Abstract
Purpose of Review: The term mild cognitive impairment (MCI) is used to describe older subjects with demonstrable cognitive impairment who have not crossed the threshold for dementia. Because patients with MCI have an increased risk of developing dementia, especially Alzheimer disease (AD), there is significant interest in the clinical characterization of these subjects and in understanding the pathophysiology of the transition from MCI to AD.
Recent Findings: The MCI syndrome, as an expression of an incipient disorder that may lead to dementia, is extremely heterogeneous and may coexist with systemic, neurologic, or psychiatric disorders that can cause cognitive deficits. Recent clinical criteria were designed to take into account the different forms of clinical presentation of the syndrome, and introduced the possible contribution of biomarkers to the clinical diagnosis. Bedside diagnosis of MCI can be difficult, since patients who report having cognitive problems may have normal scores in global cognitive scales or in brief neuropsychological instruments.
Summary: This article presents the evolution of the clinical concept of MCI, the operationalization of its current definitions, the development of biomarkers that can help to identify an underlying neurodegenerative process as the etiology of the syndrome, and its proposed treatments.
INTRODUCTION
Aging is associated with cognitive decline,1,2 and older subjects can have demonstrable cognitive impairment without crossing the threshold for dementia. This condition has been termed “mild cognitive impairment” (MCI), and these patients have an increased risk of developing dementia, especially Alzheimer disease (AD).3–6 Studies conducted in referral clinics have shown that patients with MCI progress to AD at a rate of 10% to 15% per year,5,7 and 80% of these patients have converted to AD after approximately 6 years of follow-up.5
The identification and classification of MCI can be a major challenge. The MCI syndrome, as an expression of an incipient neurodegenerative disorder that may lead to dementia, is extremely heterogeneous and may coexist with systemic, neurologic, or psychiatric disorders that can cause cognitive deficits. In addition, data from community-based studies have shown a greater variability in the clinical course of the syndromes than that observed in referral clinics: some patients with MCI progress to dementia, some remain stable, some improve over time,8,9 and some who return to normal can go back to MCI and eventually develop dementia.10 This article discusses the evolution of the clinical concept of MCI, the operationalization of the current definition of MCI, the development of biomarkers that can help to identify an underlying neurodegenerative process as the etiology of the syndrome, and its treatment.
MILD COGNITIVE IMPAIRMENT CRITERIA
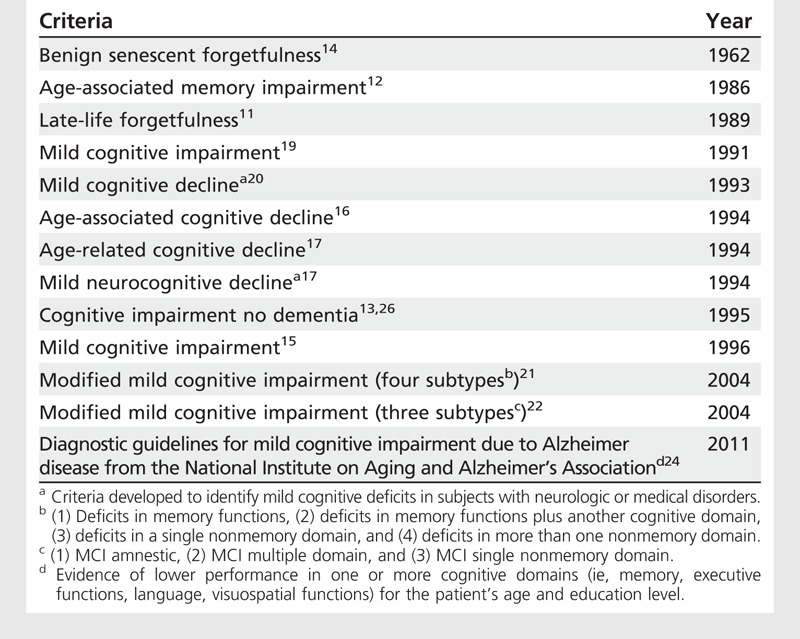
Initially, the criteria for MCI followed two conceptual models: one associated only with memory deficits, and the other with a broader range of deficits (memory and other areas of cognition). Because memory deficits are the clinical hallmark of AD, most of the criteria developed to characterize MCI required the presence of memory deficits in isolation.11–15 However, other clinicians felt that the memory-centered definition of MCI was too restrictive because it did not capture other cognitive problems that often occur in the elderly.16,17 For example, the International Psychogeriatric Association and the World Health Organization proposed the term “age-associated cognitive decline” (AACD) to describe subjects with a wider range of cognitive deficits.16 In addition, longitudinal studies showed that patients with MCI with or without memory deficits can progress to AD,18 and epidemiologic studies showed that the prevalence of the MCI syndrome with isolated memory deficits was lower than that observed in subjects who presented with a wider range of cognitive problems.10 Table 7-111–17,19–22 shows the different diagnostic criteria used to identify subjects with MCI.
