-Bac polypeptide at 10 μM concentration caused less than 20% inhibition compared to almost 60% inhibition caused by thermally responsive p21-ELP1-Bac (Fig. 3C, 3D). The effect of hyperthermia alone was negligible in both cell lines (data not shown) which is consistent with other reports [20,22]. These data confirm that the ELP1-bound p21-mimetic peptide inhibits androgen-independent prostate cancer cell proliferation, and that inhibition of proliferation may be further enhanced due to the thermally induced internalization of the p21-mimetic peptide by the elastin-like polypeptide carrier.
Figure 4. Effect of polypeptide treatment on DU-145 (A, C) and PC-3 cell proliferation (B, D) and the effect of bortezomib and p21-ELP1-Bac combination treatment on DU-145 (E) and PC-3 cell proliferation (F). Intracellular uptake of scrambled p21-ELP1-Bac polypeptide in DU-145 (G) and PC-3 (H) cells.
DU-145 (A) and PC-3 (B) cells were treated for 1 h at 37 °C and 42 °C with various concentrations of p21-ELP1-Bac. Cell viability was determined 72 h after the polypeptide treatment. Data for DU-145 (C) and PC-3 (D) cells presents an overlay of 42 °C data, 72 h after the treatment with p21-ELP1-Bac, scrambled p21-ELP1-Bac or p21-ELP2-Bac. The concentrations used for scrambled p21-ELP1-Bac and p21-ELP2-Bac were those of p21-ELP1-Bac polypeptide that did not have significant influence on DU-145 and PC-3 cell proliferation after the treatment at 37 °C. For the combination treatments, DU-145 (E) and PC-3 (F) cells were pretreated with various concentrations of bortezomib, and 24 h later, incubated for 1 h at 42 °C with indicated concentrations of p21-ELP1-Bac. Cell viability was determined 48 h after the polypeptide treatment using MTS assay. Cell survival was calculated as % of the untreated control that was not heated. Results are presented as mean ± SEM of 3 independent experiments. DU-145 (G) and PC-3 (H) cell lines were incubated with 20 μM FITC-scrambled p21-ELP1-Bac and 20 μM FITC-p21-ELP1-Bac polypeptide at 42 °C and the fluorescence intensity was monitored by flow cytometry as described above. Data represents mean ± SEM of at least 3 experiments. For (A, B) p<0.01, for (E, F) *p<0. 01, #p<0.005.
The combination treatment with bortezomib and p21-ELP-Bac led to increased inhibition of the CRPC cell lines proliferation
To examine the effect of a combination treatment of bortezomib and the thermally targeted p21-mimetic polypeptide on CRPC cells, DU-145 and PC-3 cells were pre-treated with various concentrations of bortezomib, which was removed 24h later and replaced with different concentrations of p21-ELP1-Bac (Fig. 4E, 4F). For both cell lines, all the combinations of bortezomib and p21-ELP1-Bac produced a CI of ~1, indicative of an additive effect. However, we detected a statistically significant decrease in DU-145 and PC-3 cell proliferation (p<0.001 and p<0.01, respectively) after the incubation with low concentrations of bortezomib followed by the treatment with p21-ELP1-Bac at 42 °C compared to each treatment alone. The IC50 values of bortezomib in DU-145 and PC-3 cells in combination with different concentrations of p21-ELP1-Bac polypeptide are presented in Table 1.
Table 1.
IC50 values of bortezomib after the incubation of DU−145 (A) and PC−3 (B) cells with different concentrations of p21-ELP1-Bac polypeptide
| A. |
| p21-ELP1-Bac/μM | IC 50 bortezomib/nM |
|---|---|
| 0 | 15.9 ± 0.7 |
| 7.7 | 10.9 ± 0.7 |
| 15.4 | NA * |
| B. |
| p21-ELP1-Bac/μM | IC 50 bortezomib/nM |
|---|---|
| 0 | 36.1 ± 6.7 |
| 7.2 | 33.7 ± 7.1 |
| 14.4 | 22.0 ± 2.9 |
Not available
The combination treatment with bortezomib and p21-ELP1-Bac leads to increased inhibition of cell cycle progression as well as apoptosis induction
The inhibition of cancer cell growth may be due to the inhibition of cell cycle progression and/or apoptosis. Therefore, we compared the effects of single drugs with the combination treatment on cell cycle progression and the induction of apoptosis.
