Abstract
The management of inoperable lung cancer remains a challenge. It has been proven that computed tomography (CT)-guided iodine-125 (125I) seed implantation is a safe and efficient method for treating lung cancer. Computed tomographic fluoroscopy (CTF) is superior to traditional CT for percutaneous management of lung lesions, due to the real-time guidance and accurate localization of the lesions. The aim of the present prospective study was to evaluate the feasibility, safety and efficacy of CTF-guided percutaneous permanent implantation of 125I seeds for the treatment of selected patients with inoperable stage T1-3N0M0 non-small-cell lung cancer (NSCLC). A total of 24 patients with resectable but inoperable stage T1-3N0 NSCLC, with a total of 28 lesions, underwent CTF-guided percutaneous implantation of radioactive 125I seeds. A prescription dose of 100–120 Gy was delivered to each lesion. The complications and local tumor control rates were documented. Survival was estimated using the Kaplan-Meier method. All the patients successfully completed the procedure, with a mean procedure duration of 45.7 min (range, 30–75 min). No severe complications occurred. Small asymptomatic pneumothorax with lung volume compression of <10% and minor hemorrhage along the needle track without hemoptysis occurred immediately after the procedure in 3 (12.5%) and 4 (16.7%) of the 24 patients, respectively. At a median follow-up of 31.5 months (range, 8–46 months), the local control rate (LCR) of the lesions was 78.6% (22/28). The 1-, 2- and 3-year overall survival rate was 95.8, 78 and 55%, respectively. In conclusion, CTF is the favourable imaging guidance method for the percutaneous implantation of 125I seeds. CTF-guided brachytherapy with implantation of 125I seeds is a safe, feasible and effective modality for the treatment of inoperable early-stage NSCLC and may be considered an alternative option in selected patients with medically inoperable NSCLC.
Keywords: non-small-cell lung cancer, iodine-125 seed, brachytherapy, computed tomography fluoroscopy, image guidance
Introduction
Primary lung cancer is the leading cause of mortality among the oncologic patient population. Approximately 1.5 million new cases are diagnosed annually worldwide (1). Despite the advances in early detection, accurate staging and treatment, the survival rate of lung cancer patients has not significantly improved over the past 30 years, with an average 5-year survival rate of ∼15% (1,2). Surgery remains the main curative selection for patients with early-stage non-small-cell lung cancer (NSCLC). However, not all patients with resectable tumors are suitable candidates for surgery, due to certain contraindications. Radiotherapy (RT) and/or chemotherapy are commonly used for patients who are not considered surgical candidates. However, these modalities are not usually curative and are almost always accompanied by various toxic complications. Thus, there is a need for effective alternatives to surgery in patients with medically inoperable early-stage lung cancer.
Intraoperative brachytherapy with radioactive iodine-125 (125I) seed implantation has been proven to be an effective therapeutic modality and an alternative to external beam radiation therapy (EBRT) for patients with lung cancer (3–6). However, intraoperative 125I seed implantation requires an open-chest approach, which may result in more prolonged hospitalization and a significant economic burden for the patients.
Computed tomography (CT)-guided percutaneous delivery of 125I seeds for brachytherapy of malignant lung tumors has already been performed (7). However, the conventional CT guidance exhibits certain disadvantages, such as the lack of real-time visualization, prolonged procedure time and a high incidence of complications.
In the present study, we assessed the advantages of the combined use of computed tomographic fluoroscopy (CTF) guidance with transthoracic 125I brachytherapy for the treatment of selected patients with early-stage NSCLC.
Materials and methods
Patient information
Between January, 2006 and March, 2011, 24 patients with a total of 28 primary lung cancer lesions were enrolled in this prospective study. All the patients were histopathologically diagnosed by bronchoscopy and/or transthoracic needle biopsy (TNB) and staged according to the American Joint Committee for Cancer Staging manual (7th edition). The inclusion criteria were: i) patients with stage T1-3N0M0 NSCLC not considered appropriate for resection due to high-risk factors, such as poor heart and/or lung function; ii) patients with resectable lung cancer who refused surgical treatment and; iii) long diameter of the lesion of <8 cm. The patient characteristics are presented in Table I. The protocol for the present study was approved by our Institutional Review Board. The patients were informed about the potential risks, benefits and complications of the procedure and written consent was provided on the day prior to the treatment.
