Abstract
BACKGROUND
Aberrant activation of the androgen receptor (AR) is a major factor highly relevant to castration-resistant progression of prostate cancer (PCa). FOXO1, a key downstream effector of PTEN, inhibits androgen-independent activation of the AR. However, the underlying mechanism remains elusive.
METHODS
The inhibitory effect of FOXO1 on full-length and constitutively active splice variants of the AR was examined by luciferase reporter assays and real-time reverse transcription polymerase chain reaction (RT-qPCR). In vitro protein binding assays and western blot analyses were used to determine the regions in FOXO1 and AR responsible for their interaction.
RESULTS
We found that a putative transcription repression domain in the NH2-terminus of FOXO1 is dispensable for FOXO1 inhibition of the AR. In vitro protein binding assays showed that FOXO1 binds to the transcription activation unit 5 (TAU5) motif in the AR NH2-terminal domain (NTD), a region required for recruitment of p160 activators including SRC-1. Ectopic expression of SRC-1 augmented transcriptional activity of some, but not all AR splice variants examined. Forced expression of FOXO1 blocked the effect of SRC-1 on AR variants’ transcriptional activity by decreasing the binding of SRC-1 to the AR NTD. Ectopic expression of FOXO1 inhibited expression of endogenous genes activated primarily by alternatively spliced AR variants in human castration-resistant PCa 22Rv1 cells.
CONCLUSIONS
FOXO1 binds to the TAU5 motif in the AR NTD and inhibits ligand-independent activation of AR splice variants, suggesting the PTEN/FOXO1 pathway as a potential therapeutic target for inhibition of aberrant AR activation and castration-resistant PCa growth.
Keywords: androgen receptor, FOXO1, prostate cancer, PTEN
INTRODUCTION
Prostate cancer (PCa) cells appear to be addicted to activity of the androgen receptor (AR) for growth and survival [1]. Androgen ablation therapy is the standard treatment option for advanced/metastatic PCa. Unfortunately, majority of prostate cancers eventually relapse with a disease termed castration-resistant prostate cancer (CRPC). Recently, various new treatments have been developed for treating CRPC patients, including taxane-based chemotherapy, androgen/AR targeted therapies such as abiraterone and enzalutamide, and immunotherapies such as sipuleucel-T. However, none of these new treatment options are curative and ongoing disease progression remains a major clinical issue.
The AR is implicated in the castration-resistant PCa progression. AR protein is expressed in castration-resistant prostate cancers [2]. In agreement with this observation, serum levels of prostate-specific antigen (PSA), a well-studied transcriptional target of the AR, invariably rise along with the emergence of castration-resistant disease, indicating that the AR is functionally active in CRPC. This notion is supported by further studies that the AR is required for the proliferation of CRPC cells in culture and in animals [3,4].
A number of mechanisms have been proposed to explain aberrant activation of the AR in CRPC. Increasing evidence suggests that CRPC cells produce androgens through an intracrine mechanism [5,6]. It is believed that the residual amount of locally synthesized androgens may provide sufficient ‘‘fuel’’ to support AR function and CRPC growth. The AR gene itself is amplified at the genomic level in a subset of CPRC tumors [7]. Elevated AR protein may therefore compensate for the absence or reduced levels of androgens following hormonal therapy [4]. Many signaling pathways activated by growth factors and cytokines such as interleukin-6 (IL-6) are also implicated in the activation of the AR in the absence or presence of low levels of androgens [8–10]. Most recently, several alternatively spliced AR variants have been identified [11–15]. These AR splice variants lack the COOH-terminal ligand-binding domain and are constitutively active without the need of androgen stimulation. Importantly, expression of some of the AR variants is sufficient to allow PCa cells to grow in culture and in animals under androgen-depletion conditions [13–15]. While continued/persistent AR signaling following AR-directed therapies is the major challenge for the management of CRPC, it also provides an opportunity for the development of novel therapeutics for this disease.
The tumor suppressor gene PTEN is one of the frequently mutated or deleted genes in human prostate cancers. Genome-wide high-density single-nucleotide polymorphism (SNP) array and integrative genomic profiling analyses show that PTEN loss occurs in 40–50% of metastatic prostate cancers [16,17], implying a role of PTEN inactivation in PCa metastasis and castration-resistant progression. This hypothesis is further supported by mouse studies demonstrating that deletion of the PTEN gene promotes development of CRPC [18,19]. We and others have demonstrated that the transcription factor FOXO1, a key downstream effector of PTEN, binds to and inhibits both androgen-dependent and independent activation of the AR [20–23]. Importantly, the inhibitory effect of FOXO1 on the AR is independent of the DNA-binding activity of FOXO1 [22,23], although the underlying mechanism remains elusive. In the present study, we show that FOXO1 directly binds to the transcriptional activation unit 5 (TAU5) motif in the NH2-terminal domain of the AR, competes for binding of AR with the transcriptional coactivator SRC-1, and thereby inhibits the androgen-independent activation of the full-length AR and truncated AR splice variants in PCa cells.
MATERIALSANDMETHODS
Cell Lines and Cell Culture
LNCaP, PC-3, and DU145 cells were purchased from the American Type Culture Collection (Manassas, VA). 22Rv1 cells were kindly provided by Dr. C.Y. Young (Mayo Clinic, Rochester, MN). Cells were cultured in RPMI 1640 containing 10% fetal bovine serum, 100 μg/ml streptomycin, 100 units/ml penicillin, and 0.25 μg/ml amphotericin B.