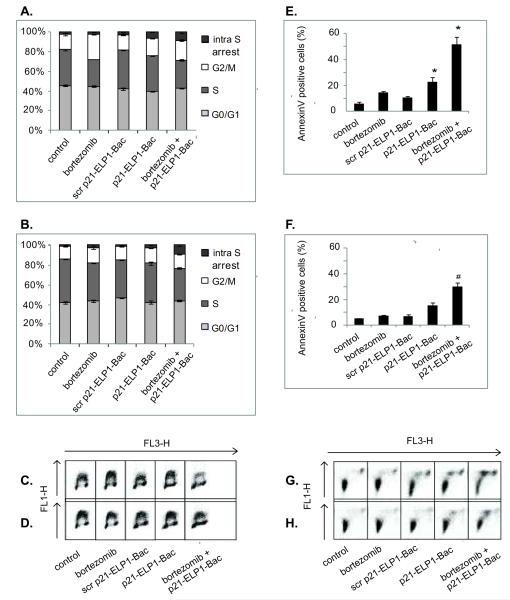
Labeling with BrdU enables accurate distinction of the cells in the S phase of the cell cycle after staining the DNA with propidium iodide. It also allows for the detection of the cells in the S phase of the cell cycle that do not actively replicate their DNA and therefore do not incorporate BrdU (intra-S phase arrest). In the DU-145 cell line the p21-ELP1-Bac led to a substantial intra-S phase cell cycle arrest. In the same cell line, bortezomib as a single treatment induced G2/M arrest on the account of the S phase. However, the combination treatment of the DU-145 cells induced an increase in the intra-S phase arrested cells compared to the single treatments (p<0.0002, Fig. 5A, 5C). In PC-3 cells, all the treatments induced a decrease in S phase; however, that decrease was most evident in the cells treated with bortezomib followed by p21-ELP1-Bac. In the same sample, a significant intra-S phase arrest was also detected (p<0.0005, Fig. 5B, 5D). In both cell lines, p21-ELP1-Bac induced a statistically significant intra-S phase arrest with regards to the scrambled p21-ELP1-Bac, confirming that the scrambled p21 polypeptide does not possess the p21 peptide related activity (p<0.0002 for DU-145 and p<0.0005 for PC-3 cell line, Fig. 5A-D).
Figure 5. Effect of bortezomib and p21-ELP1-Bac combination treatment on cell cycle distribution and the induction of apoptosis in DU-145 and PC-3 cells.
For the assessment of the cell cycle distribution, DU-145 (A, C) and PC-3 (B, D) cells were pretreated with 7.5 nM bortezomib. 24 h after bortezomib treatment, cells were treated for 1 h at 42 °C with 20 μM p21-ELP1-Bac. 24 h after cells were stained with anti-BrdU Alexa 488 labeled antibodies and propidium-iodide and analyzed by flow cytometry. Alexa 488 fluorescence was measured in channel FL1 and propidium-iodide fluorescence was measured in channel FL3 A scatter plot of forward scatter vs. FL3 intensity was used to exclude cell debris and cell aggregates from the analysis. The plots of propidium-iodide and Alexa 488 fluorescence intensity were gated into regions representing cell cycle phases to determine the percentage of cells in each phase of the cell cycle. For the apoptosis induction experiment, DU-145 (E, G) and PC-3 (F, H) cells were pretreated with 15 nM bortezomib and 24 h later incubated for 1 h at 42 °C with 10 μM p21-ELP1-Bac or scrambled p21-ELP1-Bac polypeptide. After 24 h, cells were co-stained with Alexa-488 labeled Annexin and propidium-iodide. Raw data from single representative experiment are presented in panels (C) and (G) for DU-145 cell line and (D) and (H) for PC-3 cell line. The ratio of AnnexineV positive cells was calculated as % of the untreated control that was heated at 42 °C. Results are presented as mean ± SEM of 3 independent experiments. *p<0.01
To assess the induction of apoptosis, DU-145 and PC-3 cells were stained with flourescein-isothiocyanate labeled Annexin V after the treatment. Annexin V binds to phosphatidylserine (PS) externalized early in the induction of apoptosis. In DU-145 cell line, p21-ELP1-Bac as a single treatment induced the 3.8-fold increase in the PS externalization with the respect to the cells exposed only to hyperthermia conditions (p<0.001, Fig. 5E). PS externalization was significantly increased when both, DU-145 (Fig. 5E) and PC-3 (Fig. 5F) cell lines were treated with bortezomib and p21-ELP1-Bac polypeptide (8.6-fold and 5.7-fold, p<0.001 and p<0.02 respectively). The incubation of tested cell lines with scrambled p21-ELP1-Bac polypeptide or bortezomib alone did not induce significant PS externalization with the respect to the control (Fig. 5E, 5F).