Table I.
Patient characteristics prior to CTF-guided 125I seed implantation.
| Characteristics | Value |
|---|---|
| Age, years [mean (range)] | 65.4 (54–81) |
| Gender | |
| Male | 18 |
| Female | 6 |
| Histopathology, n | |
| Squamous carcinoma | 9 (11) |
| Adenocarcinoma | 15 (17) |
| Tumor T stage, n | |
| T1 | 7 (8) |
| T2 | 12 (15) |
| T3 | 5 (5) |
| Maximum lesion diameter, cm [mean (range)] | 4.0±1.5 (2.1–7.6) |
The values in parentheses are the number of lesions unless otherwise specified. CTF, computed tomography fluoroscopy; T1, tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura; T2, tumor >3 but ≤7 cm, or any of the following: >2 cm distal to the carina or invasion of the visceral pleura; T3, tumor >7 cm, or direct invasion of the parietal pleural or the chest wall, or tumor located in the main bronchus <2 cm distal to the carina but without involvement of the carina.
Instruments and implant treatment planning
A multidetector CT scanner with an 85-cm gantry bore (Brilliance CT-Big Bore Oncology, Philips Medical Systems (Cleveland), Inc., Cleveland, OH, USA) was used to guide the 125I seed implantation. A monitor for monitoring the procedure and a foot pedal for operating the CTF were available within the scanner room.
The 125I seeds (registered no., H20041350) were purchased from Shanghai GMS Pharmaceutical Co., Ltd. (Shanghai, China) and approved by the United States Food and Drug Administration. The 125I isotope was absorbed in minute silver rods and furnished in 4.5×0.8-mm cylindrical titanium capsules welded by laser at both ends. The pre-planned 125I seeds were housed into the cartridge chamber of an implantation gun that provided complete shielding and were sterilized with auto-claving prior to use.
The pre-implant dosimetric planning was performed using the Seed Interstitial Radiotherapy Planning System (SIRPS, KeLinZhong Medical Technique Institute, Beijing, China). The prescription dose was defined as a minimal peripheral dose of 100–120 Gy. A CT scan spanning the entire lung with a 5-mm slice thickness was performed in all the patients 1–2 weeks prior to the seed implantation. The continuous axial images were transferred to the Treatment Planning System (TPS). The planning target volume, defined as the gross tumour volume (GTV) with a 0.5–1-cm safety margin, was outlined on each axial image. The prescribed D90 (the dose delivered to 90% of the target volume) was calculated through the TPS. Postoperative dosimetric verification was routinely performed for all the patients. A chest CT scan with the same parameters as those of pre-operation one was obtained immediately after the seed implantation. The axial CT images were transferred to the TPS. Based on the recommendations of the American Brachytherapy Society (ABS) (8), the D90 dose, the isodose curves for each slice and the dose-volume histograms (DVH) of the target were generated on the TPS.
Implant technique protocol
The patients were placed on the CT table in a comfortable position that facilitated the pre-planned access route. A sequential axial CT scan of the region of interest was performed with a slice thickness of 5 mm. The access path of the puncture needle was assessed on the axial images combined with the pre-implant plan. Under local anesthesia, the 18-G implantation needles (Hakko Medical Co., Ltd, Nagano-ken, Japan) were inserted through the intercostal space closely below the ribs at the end of a soft expiration phase and were then advanced into the lesion under CTF guidance. During the entire procedure, the patients were instructed to maintain gentle breathing. Four frames of contiguous, cross-sectional fluoroscopic images with a slice thinkness of 3 or 6 mm were generated as the foot pedal was pressed. The 125I seeds were subsequently deposited while withdrawing the needle from the distal to the proximal portion of each lesion at 0.5–1-cm intervals.