Table 7-1.
Criteria Developed to Characterize Cognitive Impairments in Nondemented Elderly Subjects
The most recent criteria for MCI encompassed all possible cognitive manifestations of the syndrome and four subgroups have been proposed: deficits only in memory functions; memory deficits plus deficits in another cognitive domain; deficits in a single nonmemory domain; and deficits in more than one nonmemory domain.21,22 This has expanded the knowledge of the MCI syndrome and allowed examination of the relationship between MCI syndromes and other dementias that do not have memory deficits. For example, the MCI with isolated executive deficits has been reported to be associated with cerebrovascular disease and a predictor of vascular dementia.23
The National Institute on Aging and Alzheimer’s Association (NIA-AA) criteria for MCI were created to characterize a syndrome that is most likely associated with AD pathology.24 The purpose of these criteria was to identify subjects in the pre-AD state, and they require that patients with MCI must have impairments in one or more cognitive domains. Although the criteria emphasized the presence of memory deficits as the central characteristic of the syndrome that can progress to AD, they recognized that there are forms of AD (eg, visual or language variants) that do not have memory deficits in their early stages. In addition, the syndrome should not be present in the context of vascular, traumatic, or other causes of mild CNS dysfunction. However, while these criteria maximized the likelihood that subjects with MCI who participate in prevention trials for AD or other research studies will have an underlying AD pathology, the use of the criteria in clinical practice is difficult. MCI subjects with multiple disease processes that can affect cognition can progress to AD,18 and not all AD patients have memory loss at initial presentation;25 usually, mild nonmemory function deficits are difficult to detect with brief cognitive evaluations (Case 7-1).
Case 7-1
A 65-year-old man with 16 years of education presented with a 2-year history of progressive memory problems that affected his job performance. His colleagues had not noticed these problems, nor had his wife reported him having any cognitive problems at home. He had recently been diagnosed with hypertension, but his vital signs were normal. He denied any symptoms of major depression. The results of his neurologic examination were normal, and his Mini-Mental State Examination (MMSE) score was 29/30 (he forgot the floor on which the doctor’s office was located in the orientation subtest). His clock-drawing test results were normal, and his verbal fluency for animals was 11. Laboratory tests and brain MRI showed no abnormalities. Because of the patient’s concerns about his progressive memory problems, he was referred for a comprehensive neuropsychological assessment. The test showed that his memory functions were 1.5 standard deviations (SD) below the mean adjusted for people of his age and education level, and his executive functions were between 1.0 and 1.5 SD below the mean. He had normal language, visuospatial, and visuoconstructional functions.
Two years later, the patient retired from his job due to the worsening in his cognition, and his wife noticed problems in his instrumental activities of daily living (eg, the patient forgot to pay bills and got lost while driving). His MMSE score dropped to 25/30, and his comprehensive cognitive evaluation showed deficits in memory, language, and executive functions, consistent with a dementia syndrome, most likely Alzheimer disease.
Comment. This is a highly educated man who noticed that his cognitive functions were severe enough to interfere with his occupational affairs. Initially, his wife and colleagues did not detect these problems, and the brief neuropsychological evaluation conducted at the neurologist’s office was within normal limits. However, detailed cognitive assessment by a neuropsychologist revealed the presence of memory and executive function deficits. This patient presented with the memory plus other cognitive domain subtype of mild cognitive impairment (MCI), which later progressed to dementia. This case illustrates two important aspects of the clinical diagnosis of MCI: (1) it is difficult to detect the syndrome with bedside cognitive instruments, especially in highly educated people; and (2) additional cognitive evaluations are an important tool to detect not only impaired cognitive domains during the MCI stage, but also during the early stages of Alzheimer disease.