Proteosomal inhibition leads to enhanced intracellular levels of p21-ELP1-Bac polypeptide
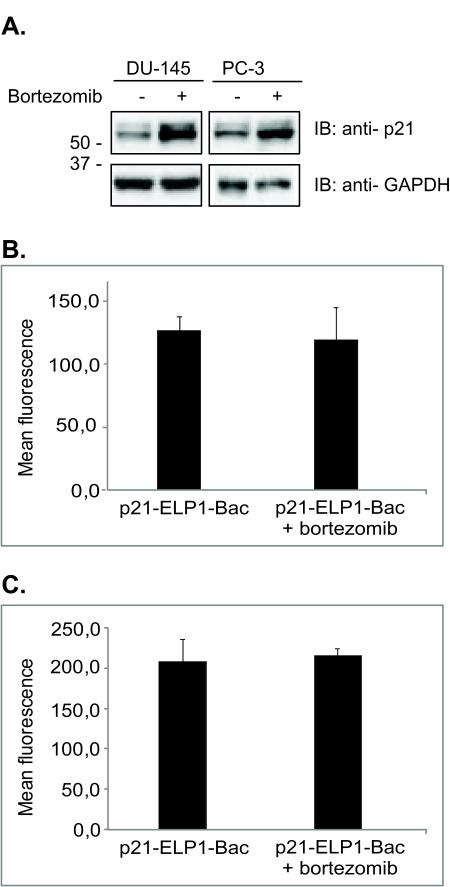
To investigate why the combination treatment of bortezomib and p21-ELP1-Bac led to increased inhibition of cell proliferation as well as increased apoptosis induction, we analyzed the levels of p21-ELP1-Bac protein in the cells that had been pretreated with bortezomib. Immunoblotting experiments revealed that pretreatment of the cells with bortezomib led to increased levels of p21-ELP1-Bac compared to the cells that had not been pretreated with bortezomib (Fig. 6a). To confirm that the increased levels of p21-ELP1-Bac polypeptide after bortezomib treatment were not consequence of the increased polypeptide, the uptake experiment was repeated in the presence of bortezomib. The results in the Fig. 6B clearly indicate that bortezomib did not alter the polypeptide intracellular uptake. These results provide an insight into the additive effect observed with bortezomib and p21-ELP1-Bac combination treatment.
Figure 6. The effect of bortezomib on intracellular levels of p21-ELP1-Bac.
(A) DU-145 and PC-3 cells were pretreated with 15 nM bortezomib as indicated in the figure and after 24 h, incubated at 42 °C with 10 μM p21-ELP1-Bac polypeptide for 1 h. 24 h later, both floating and attached cells were harvested and whole cell lysates were examined for p21-ELP1-Bac levels by Western blotting. Anti-GAPDH antibody was used as a loading control. To confirm that pretreatment with bortezomib did not alter the intracellular uptake of p21-ELP1-Bac polypeptide, DU-145 (B) and PC-3 (C) cells were treated with 15 nM bortezomib. After 24 h the cells were incubated for 1 h at 42 °C with 20 μM FITC-labeled p21-ELP1-Bac polypeptides the fluorescence intensity was monitored by flow cytometry as described above. Data represents mean ± SEM of 3 experiments, *p<0.05.