After the procedure, a CT scan of the entire chest was obtained to confirm the seed distribution and exclude any procedure-related complications. The procedure time was defined as the duration from the initiation of the CT scan for the localization of lesion to the successful implantation of all the 125I seeds. Technical success was defined as the pre-operatively planned 125I seeds being implanted in the appropriate area within the tumor based on the pre-treatment plan formulated with the TPS. After the procedure, the patients were instructed to maintain bed rest for 6 h, lying on the puncture site, with routine monitoring of the vital signs.
Follow-up assessment and response criteria
At post-procedure day 1, a chest radiograph was routinely performed to identify possible complications. A CT scan was performed when pneumothorax, hemorrhage or other complication was suspected. Patients were followed up with physical examinations and thoracic CT scans at 2, 4 and 6 months post-125I seed implantation. The follow-up period was prolonged by 3–6 months if there was no evidence of disease progression after the first 6 months post-procedure. CT images were simultaneously reviewed by all the interventional radiologists involved in this study and a consensus was obtained for each session of the examination. The longest diameter of the lesion was measured with calipers on the CT workstation.
The response criteria were based on the measurement of the longest diameter of all the target lesions and were classified as follows: i) complete response (CR), disappearance of the target lesion; ii) partial response (PR), ≥30% decrease in the longest diameter of the target lesion; iii) progressive disease (PD), ≥20% increase in the longest diameter of the target lesion; and iv) stable disease (SD), neither sufficient shrinkage of the lesion to qualify for PR nor sufficient increase to qualify for PD (9). The local tumor control rates were defined as the percentage of the sum of CR, PR and SD of all the target lesions. Local control failure was determined by PD. Cancer-related death was defined as the endpoint.
Statistical analysis
The local tumor control rates, local control failure rates and complications were documented at the end of the present study. The statistical analyses were performed with SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier method was used to estimate survival curves.
Results
Technical outcomes
All the patients successfully completed the CTF-guided 125I seed implantation, with a mean procedure time of 45.7 min (range, 30–75 min). The radioactivity of the 125I seeds was 0.7 mCi. All the required seeds were deposited in the corresponding areas based on the pre-planned dosimetry. The median number of needle tracks was 14 (range, 6–53) and the median number of implanted 125I seeds was 30 (range, 10–100). The post-treatment dosimetric measurement demonstrated that the actual D90 ranged from 69 to 132 Gy (median, 107 Gy) and the median follow-up time was 31.5 months (range, 8–46 months).
Local control rate (LCR)
At the end of follow-up period, the number of the target lesions exhibiting CR, PR and SD was 8, 10 and 4, respectively. Thus, the tumor LCR was 78.6% (22/28) (Figs. 1 and 2). Local control failure (or PD) occurred in 6 lesions (21.6%, 6/28), demonstrated as either lesion progression after treatment or local recurrence during the follow-up period. Distant metastases were confirmed in 3 patients.
Figure 1.
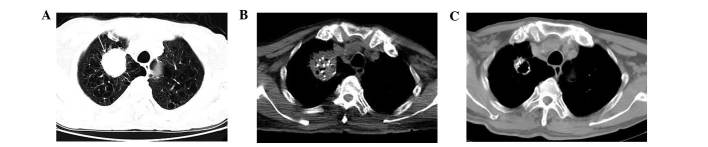
Computed tomographic fluoroscopy (CTF)-guided implantation of 125I seeds in an 81-year-old man with squamous carcinoma who did not undergo chemotherapy. (A) Pre-treatment lung window image shows an irregular oval mass in the right upper lobe (arrow), with severe emphysema. (B) Computed tomography (CT) image immediately post-treatment shows multiple high-density 125I seeds distributed within the lesion. (C) CT image obtained 3 months post-treatment reveals that the lesion is significantly decreased in size and partially cavitated.
Figure 2.