Operationalization of Mild Cognitive Impairment Criteria
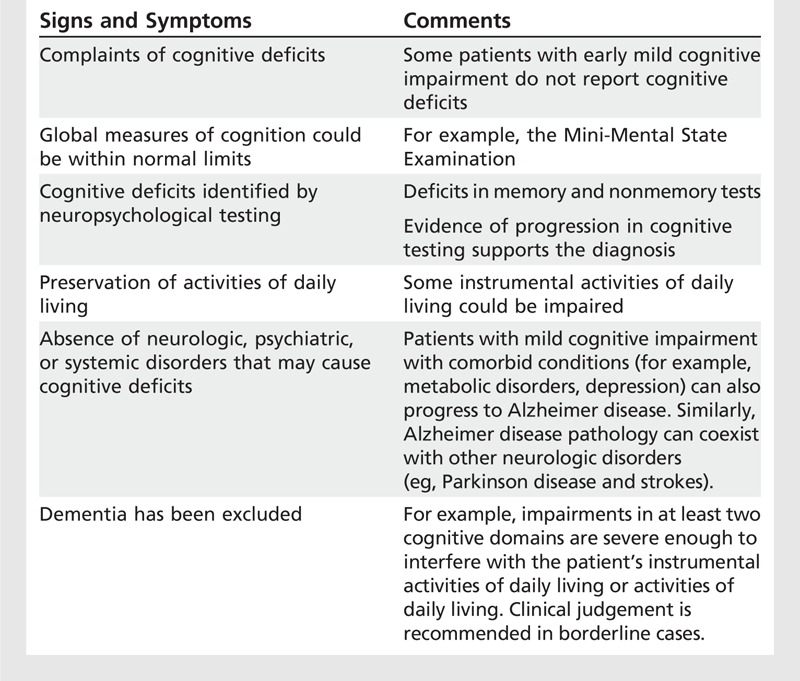
The MCI syndrome is centered on the presence of an abnormal neuropsychological performance associated with other signs and symptoms26 (Table 7-2), specifically: (1) complaints of cognitive deficits (or awareness of cognitive problems); (2) deficits in memory or other cognitive domain function demonstrable by testing; and (3) intact activities of daily living (ADLs), although mild problems in instrumental activities of daily living (IADLs) could be present. The majority of the published criteria exclude cases with other medical conditions that may cause cognitive deficits.26
Table 7-2.
Components of the Mild Cognitive Impairment Syndrome Associated With Alzheimer Disease Required by the Majority of the Criteria
Complaints of memory deficits. A certain level of concern about their cognitive problems should be present in these patients, which in turn motivates the visit to a memory clinic. Indeed, all of the criteria for MCI require that the patient report having cognitive problems, and studies conducted with subjects who have no dementia have shown that complaints of cognitive problems were predictors of incident dementia.7,27 However, there are studies that have not identified complaints of cognitive problems as risk factors for dementia,28 and mood-related complaints were found to be better predictors of cognitive decline.29 A study conducted in a German population found that subjects who reported memory problems and were worried about these problems had an increased risk of progressing to dementia compared to those who did not report or worry about memory problems.30
Examination of the types of subjects enrolled in MCI studies is critical to understand the validity of complaints of memory problems as a predictor of dementia and as a component of the syndrome. The fact that subjects request evaluations through specialized clinics is an indication that they or their families or physicians have noticed cognitive problems. By contrast, population-based studies identify a more heterogenous group, including those whose symptoms are minimal; those who think that their cognitive problems are not relevant, in spite of the presence of a more advanced syndrome; those with a more advanced syndrome who do report cognitive problems (similar to those seen in referral clinics); and those with multiple comorbidities that explain the presence of cognitive problems. Therefore, the subjects seen in referral clinics are likely to have a more advanced disease (possibly related to neurodegeneration) than those identified in population-based studies. The NIA-AA MCI guidelines have addressed this issue and stated that the subjective cognitive complaints or reports of cognitive change can come from the patient, an informant, or the physician observing the patient.