Discussion
The major limitations of the antineoplastic therapy are poor efficacy and/or severe side-effects. Recently, TPs have emerged as a new and promising class of anticancer drugs [10,11]. These peptides are specific for their targets, enabling reduction of the administered dose and, consequently, the unwanted side effects. The biggest obstacle in the utilization of peptides as anticancer drugs, however, is their poor pharmacokinetic parameters. A thermally responsive delivery vector based on ELP has previously been utilized to deliver bioactive peptides into cells [12,13]. ELP is advantageous as a drug carrier because it is a macromolecule and therefore has increased drug solubility, extended plasma half life, passive tumor accumulation, and reduced drug toxicity [22]. ELP has an additional advantage of being actively targeted to the tumor through induced hyperthermia causing aggregation at the tumor site. Recently, in an in vivo study Bidwell et al have shown that thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth [16].
In the present study, we examined the effect of proteosomal inhibition combined with the thermally targeted delivery of modified p21-ELP1-Bac polypeptide on the proliferation of CRPC cell lines. We report here that, though modified, the p21-ELP-Bac polypeptides retained Tt in the desired temperature range (Fig. 1B and 1C). In the presence of hyperthermia cellular uptake of thermally responsive ELP1 polypeptide was more than 15-fold higher than the thermally non-responsive ELP2 polypeptide in both cell lines leading us to the conclusion that the observed enhanced cellular uptake of ELP1 polypeptide is due to its thermally-induced phase transition (Fig. 3). Of the two cell lines tested, PC-3 cell line showed a higher degree of the p21-ELP1-Bac internalization after the hyperthermia which is most probably due to the already reported differences in susceptibility of different cell lines to protein transduction [12].
The incubation of CRPC cells with the p21-ELP1-Bac polypeptide in the presence of hyperthermia resulted in a concentration-dependent inhibition of proliferation (Fig. 4A, B). In both CRPC cell lines scrambled p21-ELP1-Bac polypeptide uptake at 42 °C was comparable to that of p21-ELP1-Bac polypeptide (Fig. 4G, 4H); however, its effect on the proliferation of CRPC cells was small in comparison to the effect seen with p21-ELP1-Bac peptide (Fig. 4C, 4D). The p21WAF1/CIP1 protein is a cell cycle regulator that inhibits cyclin E/cdk2 kinase activity required for Rb protein phosphorylation and subsequent release and activation of E2F-dependent gene expression [23]. p21WAF1/CIP1 can also bind chromatin-bound PCNA which leads to the loss of the PCNA interaction with the p125 catalytic subunit of pol δ resulting in the inhibition of the DNA synthesis [24,25]. The ELP moiety in the p21-ELP-Bac polypeptide is dominant in its size compared to both p21-peptide and Bac CPP and therefore, using the thermal pull-down assay we have confirmed that the observed effect of the p21-ELP1-Bac polypeptide was due to specific interactions of the p21 domain with its molecular targets cyclin-CDK complexes and/or PCNA(Fig. 2). The cyclin E-CDK2 complex inhibition as well as the PCNA protein inhibition with p21-.ELP1-Bac polypeptide was later indirectly confirmed by the cell cycle arrest observed after the incubation with both p21-ELP1-Bac polypeptide and its combination with bortezomib (Fig. 6 A-D). The observed difference in the p21-ELP1-Bac IC50 values in the DU-145 and PC-3 cell lines (5.4±0.7 and 12.2±2.3, respectively) is likely due to the different cellular context of the two cell lines, the most obvious difference being the lack of functional Rb protein in the DU-145 cells [26]. Mild polypeptide toxicity was observed in the absence of hyperthermia which is consistent with the results of the polypeptide uptake (Fig. 3). This phenomenon has already been described and attributed to the initial phase transition [12].
The overall strategy in developing anticancer regimens is to identify a drug combination that will allow the administration of a lower drug dose to prevent undesirable side effects in patients while preventing the development of chemoresistance, and maintaining a high amount of specific toxicity to the tumor cells. In a previous in vitro research, Bidwell et al reported that ELP-based c-Myc inhibitory peptide led to an enhancement of antiproliferative effects of topoisomerase II inhibitors doxorubicin and etoposide [27]. Tumor cells are known to be more susceptible to proteasome inhibition, although a well defined mechanism of normal cell resistance is yet to be established [28]. However, despite promising initial data on the efficiency in hematological tumors, bortezomib has a limited potential to be used as a treatment for solid tumors due to a narrow therapeutic window. We have extended our research on the p21-mimetic peptide to determine whether there is a rationale to use it in combination with proteosomal inhibition. We found that bortezomib in combination with thermally targeted p21-ELP1-Bac had a greater potential to inhibit CRPC cell growth (Fig. 4E, F). The IC50 values of bortezomib decreased in both cell lines after the treatment with p21-ELP1-Bac at hyperthermia temperatures. This combination treatment produced a CI ~ 1, indicative of an additive effect [19].