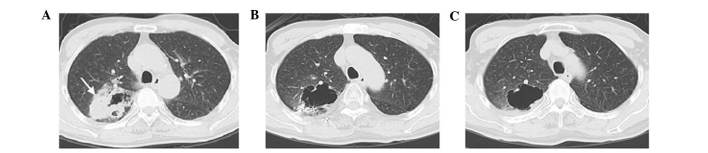
Computed tomography (CT) images pre- and post-implantation of 125I seeds in a 65-year-old man with adenocarcinoma of the right upper lobe who did not undergo chemotherapy. (A) Pre-procedure CT image reveals an irregular mass (arrow) with an eccentric cavity. (B) CT image obtained 6 months post-treatment shows the solid part of the lesion is markedly shrunk with enlargement of the cavity. (C) CT image obtained 12 months post-treatment reveals that the solid mass has almost resolved, with the cavity being unchanged compared to (B).
Survival outcomes
The overall 1-, 2- and 3-year survival rate was 95.8, 78 and 55%, respectively. The median survival time was 38 months (range, 8–46 months) (Fig. 3). At the end of the follow-up period, 19 patients had died, whereas 3 patients were lost to follow-up and 2 patients remained alive. Of the 19 deceased patients, 14 succumbed to cancer-related causes, including local recurrence and metastasis, whereas 5 patients succumbed to other diseases.
Figure 3.
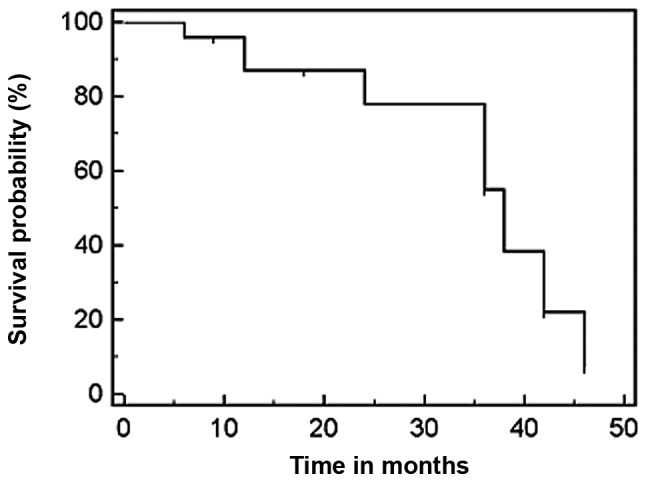
Kaplan-Meier analysis of patient survival rate. The graph shows the survival curve of all the patients with NSCLC included in this study. The median survival time was 38 months.
Complications
No severe complications occurred during the follow-up period. Of the 24 treated patients, 3 (12.5%) experienced asymptomatic pneumothorax with a lung volume compression of <10% and 4 (16.7%) developed mild hemorrhage along the needle tracks after the procedure, without hemoptysis. The pneumothorax and hemorrhage were treated conservatively and resolved within 1 week. The post-procedure chest CT revealed no significant radiation pneumonitis, whereas regional emphysema was identified in the location of the brachytherapy seeds in 2 patients, which required no further treatment. No seed migration was observed.
Discussion
Lung cancer remains one of the leading causes of mortality worldwide. Although surgery remains the gold standard for the treatment of early-stage NSCLC, a significant proportion of patients with otherwise resectable lung cancer may exhibit other comorbidities, precluding surgical resection. In patients with medically inoperable early-stage lung cancer, conventional chemotherapy and/or EBRT is commonly the treatment choice. However, despite aggressive multiple-drug regimens and the addition of radiation treatment, survival remains poor without surgery and recurrence is the rule, regardless of the initial treatment selection.
Radiofrequency ablation (RFA), a commonly used thermal ablation technique and stereotactic body radiation therapy (SBRT) are emerging treatment modalities. These methods have been applied in high-risk patients with early-stage lung cancer. However, although these modalities have recently attracted attention due to their promising results (10), they have certain limitations.