Plasmids and Small Interference RNAs (siRNAs)
Expression vectors for the AR variants including 1/2/3, 1/2/3/2b, CE1, CE2, and CE3 (the later three variants are also named as AR-V1, AR-V5, and AR-V7, respectively [12]) and deletion mutants of the AR AF-1 domain were generated as described [11,12,24,25]. The AF-1 domain of the deletion mutants was amplified by PCR and ligated with the pGEX-4T-1 vector (GE Healthcare Life Sciences) to generate GST-AF-1 constructs. The renilla luciferase reporter vector was purchased from Promega (Madison, WI). The PSA promoter luciferase reporter containing a ~5.8 kb genomic fragment from the promoter of the PSA gene was provided by Dr. C.Y. Young. siAR-1 (GGAACTCGATCGTATCATT) and siAR-4 (GAAATGATTGCACTATTGA) were purchased from Dharmacon (Chicago, IL). Expression vectors for FLAG-tagged wild-type (FOXO1-WT), constitutive active (FOXO1-A3), and various COOH-terminal truncated FOXO1 are described previously [26]. V5-tagged wild-type FOXO1 was constructed by subcloning full-length FOXO1 into the backbone vector pcDNA3. 1D (Invitrogen, Carlsbad, CA). The lucif-erase reporter constructs PSA-Luc and 3xARE-Luc are described previously [22]. Internal deletion constructs ΔSID1 (Δ54–58), ΔSID2 (Δ102–106), ΔSID3 (Δ141–145), and ΔSID1, 2 and 3 of truncated FOXO1 (1–267) were generated by mutagenesis.
Cell Transfection and Luciferase Reporter Assay
Transient transfection was performed as previously described [27]. Approximately 75–90% transfection efficiency was routinely achieved. For luciferase reporter assays, cells were harvested at 24–48 hr after transfection. Firefly and renilla luciferase activities were determined using a dual luciferase kit (Promega).
GST Purification and Pull Down Assay
GST and FOXO1 or AR fusion proteins were expressed in BL21 cells (Invitrogen) and induced with IPTG for 3 hr at 37°C. Cells were sonicated in PBS with protease inhibitors. After spinning, the supernatant was incubated overnight with Glutathione Sepharose 4B beads (GE Healthcare Life Sciences). Beads were washed with PBS and samples were run on a 10% SDS–PAGE gel and stained with GelCode Blue Staining Reagent (Thermo Scientific, Rockford, IL). AR, FOXO1, and SRC-1 proteins were produced using 35S-methionine and the TNT Quick Coupled Transcription/Translation System (Promega) according to the manufacturer’s protocol. FOXO1 was incubated with the GST-AR proteins overnight. Beads were washed and samples were run on NuPAGE 4–12% bis–tris gels (Invitrogen).
Western Blot Analysis and Antibodies
Protein samples were prepared by lysing cells in modified RIPA buffer (1× PBS, 1% NP-40, 0.1% SDS, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO)). Lysates (50 μg) were separated on a 7.5% SDS–PAGE gel and transferred to a nitrocellu-lose membrane. The membrane was probed with the specific primary antibody and HRP-conjugated secondary antibody and then visualized by chemiluminescence. Antibodies against AR (N-20) and ERK2 (D-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody against FOXO1 (C29H4) was purchased from Cell Signaling Technology (Danvers, MA). The FLAG antibody was purchased from Sigma-Aldrich.
Real-Time RT-PCR
Total RNA was isolated with Trizol reagent (Invitrogen). cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen). Real-time RT-PCR was done with cDNA samples using the iQ SYBR Green Supermix and ABI Prism 7900 platform (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. The 2−ΔΔCt method was used to calculate the relative expression level by normalizing to GAPDH levels. The following primer sequences were used: PSA (5′-AGGCCTTCCCTGTACACCAA-3′) and (5′-GTCTTGGCCTGGTCATTTCC-3′); hK2 (5′-CAAAGT-GACAGTGGGTGTGG-3′) and (5′-GCCAGGTCCTT-CACTGTCTC-3′); NKX3.1 (5′-GTACCTGTCGGCCCC TGAAC-3′) and (5′-GGAGAGCTGCTTTCGCTTAG-3′); and MASPIN (5′-CCCTATGCAAAGGAATTGG A-3′) and (5′-CAAAGTGGCCATCTGTGAGA-3′); GAPDH (5′-GAAGGTGAAGGTCGGAGTC-3′) and (5 ′-GAAGATGGTGATGGGATTTC-3′).