Neuropsychological assessment. Detailed cognitive assessments are the most important tool in the diagnosis of MCI and its subgroups, since these patients tend to perform normally in global cognitive measures such as the Mini-Mental State Examination.31 The neuropsychological battery should cover a range of areas of cognition and must be sensitive enough to detect MCI subgroups as well as the possible contributions of other disease processes (eg, cerebrovascular disease) to the manifestation of the syndrome. Although no consensus has been reached about the components of the neuropsychological battery to identify MCI, the battery should include tests that can clearly identify performance in at least four cognitive domains: memory, language, attention/executive, and visuoperceptual/visuoconstructional functions. This represents a limitation to the bedside diagnosis of the syndrome, since extensive cognitive evaluations are difficult to perform in a busy clinical practice. Consequently, these patients should be referred for neuropsychological evaluation.
Memory loss predicts progression to AD in the amnestic form of MCI.3,7,15,32,33 However, there have been studies demonstrating that verbal fluency, attentional, and executive function deficits are also predictors of dementia,8,34 and others found the severity of deficits in memory and verbal fluency8 or executive function35 in MCI to be the best predictors of incident AD. In addition, patients with MCI who presented with a demonstrable progression of their deficits were shown to be more likely to develop dementia than those whose deficits remained stable.19 Indeed, the NIA-AA guidelines stated that support for the diagnosis of MCI could be provided by evidence of a decline on serial testing.
The overall prevalence of MCI in the general elderly population ranges from 2% to 20%,6,9 and the neuropsychological definition of MCI has been instrumental in the determination of the prevalence of the syndrome in epidemiologic studies. The MCI memory-only subtype has a lower prevalence (eg, 2% to 4%)9 compared to the much broader MCI definition that includes all subtypes (eg, 18% to 21%).6 The prevalence of the MCI syndrome is very dynamic, and it has been reported that up to 30% to 55% of the cases can return to normal during follow-up,9 although 19% to 20% is a more conservative estimate.10 The MCI memory-only subtype is more frequent in specialized referral clinics and consequently has been the most extensively examined MCI syndrome in neuroimaging and biomarker studies.
Activities of daily living. Patients with MCI should have normal ADLs (eg, getting dressed or controlling sphincters), although they can have mild deficits in IADLs (eg, problems in job performance, forgetting to pay a bill). However, the determination of abnormal IADLs can be difficult in an older population. For example, subjects who remain active in their careers (eg, physicians) may report mild changes affecting their professional activities. By contrast, subjects with sedentary lifestyles after retirement (or with lower-skill jobs that they have practiced throughout their lives), who perform activities that do not require significant intellectual challenge, may not report IADL problems (Case 7-2). These examples represent the two extremes generally encountered in clinical practice: high-functioning patients may be overly sensitive in reporting IADL deficits, whereas less challenged subjects may be unaware of changes. Finally, it has been shown that the neuropsychological characteristics of the MCI syndrome can be present in subjects who do not report memory deficits.36
Case 7-2
A 78-year-old woman with 12 years of education presented for evaluation with a 3-year history of cognitive decline associated with symptoms of major depression. According to her family, she had depression associated with cognitive problems, which had worsened over the past 6 months. She had depressed mood, apathy, anxiety, crying spells, suicidal ideation, insomnia, and lack of appetite; she had lost 15 pounds in 1 year. She also had short-term memory problems and difficulty managing her finances and using the pillbox for her daily medication. However, the effects of her cognitive problems on her activities of daily living were difficult to determine, since she did not do much at home because of her depressed mood and lack of motivation. She had a history of breast cancer (treated with mastectomy), coronary artery disease, glaucoma, and arthritis, but no history of depression or other psychiatric illness. The patient was medicated with sertraline 25 mg/d for her symptoms of depression 3 months ago. Her laboratory test results were normal and MRI of her brain showed mild atrophy. No infarcts or white matter lesions were noted.
On examination, the patient looked tearful and had minimal bradykinesia. Her Mini-Mental State Examination (MMSE) score was 24 (1/3 recall and 1/5 in attention subtests). Her clock-drawing test results were normal, and verbal fluency for animals was 7. Her Hamilton Depression Rating Scale score was 20; she met criteria for major depression.