The successful treatment of hepatic tumors using RFA has resulted in this method being used for other solid tumors, such as lung cancer. However, regarding lung cancer, available data on the histomorphological effects of this method are limited. Although RFA may generally be considered as an effective alternative to surgical resection for the treatment of stage I NSCLC, it remains controversial. Early immunohistochemical findings following RFA revealed complete tumor cell necrosis in only 38% of the cases (11). The high proportion of remaining viable tumor cells following ablation casts doubt on RFA as a curative concept. This approach may be considered with extreme caution as a palliative treatment option for lung cancer (11). In addition, RFA is not suitable for lung tumors that are >3 cm in diameter or centrally located (12–14).
SBRT employs external fixation and hypofractionation to deliver a high dose per fraction of radiation to a small target volume. Therefore, it appears to be a valid alternative to conventional 3D-CRT, with high rates of local control and promising survival rates according to recent reported series (15–18). Local failure was associated with tumor size, target definition and central or pleural proximity (15). The most appropriate tumor size for SBRT was considered to be ∼2–3 cm. When the GTV was >65 cm3, SBRT was ineffective (16). Although a higher biological equivalent dose (BED) may result in improved LCR, the optimal BED range for SBRT was 83.2–146 Gy for stage I NSCLC, due to the toxicity associated with higher doses (17). Even within this dose range, however, different types and rates of toxicity have been reported. A 39.9% rate of adverse effects and 10% of grade 3–4 toxicity according to the Radiation Therapy Oncology Group (RTOG) were reported by Baumann et al (15). It was previously reported that toxicity may be associated with tumor location. Grade 3–5 toxicity was observed in 10.4 and 27.3% of patients with peripheral and central lung tumors, respectively (18). Acute ≥grade 2 pulmonary toxicity developed in 6.5% and symptomatic pneumonia in 10% of the patients after a median interval of 5 months (19). Furthermore, SBRT may be associated with significant skin toxicity. Hoppe et al reported that, after a minimum of 3 months of follow-up, 38% of the patients developed grade 1, 8% grade 2, 4% grade 3 and 2% grade 4 acute skin toxicity (20). Additional side effects, such as fatigue, dyspnea/cough or transient thoracic pain were recorded in approximately 10% of the patients (21).
Iodine-125 (125I) is a commonly used radionuclide with a half-life of approximately 60 days, emitting gamma-rays with a maximum energy of 35 keV. Intraoperative brachytherapy is an effective therapeutic modality for patients for whom surgical resection is contraindicated (3,4). The primary aim of radiation therapy is the eradication of the tumor without damage to healthy tissue and adjacent organs. Intraoperative 125I seed implantation is able to deliver a higher radiation dose to the tumor and a lower radiation dose to the surrounding healthy tissues compared to EBRT. Intraoperative implantation of 125I seeds has been used for the treatment of lung cancer, either as an alternative to or in combination with surgical resection. The implantation techniques and purposes may vary according to different studies. Previous studies reported that a vicryl mesh containing 125I seeds may be inserted over the tumor bed or the resection margins following video-assisted thoracoscopic resection (VATR) for early-stage NSCLC deemed unsuitable for conventional surgery due to high risk (3,4), whereas others reported that 125I seeds were implanted along the resection margin following limited resection in NSCLC patients who were not considered candidates for lobectomy or pneumonectomy (5). It was demonstrated that intraoperative 125I seed brachytherapy for early-stage NSCLC patients resulted in improved LCR, decreased local recurrence and prolonged survival (3–5,22). However, intraoperative 125I seed implantation exhibits two disadvantages. First, it lacks the standard pre-implantation treatment plan indicating the number, location, dose and activity of the seeds, which is critical for successful brachytherapy. Second, it is an invasive procedure, involving open-chest surgery, even with the VATR approach.
CT-guided TNB is currently an established technique for diagnostic evaluation of pulmonary nodules. The advent of CT fluoroscopy has rendered TNB more facile. It allows near real-time monitoring of the placement of the TNB needles and thereby significantly shortens the duration of the TNB procedure (23). Therefore, CTF may facilitate the percutaneous implantation of 125I seeds for the treatment of lung cancer. Until recently, the percutaneous delivery of 125I seeds for brachytherapy of malignant lung tumors was performed mainly under conventional CT guidance (7). The disadvantages of conventional CT-guided 125I seed implantation include lack of real-time visualization, a high incidence of complications (such as pneumothorax) and prolonged patient discomfort due to the longer duration of the procedure (1–2 h).