RESULTS
The Putative Repression Domain in the NH2-Terminus of FOXO1 Is Dispensable for FOXO1 Inhibition of AR Transcriptional Activity
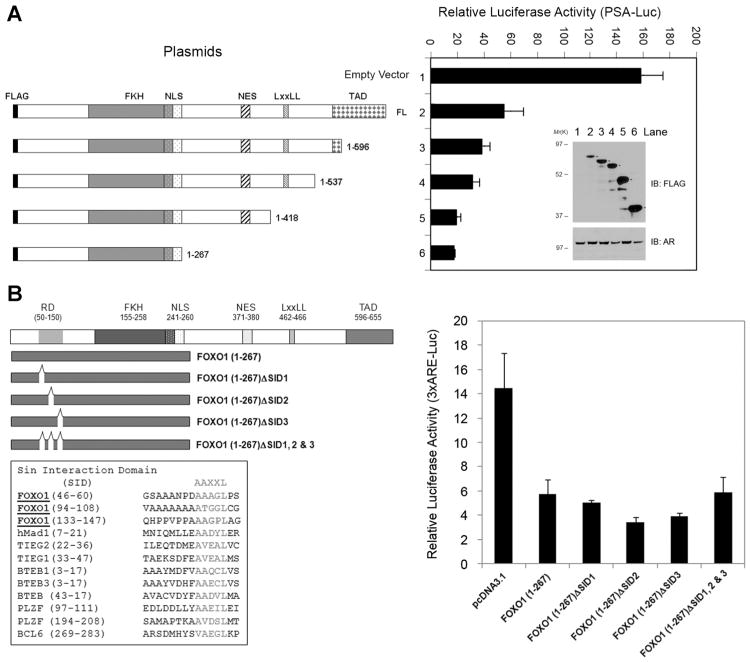
To define which region in FOXO1 mediates inhibition of AR transcriptional activity, we generated a series of expression vectors for FOXO1 truncated from the COOH-terminus. We demonstrated that the NH2-termimal 267-amino-acid fragment of FOXO1 was sufficient in mediating AR inhibition in LNCaP PCa cells (Fig. 1A), suggesting that the AR inhibitory effect of FOXO1 is mediated by the region located in the NH2-terminus of FOXO1. It has long been suspected that an alanine rich sequence (also termed repression domain, RD) in the NH2-terminus of FOXO1 (Fig. 1B, left) may have a transcriptional repression function [28,29]. Protein sequence analysis indicated that in the putative RD of FOXO1 (Fig. 1B, left), there are three consensus mSin3A interaction domains (SIDs), which are present in a number of transcription repressors such as hMad1, TIEG1, TIEG2, BTEB1, BTEB3, BTEB, PLZF, and BCL6 [30]. Expression of FOXO1 (1–267) mutants, in which each SID domain was deleted individually or in combination, resulted in inhibition of AR transcriptional activity to a degree similar to unmutated FOXO1 (1–267) (Fig. 1B, right). These data suggest that the putative RD in the NH2-terminus of FOXO1 is dispensable for FOXO1 inhibition of AR transcriptional activity in LNCaP PCa cells.
Fig. 1.
FOXO1 inhibition of AR transactivation independent of the putative repression domain in the NH2-terminus. A: Left, schematic diagram showing a series of COOH-terminal truncated FLAG-tagged FOXO1 mammalian expression constructs. FKH, forkhead (DNA binding) domain; NLS, nuclear localization signal; NES, nuclear exportation signal; LxxLL, nuclear receptor interaction motif; TAD, transcription activation domain. Right, inhibition of AR transcriptional activity by the NH2-terminus of FOXO1. LNCaP cells were transfected with the PSA-Luc reporter construct in combination with the empty control vector or different FOXO1 mutants. At 24 hr after transfection, cells were treated with 1 nM of R1881 (a synthetic androgen) for 12 hr and harvested for luciferase activity measurement as described in the Materials and Methods Section. Columns, mean among three individual experiments; bars, SD. Expression of FLAG-tagged FOXO1 and AR proteins were analyzed by western blot using the indicated antibodies (inset).B: Left, schematic diagram showing mutants of FOXO1 NH2-terminus (amino acids 1–267) with deletion of different mSin3a interaction domains (SIDs) identified in many transcription repressors. Right, inhibition of AR transcriptional activity by FOXO1NH2-terminus (1–267) is independent of the SID domains. LNCaP cells were transfected with the AR reporter gene 3xARE-Luc and FOXO1 mutants as indicated. Cells were treated and luciferase activities in cells were measured as in(A).
FOXO1 Directly Interacts With the AR Through a Motif That Commonly Interacts With Other Proteins
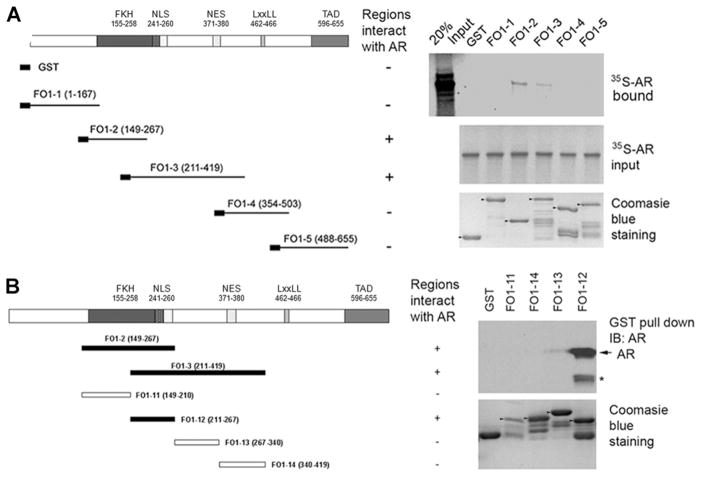
To further explore the molecular mechanism by which FOXO1 inhibits AR activity, we sought to determine which region of FOXO1 interacts with AR. To this end, we generated GST fusion proteins with various fragments of the FOXO1 protein (Fig. 2A, left). 35S-labeled AR proteins produced by in vitro transcription and translation were incubated with GST or GST–FOXO1 fusion proteins, and GST pull-down assays were performed. As shown in Figure 2A (right), AR proteins directly interacted with the second (FO1-2, a.a. 149–267) and third (FO1-3, a.a. 211–419) fragments of FOXO1. Further analysis confirmed that a smaller region (FO1-12, a.a. 211–267) overlapped by FO1-2 and FO1-3 specifically interacted with the AR (Fig. 2B). Intriguingly, several proteins examined including CDK2, CDK1, and cyclin D1 also interact with FOXO1 through the same region that interacts with the AR [31–33]. Thus, FOXO1 directly interacts with the AR through a motif that commonly interacts with many proteins. Importantly, the AR interaction region in FOXO1 (a.a. 211–267) overlaps with the region (a.a. 1–267) required for FOXO1 inhibition of AR transcriptional activity (Fig. 1A).