In a previous study, Massodi et al, have shown that Bac-ELP1-p21 predominantly arrests SKOV-3 cells in the S and G2/M phase of the cell cycle due to the inhibition of the Rb protein phosphorylation [20]. In our research, of the two CRPC cell lines tested, p21-ELP1-Bac polypeptide alone led to statistically significant intra-S-phase arrest on the account of actively proliferating cells in DU-145 cells. When those as well as PC-3 cells were treated with bortezomib 24h prior to the incubation with p21-ELP1-Bac, a further increase in the arrested cells was noticed (Fig. 5A-D). The difference between our data and Massodi et al ‘s experimental data on cell cycle phase distribution is probably due to differences in cellular context of the cell lines used in the two studies. The scrambled p21-ELP1-Bac polypeptide used in this research did not induce an intra-S phase arrest neither in the DU-145 nor in the PC-3 cell line, indicating that the p21-mimetic portion of the ELP polypeptide is indeed responsible for it. The obtained data on the cell cycle distribution provides an additional proof of the ability of p21 portion within the used p21-ELP1-Bac polypeptide to interact with its molecular targets, mostly PCNA which led to the observed intra-S-phase arrest.
Besides cell cycle regulatory function, the p21WAF1/CIP1 protein is a known modulator of apoptosis induction. Whether overexpressed ectopically or induced by various therapeutic agents p21WAF1/CIP1 was shown to induce apoptosis in various cancer cell lines as well as xenograft models regardless of p53 status [29,30]. Incubation with the p21-ELP1-Bac polypeptide led to induction of apoptosis only in the DU-145 cell line, which could be the result of a different molecular pathway, since these cells do not have a functional Rb protein. This finding explains the stronger proliferation inhibition detected after the treatment with the p21-ELP1-Bac in the DU-145 cell (Fig. 4A-4D) despite the observed increased intracellular uptake of the polypeptide in the PC-3 cells (Fig. 3). Apoptosis induction in response to bortezomib treatment has been reported to be dependent on the p53 status in different cell lines [4]. In our experiments, bortezomib as a single treatment did not induce apoptosis in either the DU-145 that harbors the mutant p53 protein nor in the p53-null PC-3 cell line [31] (Fig. 5E-H). However, when both cell lines were treated with p21-ELP1-Bac after the bortezomib treatment, we observed a statistically significant increase in the amount of cells that underwent apoptosis with respect to single treatment as well as the scrambled p21-ELP1-Bac polypeptide (Fig. 5 E-H).
In an attempt to elucidate the underlying mechanism by which bortezomib and p21-ELP1-Bac combination treatment increased the inhibition of prostate cancer cell proliferation, we determined the levels of internalized p21-ELP1-Bac polypeptide with and without bortezomib pretreatment. We found that bortezomib led to increased levels of the intracellular p21-ELP1-Bac levels with respect to the cells that were not pretreated with bortezomib (Fig.6A) which was not the consequence of the increased intracellular uptake but most probably due to decreased proteasomal degradation of the p21-ELP1-Bac polypeptide (Fig. 6B, 6C). The used p21-ELP1-Bac polypeptide encompasses the C-terminal region (aa 148-157) of the p21WAF1/CIP1 protein that is necessary for its efficient ubiquitination and subsequent proteosomal degradation [32]. Therefore, we believe that pretreatment with bortezomib lead to decreased degradation of the p21-ELP1-Bac polypeptide. This finding could have an immense impact on peptide-based therapy because clinically approved proteasome inhibitors might be able to prevent or reduce the degradation of therapeutic peptides, resulting in their prolonged and higher cellular concentration, and consequently in higher therapeutic efficacy.