In the present study, we attempted to combine the advanced CTF-guidance technique and percutaneous brachytherapy with 125I seed implantation for the treatment of lung cancer, which achieved near real-time visualization and accurate localization of the accessed needles and implanted seeds. The results of our study demonstrated that the mean duration of the procedure was 45.7 minutes, which was significantly shorter compared to that under conventional CT-guidance. The incidence of procedure-related complications was also markedly reduced. It was previously demonstrated that the most common complications of conventional CT-guided biopsy and 125I seed implantation included pneumothorax and hemorrhage in 12.5–31 and 9.2–46.9% of the treated patients, respectively (7). In our study, small asymptomatic pneumothorax developed in 3 patients (12.5%) and mild, self-limited hemorrhage along the needle tracks in 4 patients (16.7%), without hemoptysis. These complications spontaneously resolved within 1 week after the intervention, without requiring special management. Under CTF-guidance, the inserted needle may be observed as it advances. The near real-time monitoring by CTF may help the operators avoid the puncture of bullae, interlobar fissures and vessels, decrease the number of pleural punctures, shorten the duration of the procedure and, thereby, reduce the incidence of pneumothorax and hemorrhage. Therefore, percutaneous 125I seed implantation may be performed more conveniently, efficiently and safely under CTF-guidance. Additionally, radioactive complications, such as lung fibrosis, loss of pulmonary function and cardiac toxicity, which are common following EBRT (24,25), have not been reported following intraoperative 125I brachytherapy.
Although 125I seed implantation has been applied for the treatment of lung cancer for more than two decades, the number of studies pertaining to long-term results is limited. To the best of our knowledge, there are no available studies on the treatment of early-stage NSCLC with CTF-guided 125I seed implantation with a curative intent. Zhang et al (7) reported the results of CT-guided radioactive 125I seed implantation in the treatment of localized advanced pulmonary carcinoma. The results of that study demonstrated that the LCR was 78.1% at 2 months of follow-up, with a 1-year survival rate of 65.0%, which were significantly higher compared to that of the control group treated by chemotherapy. Optimal outcomes for early-stage NSCLC have been achieved with SBRT thus far, with a 3-year LCR of 40-89% and a 3-year overall survival (OS) of 42.7–57.1% (16,19,22). In the present study, the lesion LCR was 78.6% at the end of a median follow-up period of 31.5 months and the 1-, 2- and 3-year OS for stage T1-3N0 NSCLC was 95.8, 78 and 55%, respectively. In addition, we prescribed a radiation dose of 100–120 Gy, which was higher compared to that of conventional EBRT. That dose contributed to the satisfactory local lesion control, without any evidence of radiation damage to the surrounding healthy tissues and organs during the follow-up period.
In conclusion, the present study demonstrated that CTF is able to provide real-time guidance for percutaneous implantation of high-dose 125I seeds within the lung tumor. CTF-guided percutaneous implantation of 125I seeds appears to be a feasible, safe and effective modality for the treatment of inoperable early-stage NSCLC. However, our study had certain limitations and further studies, including larger patient samples and longer follow-up periods are required.