Fig. 2.
FOXO1 directly interacts with the AR through a small region in the forkhead domain. A: In vitro protein binding assays with GST– FOXO1 recombination proteins and 35S-labeled in vitro transcribed and translated AR. Left, schematic diagram of GST–FOXO1 recombination proteins. Right, purified GST and GST–FOXO1 fusion proteins (lower panel) were incubated with 35S-labeled in vitro transcribed and translated AR proteins(middle panel)and pull-down assays were performed. Pull-down proteins were subjected to SDS–PAGE analysis and auto radiography (upper panel).B: GST pull-down assays with whole cell lysates of LNCaP cells and GST–FOXO1 recombination proteins. Left, GST–FOXO1 recombination protein diagram. Right, purified GST and GST–FOXO1 fusion proteins (lower panel) were incubated with whole cell lysate of LNCaP and pull-down assays were performed. The pull-down proteins were analyzed by western blot using anti-AR antibody (upper panel). The asterisk indicates a non specific band recognized by the anti-AR antibody.
FOXO1 Directly Binds to the AR Transcriptional Activation Unit 5 (TAU5) Motif
Next we sought to determine which region in AR is bound by FOXO1. We demonstrated previously that FOXO1 inhibits androgen-independent activation of the AR [22] and this function of the AR is primarily attributed to the activation function 1 (AF1) in the NH2-terminus of the AR [24]. Because FOXO1 only interacts with the NH2-terminal domain (NTD) of the AR in the absence of androgens [34], we were interested in determining the FOXO1-binding site in the AR NTD. To this end, a series of internal deletion mutants of the AR NTD were generated (Fig. 3A) and fused with the GST protein as described previously [24]. 35S-labeled FOXO1 proteins produced by in vitro transcription and translation were incubated with GST or GST-AR fusion proteins, and GST pull-down assays were performed. As shown in Figure 3B, FOXO1 directly interacted with all the GST-AR fusion proteins except a mutant of the AR NTD in which a region of a.a. 361–490 was deleted. Importantly, this region contains the TAU5 motif that is required for the AF1 function of the AR NTD [24,35]. Thus, FOXO1 may inhibit androgen-independent activation of the AR through binding to the TAU5 motif in the NTD. No binding of FOXO1 to GST alone was detected in our assay, suggesting that FOXO1-AR binding is specific.
Fig. 3.
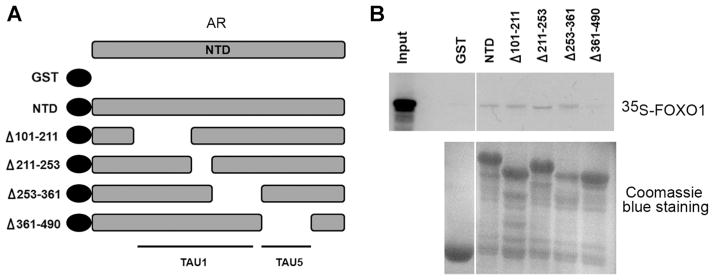
FOXO1 directly binds to AR at the TAU5 domain. A: Schematic diagram of AR NTD deletion mutants of AR fused to GST. NTD, NH2-terminal domain. TAU 5, transactivation unit 5.B: Purified GST and GST-AR fusion proteins (lower panel) were incubated with in vitro transcribed/translated 35S-labeled FOXO1. Bound FOXO1 proteins were subjected to SDS–PAGE analysis and autoradiography (upper panel).