Because of the severity of the patient’s depression, sertraline was gradually increased to 150 mg/d. She was re-examined 6 months after her initial examination, after she had remained stable on sertraline 150 mg/d for a month. Her MMSE score was 30, her clock-drawing test results were normal, and her verbal fluency for animals was 14. Her Hamilton Depression Rating Scale score was 5; she met criteria for major depression in full remission.
Comment. This patient developed late-life major depression that was severe enough to affect her cognition and instrumental activities of daily living. She initially presented with the memory plus other cognitive domain subtype of mild cognitive impairment (MCI). After treatment with antidepressant medication, her cognition and depression improved, and she returned to normal. This case illustrates that late-life mood disorders can cause an MCI syndrome, and with proper treatment these patients can return to normal. However, close follow-up of the symptoms of depression and its treatment is recommended, since they may return; repeat cognitive evaluations on an annual basis are also recommended, since depressed mood has been identified as a risk factor for incident Alzheimer disease.
Comorbid conditions. There are several neurologic, systemic, and psychiatric syndromes that can cause cognitive impairment (eg, depression, vascular disease).37,38 The distinction of MCI groups based on the possible etiology of the cognitive deficits is essential to identify a syndrome that has increased likelihood of progression to AD. However, the reality is that patients with MCI with multiple comorbidities are the most common in clinical practice and progress to AD at the same rate as those without comorbidities.18 Consequently, even in the presence of a disease process that could explain the MCI syndrome, it could be an underlying neurodegenerative process that will eventually lead to the dementia symptoms. In this case, the biomarkers have a critical role in identifying AD pathology in patients with MCI with comorbid conditions. Figure 7-1 shows that the majority of the MCI cases detected in the general population had medical processes that can explain the presence of cognitive deficits.6 In addition, other neurodegenerative disorders that cause dementia either in isolation or in combination with AD pathology can develop an MCI syndrome before progressing to dementia (eg, Parkinson disease).
Figure 7-1.
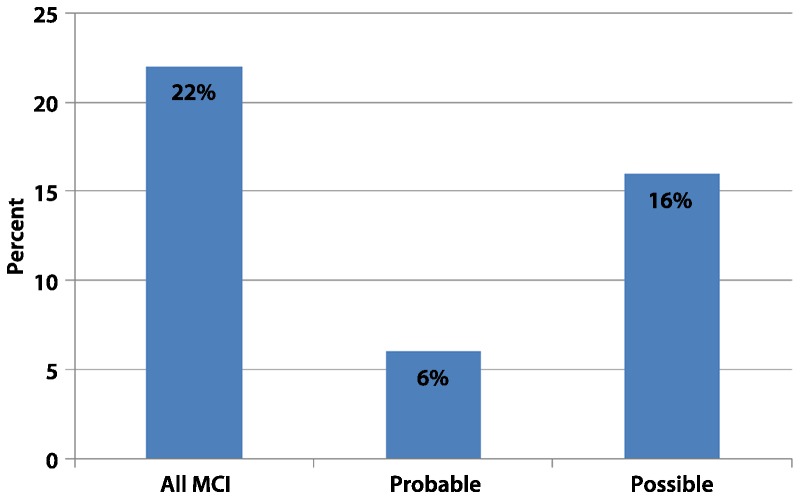
Prevalence of mild cognitive impairment (MCI) in the Pittsburgh Cardiovascular Health Study according to the presence of comorbid conditions. Probable MCI is indicated when the etiology of the MCI syndrome was most likely associated with a neurodegenerative process. Possible MCI is indicated when the etiology of the MCI syndrome could be secondary to medical (eg, cancer), psychiatric (eg, depression), or neurologic (eg, stroke) processes.
Reprinted with permission from Lopez OL, et al, Arch Neurol.6 © 2003, American Medical Association. All rights reserved. archneur.jamanetwork.com/article.aspx?articleid=784823.