References
- 1.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 2.Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75:56–63. [PubMed] [Google Scholar]
- 3.d’Amato TA, Galloway M, Szydlowski G, Chen A, Landreneau RJ. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest. 1998;114:1112–1115. doi: 10.1378/chest.114.4.1112. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Galloway M, Landreneau R, et al. Intraoperative 125I brachytherapy for high-risk stage I non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 1999;44:1057–1063. doi: 10.1016/s0360-3016(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 5.Lee W, Daly BD, DiPetrillo TA, Morelli DM, Neuschatz AC, Morr J, Rivard MJ. Limited resection for non-small cell lung cancer: observed local control with implantation of I-125 brachy-therapy seeds. Ann Thorac Surg. 2003;75:237–242. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 6.Pisch J, Belsley SJ, Ashton R, Wang L, Woode R, Connery C. Placement of 125I implants with the da Vinci robotic system after video-assisted thoracoscopic wedge resection: a feasibility study. Int J Radiat Oncol Biol Phys. 2004;60:928–932. doi: 10.1016/j.ijrobp.2004.07.680. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FJ, Li CX, Wu PH, Wu YX, Jiao DC, Liu J, Li YL. CT guided radiazctive 125I seed implantation in treating localized advanced pulmonary carcinoma. Zhonghua Yi Xue Za Zhi. 2007;87:3272–3275. (In Chinese) [PubMed] [Google Scholar]
- 8.Nag S, Beyer D, Friedland J, Grimm P, Nath R. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44:789–799. doi: 10.1016/s0360-3016(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Das M, Abdelmaksoud MH, Loo BW, Jr, Kothary N. Alternatives to surgery for early stage non-small cell lung cancer-ready for prime time? Curr Treat Options Oncol. 2010;11:24–35. doi: 10.1007/s11864-010-0119-z. [DOI] [PubMed] [Google Scholar]
- 11.Schneider T, Reuss D, Warth A, et al. The efficacy of bipolar and multipolar radiofrequency ablation of lung neoplasms - results of an ablate and resect study. Eur J Cardiothoracic Surg. 2011;39:968–973. doi: 10.1016/j.ejcts.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Abbas G, Schuchert MJ, Pennathur A, Gilbert S, Luketich JD. Ablative treatments for lung tumors: radiofrequency ablation, stereotactic radiosurgery, and microwave ablation. Thorac Surg Clin. 2007;17:261–271. doi: 10.1016/j.thorsurg.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen CL, Scott WJ, Young NA, Rader T, Giles LR, Goldberg M. Radiofrequency ablation of primary lung cancer: results from an ablate and resect pilot study. Chest. 2005;128:3507–3511. doi: 10.1378/chest.128.5.3507. [DOI] [PubMed] [Google Scholar]
- 14.Ambrogi MC, Lucchi M, Dini P, et al. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg. 2006;30:177–183. doi: 10.1016/j.ejcts.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 15.Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45:787–795. doi: 10.1080/02841860600904862. [DOI] [PubMed] [Google Scholar]
- 16.Beitler JJ, Badine EA, El-Sayah D, et al. Stereotactic body radiation therapy for nonmetastatic lung cancer: an analysis of 75 patients treated over 5 years. Int J Radiat Oncol Biol Phys. 2006;65:100–106. doi: 10.1016/j.ijrobp.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81:e305–e316. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Guckenberger M, Heilman K, Wulf J, Mueller G, Beckmann G, Flentje M. Pulmonary injury and tumor response after stereo-tactic body radiotherapy (SBRT): results of a serial follow-up CT study. Radiother Oncol. 2007;85:435–442. doi: 10.1016/j.radonc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys. 2008;72:1283–1286. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Mutyala S, Stewart A, Khan AJ, Cormack RA, O’Farrell D, Sugarbaker D, Devlin PM. Permanent iodine-125 interstitial planar seed brachytherapy for close or positive margins for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2010;76:1114–1120. doi: 10.1016/j.ijrobp.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Templeton PA, White CS, Protopapas Z, et al. Real-time continuous imaging CT guidance for lung biopsy. Radiology. 1996;201:270. [Google Scholar]
- 24.Slater JD, Ellerbroek NA, Barkley HT, Mountain C, Oswald MJ, Roth JA, Pepers LJ. Radiation therapy following resection of non-small cell bronchogenic carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:945–951. doi: 10.1016/0360-3016(91)90190-f. [DOI] [PubMed] [Google Scholar]
- 25.Choi NC, Kanarek DJ. Toxixity of thoracic radiotherapy on pulmonary function in lung cancer. Lung Cancer. 1994;10:S219–S230. doi: 10.1016/0169-5002(94)91685-3. [DOI] [PubMed] [Google Scholar]