FOXO1 Inhibits Ligand-Independent Transcriptional Activity of Alternatively Spliced AR Variants
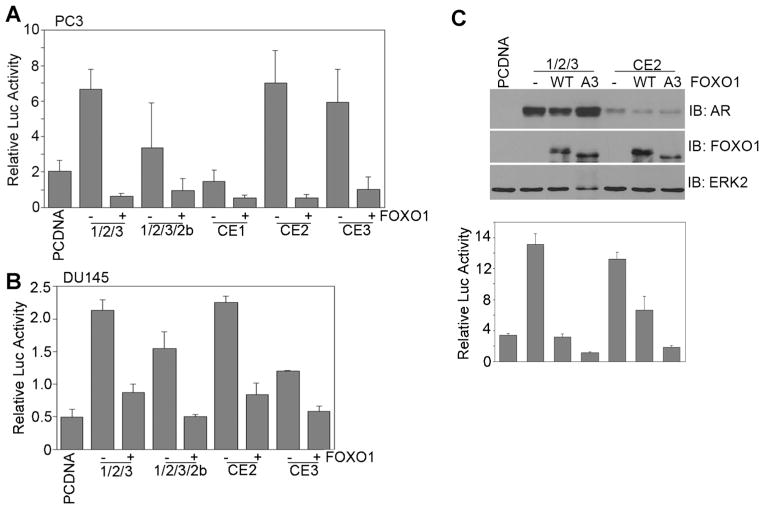
Recently, several groups independently identified a number of alternatively spliced AR isoform variants expressed in castration-resistant prostate cancer cell lines, tumors and xenografts [11–15], suggesting that these AR variants may be clinically relevant. The AR variants may contribute to castration-resistant progression of PCa since they only contain the ligand-independent AF1 function and are constitutively active in the absence of androgens. Given that FOXO1 directly binds to the TAU5 motif, which is important for AF1 function (Fig. 3), we were interested to determine whether FOXO1 was also able to inhibit the ligand-independent function of AR variants. Similar to previous reports [12,13,15], the AR variants were transcriptionally active, but the activity varied from one variant to the other when they were ectopically expressed in PC-3 PCa cells (Fig. 4A). Importantly, expression of FOXO1 invariably inhibited the transcriptional activity of all the truncated AR variants examined, including a synthetic truncated AR (AR-1/2/3) composed of just the NTD, DBD, and hinge region (Fig. 4A). It is noteworthy that the transcriptional activities of the truncated AR variants in cells transfected with FOXO1 were lower than the basal levels of the luciferase reporter gene (Fig. 4A). It has been shown previously that forced expression of FOXO1 induces cell death in PTEN mutated LNCaP and PC-3 cells [26,36–38]. Because luciferase protein is rapidly degraded after cell death [39,40], this may explain why reporter gene activity in FOXO1 transfected cells was lower than the mock transfected cells (Fig. 4A). To test this hypothesis, we repeated the experiments using DU145 cells which express endogenous PTEN and are not sensitive to FOXO1-induced cell death [36]. Ectopic expression of FOXO1 inhibited transcriptional activity of all the truncated AR variants examined in DU145 cells (Fig. 4B), but the residual activities of the truncated AR variants were not lower than that in mock transfected cells (Fig. 4B). Moreover, it has been shown previously that expression of constitutively active FOXO1 (FOXO1-A3), in which three Akt phosphorylation sites are mutated, induces much higher rate of cell death in PTEN-mutated prostate cancer cells [36]. To further test our hypothesis, we compared the inhibitory effects of wild-type FOXO1 (FOXO1-WT) and the FOXO1-A3 mutant on transcriptional activities of truncated AR variants in PC-3 cells. As shown in Figure 4C, FOXO1-A3 inhibited transcriptional activities of truncated AR variants to a greater degree, indicating that FOXO1-A3 may also induce this cell death effect. Together, these data demonstrate that ligand-independent activity of truncated AR variants can be inhibited by overexpression of FOXO1.
Fig. 4.
FOXO1 inhibits transcriptional activity of AR variants. PC-3 (A,C) or DU145 (B) cells were transfected with the PSA-Luc reporter and the plasmid for V5-tagged wild-type FOXO1 (FOXO1-WT) or FLAG-tagged constitutively active FOXO1 (FOXO1-A3). At 48 hr after transfection, luciferase activities were measured and normalized to renilla levels. Columns, mean among three individual experiments; bars, SD. Expression of AR variants and FOXO1 in transfected PC-3 cells were analyzed by western blot (C). Immunoblotting of extracellular signal-regulated kinase 2 (ERK2) was included as a loading control.
FOXO1 Blocks the Transcriptional Activity of AR Variants Augmented by the Transcriptional Coactivator SRC-1
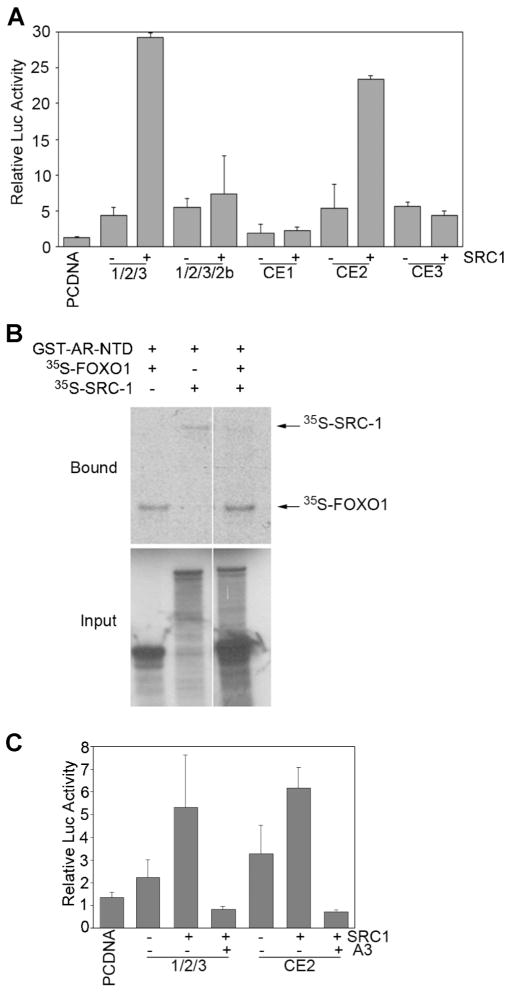
A large number of transcriptional coregulators, including coactivators in the p160 family, are important for both androgen-dependent and androgen-independent activation of the AR [41]. Intriguingly, the majority of these cofactors interact with AR NTD [42]. Of note, the recruitment of p160 coactivators such as SRC-1 to the AR is mediated through the TAU5 motif in the AR NTD [35]. We therefore sought to determine whether SRC-1 plays a role in androgen-independent activation of truncated AR variants and whether FOXO1 interferes with this function of SRC-1. As shown in Figure 5A, ectopic expression of SRC-1 markedly increased the transcriptional activity of the truncated AR variant derived from splicing of AR exons 1, 2, 3, and CE2 (also referred to as AR-V5) [12], but not other variants. SRC-1 also increased the activity of a synthetic AR variant mimicking mutant AR-1/ 2/3, which only contains AR exons 1, 2, 3, and the AR hinge region (Fig. 5A). Thus, the coactivator SRC-1 enhances transcriptional activity of select truncated AR variants, suggesting a novel regulatory role for the various unique COOH-terminal extensions. This result is consistent with previous observations that different AR variants differ in other functions such as cellular localization and transcriptional activity [12,13,43,44].