BIOMARKERS
The use of CSF and neuroimaging biomarkers can improve the identification of AD pathology in patients with MCI and predict the likelihood of progression to dementia, especially within a relatively short period.39–42 The altered pattern of CSF protein levels usually seen in patients with AD can also be seen in those with MCI (especially in those who will convert to AD): high levels of tau or phosphorylated tau (P-tau) with decreased amyloid-β42 (Aβ42) protein levels.41 However, because there is a great variability in the tau and Aβ42 levels in patients with AD,43 negative CSF studies may not completely exclude the presence of AD pathology in patients with MCI. In addition, CSF studies are an important tool to exclude other disease processes that can cause cognitive problems (eg, infections).
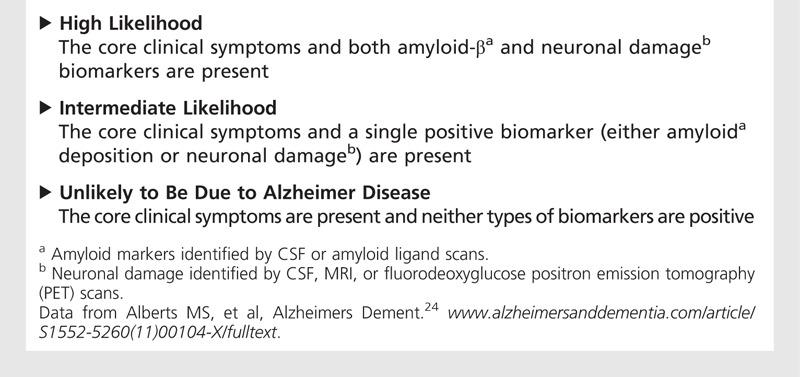
Table 7-3 shows the proposed NIA-AA biomarker criteria to grade the certainty that the MCI syndrome is due to AD. However, the authors cautioned against the use of biomarkers as predictors of incident dementia until more experience is gained, and recommended their use only for research purposes or in special clinical cases. A recent report found that single-marker models were as effective as multiple-marker models, and their accuracy was only 64%.44 It is also critical to understand that the presence of the APOE*E4 allele is not diagnostic but should be considered a risk factor for AD.
Table 7-3.
The National Institute on Aging and Alzheimer’s Association Criteria for Biomarker Certainty That the Mild Cognitive Impairment Syndrome is Caused by Alzheimer Disease
Structural MRI
The visual determination of regional or global atrophy in MCI cases is difficult because brain structure can vary greatly in elderly subjects.45 Quantitative analyses have shown that hippocampal and other brain regions are different in patients with MCI than in people who are cognitively normal.46 Patients with AD can be differentiated from normal subjects based on the volume of medial temporal lobe structures,47,48 although it is more difficult to differentiate MCI from normal aging. A study of hippocampal volumes conducted in 166 subjects (55 with AD, 43 with MCI, and 62 controls) found that hippocampal volumes could differentiate AD from controls in 92% of the cases, but there were no differences between controls and MCI.47 By contrast, other studies have shown that patients with MCI can have smaller hippocampal volumes compared to controls.49 While the amnestic type had a greater volume loss in the hippocampus and amygdala compared to normal subjects, the MCI type with impairments in multiple cognitive domains had a greater volume loss in the parietal, superior temporal, and frontal regions, with less involvement of the hippocampus.50
Hippocampal51,52 and other cerebral structure53–57 volumes can predict the development of AD in subjects with MCI, and those who progressed to AD had smaller entorhinal, superior temporal, and occipitotemporal cortices and anterior cingulate gyrus volumes compared to those who did not progress.53,54 Changes over time in hippocampal and entorhinal cortex volumes have been reported in subjects with MCI who progressed to dementia compared to those who did not,58–60 as well as an accelerated expansion of the lateral ventricles.61
Functional Imaging
Clinical fluorodeoxyglucose positron emission tomography (FDG-PET) and single-photon emission computed tomography (SPECT) studies show the “classic” AD pattern in subjects with MCI whose cognitive symptoms are thought to be related to an incipient AD-related dementia.39 The FDG-PET scan has shown a good correlation with cognitive measures in patients with MCI,62 with a sensitivity of 92% and a specificity of 89% to predict conversion to AD.63 Although the SPECT scan can be a useful tool to identify regions of hypoperfusion in MCI cases, it has shown little predictive value.64 Functional MRI studies are usually conducted in research settings and have found that subjects with MCI have a greater activation of the hippocampus compared to normal controls and subjects with AD in memory tasks.65 Studies that used executive tasks found increased activation in the parietal lobes, with decreased activation in the prefrontal cortex and anterior cingulate gyrus.66 Perfusion MRI studies have shown that subjects with MCI have increased cerebral blood flow in anterior cingulate, basal ganglia, and hippocampal regions and decreased cerebral blood flow in the precuneus and temporal-parietal regions.67
Amyloid Ligands
The approval by the US Food and Drug Administration (FDA) of florbetapir in 2012 has brought to the market an important tool for the diagnosis of AD, although it was recommended only for the differential diagnosis of AD.68 The use of clinical amyloid ligands in normal subjects or those with MCI should be avoided, since there is a dearth of longitudinal information that would allow prediction of incident dementia in amyloid-positive subjects or of whether amyloid-negative subjects can progress to AD syndromes.