Given that the p21-ELP1-Bac polypeptide can be thermally targeted to tumors in vivo, we believe that the combination of bortezomib with thermal targeting of the p21-ELP1-Bac will lead to locally enhanced bortezomib toxicity at the thermally targeted site. Considering the narrow therapeutic window of bortezomib, combination therapy with thermally targeted p21 inhibitory peptide would permit lower doses of systemically applied bortezomib, resulting in more effective treatment without subjecting patients to severe toxicity.
It has been widely accepted that cancer stem cells within tumors drive the tumor growth and recurrence. Those cells are resistant to many current cancer treatments including chemo- and radiation therapy and the treatment failure occurs mostly due to their survival. In 2009 Liu et al reported that p21 protein attenuates epithelial to mesenchymal (EMT) transition by controlling the expression of genes involved in it which led to decreased mammosphere formation in vitro [33]. On the other hand, p21 has been recognized as a molecular switch governing the entry of hematopoietic stem cells into the cell cycle which maintains a pool of quiescent leukemic stem cells by restricting their self -renewal [34] . In the future it would be very interesting to address the effect of p21-ELP1-Bac polypeptide as well as its combination with bortezomib on cancer stem cells.
In summary, the developed ELP thermally responsive drug carrier takes advantage of four principles to enhance the accumulation of cancer drugs in solid tumors: (1) passive tumor targeting by macromolecular carriers owing to the EPR effect; (2) enhanced tumor perfusion and vascular permeability with hyperthermia; (3) active targeting achieved by the thermally triggered phase transition of our genetically engineered ELP carrier; (4) enhanced tumor blood vessel and tumor cell permeability by conjugation to a cell penetrating peptide. Moreover, the proposed targeting modality is clinically feasible as it is based on an existing hyperthermia technology. Current clinical approaches for applying hyperthermia may be used with this novel targeting approach to selectively induce tumoral accumulation of a broad range of anti-cancer drugs.
Acknowledgements
We would like to thank Dr. Gene L. Bidwell III, Dr. Shama Moktan and Dr. Marijeta Kralj for inspiring discussions as well as critical reading of the manuscript, Dr. Erin White for the assistance with flow cytometry and Ms. Rowshan Begum for the technical assistance.
This work was supported by grants from the National Science Foundation (CBET-931041 and IIP-1321375) and National Institute of Health (1R21CA137418-01A2 and 1R21CA139589-01) to DR.
Footnotes
The institute at which the work was performed
Disclosure statement: D. Raucher is the president of Thermally Targeted Therapeutics, Inc., Jackson, MS
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Cunha GR, Donjacour A. Stromal-epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res. 1987;239:251–72. [PubMed] [Google Scholar]
- 3.Papandreou CN, Logothetis CJ. Bortezomib as a potential treatment for prostate cancer. Cancer Res. 2004;64:5036–43. doi: 10.1158/0008-5472.CAN-03-2707. [DOI] [PubMed] [Google Scholar]
- 4.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–22. [PubMed] [Google Scholar]
- 5.MacLaren AP, Chapman RS, Wyllie AH, Watson CJ. p53-dependent apoptosis induced by proteasome inhibition in mammary epithelial cells. Cell Death Differ. 2001;8:210–8. doi: 10.1038/sj.cdd.4400801. [DOI] [PubMed] [Google Scholar]
- 6.Ling YH, Liebes L, Jiang JD, Holland JF, Elliott PJ, Adams J, et al. Mechanisms of proteasome inhibitor PS-341-induced G(2)-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res. 2003;9:1145–54. [PubMed] [Google Scholar]
- 7.Cusack JC, Jr., Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 8.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 9.Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005;5:18. doi: 10.1186/1475-2867-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidwell GL, 3rd, Raucher D. Therapeutic peptides for cancer therapy. Part I - peptide inhibitors of signal transduction cascades. Expert Opin Drug Deliv. 2009;6:1033–47. doi: 10.1517/17425240903143745. [DOI] [PubMed] [Google Scholar]