Fig. 5.
FOXO1 blocks the transcriptional activity of AR variants augmented by the transcription coactivator SRC-1. A: PC-3 cells were transfected with the PSA-luc reporter and the indicated plasmids. At 48 hr after transfection, luciferase activities were measured and normalized to renilla levels. Columns, mean among two individual experiments; bars, SD. B: Analysis of binding of AR-NTD with in vitro transcribed/translated FOXO1 and SRC-1 proteins. Purified GST-AR NTD fusion proteins were incubated with in vitro transcribed/translated 35S-labeled FOXO1 and/or SRC-1 (lower panel). Bound FOXO1 and SRC-1 proteins were subjected to SDS–PAGE analysis and autoradiography (upper panel). C: PC-3 cells were transfected and luciferase activities were measured as in (A).Columns, mean among three individual experiments; bars, SD.
Like SRC-1 [35], FOXO1 binds to the TAU5 motif in the AR NTD (Fig. 3). Therefore, we sought to determine whether there is any competition between FOXO1 and SRC-1 for binding to the AR. To this end, we performed in vitro protein binding assays. As expected, both SRC-1 and FOXO1 directly bound to the AR NTD (Fig. 5B, lanes 1 and 2). While approximately the same amount of SRC-1 proteins was used, addition of FOXO1 proteins diminished the binding of AR-NTD to SRC-1 (Fig. 5B, lane 3). In agreement with this result, expression of constitutively active FOXO1 abrogated SRC-1-enhanced transcriptional activity of the truncated AR variant AR-CE2/AR-V5 and a synthetic variant composed of AR exons 1/2/3 and the hinge region in PC-3 cells (Fig. 5C). Thus, these results suggest that one of the mechanisms by which FOXO1 inhibits ligand-independent activation of the AR is to compete with the function of coactivators such as SRC-1.
FOXO1 Affects Expression of Endogenous Genes Regulated by AR Splice Variants
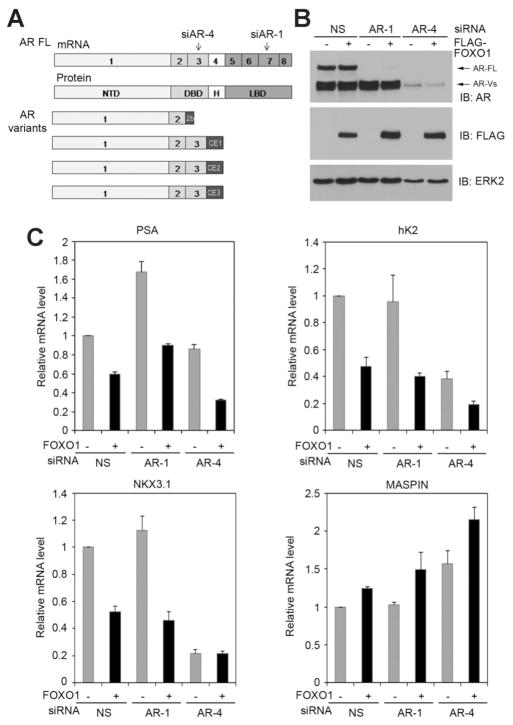
To further assess the relevance of FOXO1 inhibition of androgen-independent activation of the AR, we examined whether FOXO1 regulates the activities of endogenous full-length AR and truncated AR variants on endogenous AR target genes including PSA, hK2, NKX3.1, and MASPIN. To this end, we employed AR region-specific siRNAs to knock down full-length AR by targeting the LBD or full-length AR plus the majority of AR variants by targeting the exon 3 in 22Rv1 cells (Fig. 6A,B). Similar to a previous report [11], depletion of the full-length AR alone slightly increased PSA expression but had little or no effect on expression of hK2, NKX3.1 and MASPIN (Fig. 6C, lanes 1 and 3), while the underlying mechanism is not clear at present. In contrast, expression of AR activated genes PSA, hK2 and NKX3.1 was largely downregulated and expression of the AR repressed gene MAS-PIN was upregulated by further knockdown of the majority of AR variants (Fig. 6C, lanes 3 and 5), supporting an important role of AR variants in regulation of these AR target genes in 22Rv1 cells. As demonstrated in Figure 6B,C (lanes 1 and 2), expression of FOXO1 decreased expression of the AR activated genes PSA, hK2, and NKX3.1 in the presence of both full-length AR and AR variants in 22Rv-1 cells. In agreement with this result, ectopic expression of FOXO1 increased expression of the AR repressed gene MASPIN (Fig. 6C, lanes 1 and 2). In the absence of full-length AR, ectopic expression of FOXO1 also decreased expression of PSA, hK2, and NKX3.1 and increased expression of MASPIN (Fig. 6C, lanes 3 and 4), almost to the same degree when both the full- length AR and variants were present (Fig. 6C, lanes 1 and 2). These observations suggest that the antagonizing effect of FOXO1 on AR target gene expression appears to be primarily mediated by its inhibition of the transcriptional activities of constitutively active alternatively spliced AR variants. Furthermore, 22Rv1 cells express a broader repertoire of AR truncated variants than any other PCa cell lines and xenografts examined due to tandem duplication of a genomic segment between AR exons 2 and 4 [11,13]. Consistent with the fact that some AR variants such as AR/1/2/ 2b cannot be targeted by siAR-4 (Fig. 6A), there were certain species of AR variants retained in siAR4-transfected 22Rv1 cells (Fig. 6B). Importantly, transfection of siAR-4 expressed cells with FOXO1 further decreased expression of PSA and hK2 and increased expression of MASPIN (Fig. 6C, lanes 5 and 6). There was no effect of FOXO1 transfection on expression of NKX3.1 under these conditions (Fig. 6C, lanes 5 and 6). These data suggest that FOXO1 has differential effect on AR variants-mediated regulation of AR target gene expression in 22Rv1 cells. Together, our data show that FOXO1 inhibits expression of endogenous AR target genes regulated by alternatively spliced AR variants in 22Rv1 PCa cells.
Fig. 6.
FOXO1 regulates expression of endogenous AR target genes in 22Rv1 CRPC cells. A: Schematic diagram of AR mRNA and protein, AR siRNA targeting sites and major AR splice variants identified in 22 Rv1 cells. B,C: 22Rv1 cells were transfected with non-specific (NS) or AR siRNAs and empty vector or FLAG-tagged FOXO1. At 48 hr after transfection, cells were harvested for Western blot analysis (B) or real-time RT-PCR (C). Immunoblotting of extracellular signal-regulated kinase 2 (ERK2) was included as a loading control. Columns, mean among three individual experiments; bars, SD.
DISCUSSION
Androgen blockade and antiandrogen therapies are currently the standard treatments for advanced/ disseminated PCa. This therapy targets the ligand-binding domain in the COOH-terminus of the AR. The fact that prostate cancers invariably progress to a castration-resistant stage following androgen deprivation therapy implies that one of the possibilities for the failure of the therapy may be due to ligand-independent activation of the AR NTD. Therefore, the AR NTD represents one of the major potential targets for the treatment of CRPC. Importantly, the majority of the proteins that bind to and modulate the AR function interact with AR NTD [42]. Consistent with the finding that p160 coactivators are important for the androgen-independent activation of the full-length AR [35], we demonstrated that overexpression of SRC-1 augments ligand-independent activity of the truncated AR variant AR-CE2/AR-V5 and the synthetic variant composed of AR exons 1/2/3 and the hinge region (Fig. 5). These results suggest that like the full-length AR, the recruitment of AR coactivators appears to be important for the activation of certain alternatively spliced, truncated AR variants. It is worth noting that the transcriptional activities of different AR splice variants are variable depending on cell line and target promoter context [12,13,43–45]. Thus, transcriptional activation of different truncated AR variants may rely on different mechanisms, including differential coactivator utilization. This aspect of the truncated AR variants may depend on the identity and function of the different amino acid sequences at the COOH terminus [12,13,15,43,44]. Therefore, it will be important in the future to systematically dissect the role of various coactivators in transcriptional activation of truncated AR variants.
Previously, we demonstrated that the forkhead transcription factor FOXO1 binds to and inhibits androgen-independent activation of the AR [22]. This effect of FOXO1 is independent of its transactivation function [22]. A recent study shows that the function of the AR-V7 (termed as AR-CE3 in this study) is regulated by FOXO1 in a PTEN-PI3K-AKT-dependent manner, although it remains unknown how FOXO1 affects AR-V7’s transcriptional activity [46]. We demonstrated in the present study that the AR inhibitory function of FOXO1 is mediated through its NH2-terminus. In this region, there is a putative repression domain (RD) that has long been suspected to possess a function in transcription repression [28,29]. We further found that there are three putative mSin3A interacting motifs (SIDs) in the RD of FOXO1. However, mutagenesis studies demonstrated that deletion of the SID motifs in the RD of FOXO1 had no effect on FOXO1-mediated inhibition of the AR in PCa cells, suggesting that inhibition of the AR by FOXO1 is mediated through other mechanisms. We provide evidence that FOXO1 directly binds to the TAU5 motif in the AR NTD. Consistent with the previous observation that the recruitment of the p160 coactivator SRC-1 to the AR NTD is mediated through the TAU5 motif [35], we also found that SRC-1-augmented activation of truncated AR variants was abolished by expression of FOXO1. Moreover, we further showed that the physical interaction between SRC-1 and AR NTD was diminished by FOXO1. Based upon these observations, we envision a working model in which FOXO1 directly binds to the TAU5 motif in the AR NTD and thereby inhibits the ligand-independent, coactivator-enhanced transcriptional activity of AR variants. Thus, our data reveal a novel AR inhibitory mechanism of FOXO1.
We and others demonstrated previously that inhibition of the AR by FOXO1 requires the nuclear localization of FOXO1 [21,22]. It is well established that loss of the PTEN tumor suppressor leads to constitutive activation of Akt, which results in phosphorylation and nuclear exclusion of FOXO1 [47]. Intriguingly, Akt has been shown to be a preferential transcription target of one of the gain-of-function truncated AR variants, but not the wild-type AR in PCa cells [13]. Moreover, expression of the majority of AR splice variants identified is much higher in PTEN-positive PCa cell lines including CWR-R1, 22Rv1, VCaP, and Myc-CaP than in PTEN-null cells such as LNCaP xenografts [11,13,15]. Thus, further studies are warranted to explore if loss of PTEN and expression of AR splice variants are mutually exclusive events in human PCa specimens.