- 11.Raucher D, Moktan S, Massodi I, Bidwell GL., 3rd Therapeutic peptides for cancer therapy. Part II - cell cycle inhibitory peptides and apoptosis-inducing peptides. Expert Opin Drug Deliv. 2009;6:1049–64. doi: 10.1517/17425240903158909. [DOI] [PubMed] [Google Scholar]
- 12.Bidwell GL, 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–85. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 13.Massodi I, Bidwell GL, 3rd, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108:396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Massodi I, Raucher D. A thermally responsive Tat-elastin-like polypeptide fusion protein induces membrane leakage, apoptosis, and cell death in human breast cancer cells. J Drug Target. 2007;15:611–22. doi: 10.1080/10611860701502780. [DOI] [PubMed] [Google Scholar]
- 15.Raucher D, Massodi I, Bidwell GL. Thermally targeted delivery of chemotherapeutics and anti-cancer peptides by elastin-like polypeptide. Expert Opin Drug Deliv. 2008;5:353–69. doi: 10.1517/17425247.5.3.353. [DOI] [PubMed] [Google Scholar]
- 16.Bidwell GL, 3rd, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012;19:136–43. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moktan S, Perkins E, Kratz FRD. Thermal Targeting of an Acid-Sensitive Doxorubicin Conjugate of Elastin-like Polypeptide Enhances the Therapeutic Efficacy Compared with the Parent Compound In Vivo. Mol Cancer Ther. 2012;11:1547–56. doi: 10.1158/1535-7163.MCT-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends in molecular medicine. 2012;18:385–93. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Massodi I, Moktan S, Rawat A, Bidwell GL, 3rd, Raucher D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer. 2010;126:533–44. doi: 10.1002/ijc.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutoh M, Lung FD, Long YQ, Roller PP, Sikorski RS, O'Connor PM. A p21(Waf1/Cip1)carboxyl-terminal peptide exhibited cyclin-dependent kinase-inhibitory activity and cytotoxicity when introduced into human cells. Cancer Res. 1999;59:3480–8. [PubMed] [Google Scholar]
- 22.Bidwell GL, 3rd, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135:2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–8. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 24.Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 25.Cazzalini O, Perucca P, Riva F, Stivala LA, Bianchi L, Vannini V, et al. p21CDKN1A does not interfere with loading of PCNA at DNA replication sites, but inhibits subsequent binding of DNA polymerase delta at the G1/S phase transition. Cell Cycle. 2003;2:596–603. [PubMed] [Google Scholar]
- 26.Bookstein R, Rio P, Madreperla SA, Hong F, Allred C, Grizzle WE, et al. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci U S A. 1990;87:7762–6. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bidwell G, 3rd, Raucher D. Enhancing the antiproliferative effect of topoisomerase II inhibitors using a polypeptide inhibitor of c-Myc. Biochem Pharmacol. 2006;71:248–56. doi: 10.1016/j.bcp.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Landis-Piwowar KR, Chen D, Milacic V, Dou QP. Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci. 2008;9:227–39. doi: 10.2174/138920308784533998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy S, Gu M, Ramasamy K, Singh RP, Agarwal C, Siriwardana S, et al. p21/Cip1 and p27/Kip1 are essential molecular targets of inositol hexaphosphate for its antitumor efficacy against prostate cancer. Cancer Res. 2009;69:1166–73. doi: 10.1158/0008-5472.CAN-08-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J, Loganathan J, Eliaz I, Terry C, Sandusky GE, Sliva D. ProstaCaid inhibits tumor growth in a xenograft model of human prostate cancer. Int J Oncol. 2012;40:1339–44. doi: 10.3892/ijo.2012.1344. [DOI] [PubMed] [Google Scholar]
- 31.Scott SL, Earle JD, Gumerlock PH. Functional p53 increases prostate cancer cell survival after exposure to fractionated doses of ionizing radiation. Cancer Res. 2003;63:7190–6. [PubMed] [Google Scholar]
- 32.Fukuchi K, Hagiwara T, Nakamura K, Ichimura S, Tatsumi K, Gomi K. Identification of the regulatory region required for ubiquitination of the cyclin kinase inhibitor, p21. Biochem Biophys Res Commun. 2002;293:120–5. doi: 10.1016/S0006-291X(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, et al. p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci U S A. 2009;106:19035–9. doi: 10.1073/pnas.0910009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]