In summary, we demonstrated that the AR inhibitory effect of FOXO1 is mediated by NH2-terminal domain. While this effect of FOXO1 is independent of the putative mSin3A interaction domains identified in the NH2-terminal transcription repression domain of FOXO1, we provide evidence that FOXO1 binds directly to the TAU5 motif in the AR NTD that is known to be required for the recruitment of AR coactivators and ligand-independent activation of the AR. Consistent with this observation, we further showed that FOXO1 not only inhibits the constitutive activation of the alternatively spliced AR variants that lack the ligand-binding domain, but also abolishes the transcriptional activity of the AR variants enhanced by the AR coactivator SRC-1 by blocking the binding of SRC-1 to AR. Together, our present study demonstrates a novel role of FOXO1 in inhibition of the constitutive function of the alternatively spliced AR variants. Given the emerging role of the AR variants in castration-resistant progression of PCa, FOXO1 inhibition of AR variants may be targeted for the development of new therapeutics against castration-resistant PCa.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA134514 and CA130908 to H.H.; CA141011 to S.M.D.), the Department of Defense (W81XWH-09-1-622 to H.H. and W81XWH-10-1-0353 to S.M.D.), and the Prostate Cancer Foundation (to S.M.D.).
References
- 1.Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12:193–207. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- 2.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 3.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–1013. [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 6.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, Bremner WJ, Gleave ME, Nelson PS. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 7.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 8.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 9.Ueda T, Mawji NR, Bruchovsky N, Sadar MD. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 10.Debes JD, Schmidt LJ, Huang H, Tindall DJ. p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res. 2002;62:5632–5636. [PubMed] [Google Scholar]
- 11.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 17.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 19.Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in Nkx3. 1; Pten mice. Cancer Res. 2006;66:7929–7933. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- 20.Dong XY, Chen C, Sun X, Guo P, Vessella RL, Wang RX, Chung LW, Zhou W, Dong JT. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 21.Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- 23.Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, Bai W. FoxO1 mediates PTEN suppression of androgen receptor N-and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–225. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehm SM, Tindall DJ. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem. 2006;281:27882–27893. doi: 10.1074/jbc.M605002200. [DOI] [PubMed] [Google Scholar]
- 25.Chan SC, Li Y, Dehm SM. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J Biol Chem. 2012;287:19736–19749. doi: 10.1074/jbc.M112.352930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohrer LR, Chen S, Hallstrom TC, Huang H. Androgens suppress EZH2 expression via retinoblastoma (RB) and p130-dependent pathways: A potential mechanism of androgen-refractory progression of prostate cancer. Endocrinology. 2010;151:5136–5145. doi: 10.1210/en.2010-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, III, Emanuel BS, Rovera G, Barr FG. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–235. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 29.Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, Nawaz Z, Yee D, Barr FG, Diab SG, Brown PH, Fuqua SA, Osborne CK. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- 32.Liu P, Kao TP, Huang H. CDK1 promotes cell proliferation and survival via phosphorylation and inhibition of FOXO1 transcription factor. Oncogene. 2008;27:4733–4744. doi: 10.1038/onc.2008.104. [DOI] [PubMed] [Google Scholar]
- 33.Gan L, Liu P, Lu H, Chen S, Yang J, McCarthy JB, Knudsen KE, Huang H. Cyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikis. Cell Death Differ. 2009;16:1408–1417. doi: 10.1038/cdd.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callewaert L, Van Tilborgh N, Claessens F. Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res. 2006;66:543–553. doi: 10.1158/0008-5472.CAN-05-2389. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang H, Muddiman DC, Tindall DJ. Androgens negatively regulate forkhead transcription factor FKHR (FOXO1) through a proteolytic mechanism in prostate cancer cells. J Biol Chem. 2004;279:13866–13877. doi: 10.1074/jbc.M314143200. [DOI] [PubMed] [Google Scholar]
- 39.Coombe DR, Nakhoul AM, Stevenson SM, Peroni SE, Sanderson CJ. Expressed luciferase viability assay (ELVA) for the measurement of cell growth and viability. J Immunol Methods. 1998;215:145–150. doi: 10.1016/s0022-1759(98)00081-7. [DOI] [PubMed] [Google Scholar]
- 40.Cree IA. Luminescence-based cell viability testing. Methods Mol Biol. 1998;102:169–177. doi: 10.1385/0-89603-520-4:169. [DOI] [PubMed] [Google Scholar]
- 41.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 42.Wang G, Sadar MD. Amino-terminus domain of the androgen receptor as a molecular target to prevent the hormonal progression of prostate cancer. J Cell Biochem. 2006;98:36–53. doi: 10.1002/jcb.20802. [DOI] [PubMed] [Google Scholar]
- 43.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, Edwards J, Isaacs WB, Nelson PS, Bluemn E, Plymate SR, Luo J. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mediwala SN, Sun H, Szafran AT, Hartig SM, Sonpavde G, Hayes TG, Thiagarajan P, Mancini MA, Marcelli M. The activity of the androgen receptor variant AR-V7 is regulated by FOXO1 in a PTEN-PI3K-AKT-dependent way. Prostate. 2013;73:267–277. doi: 10.1002/pros.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]