The combination of multiple neuroimaging techniques has permitted improved examination of the pathophysiology of the transition from MCI to dementia. The finding that amyloid deposition detected with carbon-11 Pittsburgh Compound B (PiB) precedes structural changes in subjects with MCI suggests that the amyloid deposition occurs first, which is followed by neuronal loss.69 In addition, there is a positive correlation between PiB deposition and brain metabolism, as measured with FDG-PET, suggesting the presence of a compensatory process in the MCI state.70 A recent study in cognitively normal subjects showed that PiB deposition was associated with increased PET metabolism, gray matter volume loss, and visual memory measures.71 These studies indicated the presence of concomitant pathologic and (compensatory) physiologic mechanisms in subjects destined to develop AD, and that amyloid deposition has already started to exert its effect on brain function before subjects reach the MCI state (Case 7-3).
Case 7-3
An 80-year-old man with 12 years of education presented with a 3-year history of progressive memory problems, which started approximately 1 year after he had a stroke. His wife had also noticed mild problems with his instrumental activities of daily living (eg, the patient forgot to pay bills and had difficulty with hobbies). He had a history of hypertension and coronary artery disease, but his vital signs were normal. He denied any symptom of major depression. The neurologic examination showed mild weakness in his left arm, sequelae of the stroke. His Mini-Mental State Examination (MMSE) score was 27/30 (2/5 in attention subtests). The results of his clock-drawing test were borderline, and his verbal fluency for animals was 12. Laboratory test results were normal. MRI of his brain showed mild atrophy and a 3- to 4-mm infarct in the right caudate nucleus. A comprehensive neuropsychological assessment showed that his executive functions were 1.5 standard deviations (SD) below the mean adjusted for people of his age and education level, and his memory function was between 1.0 and 1.5 SD below the mean. The patient had read about the use of biomarkers in the clinical diagnosis of dementia and expressed concerns about the possible neurodegenerative etiology of his problems. Therefore, his neurologist requested a fluorodeoxyglucose positron emission tomography (FDG-PET) scan, which showed decreased metabolism in the right basal ganglia and in the temporoparietal cortex, including the posterior cingulate gyrus, bilaterally.
One year later, the patient’s wife noted progression of his difficulties in instrumental activities of daily living (eg, getting lost while driving). However, the results of his MMSE and other tests performed at the office remained unchanged. The neuropsychological examination showed that his executive function deficits remained stable, but there was progression in his memory deficits, although they remained 1.0 and 1.5 SD below the mean. The neurologist prescribed a cholinesterase inhibitor.
Comment. This is a patient with a history of cerebrovascular disease who presented with a memory plus other domain subtype of mild cognitive impairment (MCI). Because of the presence of an abnormal FDG-PET that showed an Alzheimer disease pattern, and the reported worsening of his instrumental activities of daily living, he was initiated on dementia medication. This case illustrates the complexity of the clinical aspects of the MCI diagnosis and management: (1) the bedside examination showed that this patient had the nonmemory single-domain MCI subtype, while the more extensive cognitive assessment showed a memory plus other domain subtype, which suggests that the sensitivity of the cognitive battery defined the MCI type; (2) although the patient’s family perceived a worsening of his performance on instrumental activities of daily living, the cognitive testing was not indicative of dementia, but it led the treating neurologist to initiate dementia treatment; (3) the presence of an abnormal biomarker (FDG-PET) was not associated with imminent conversion to dementia, although it supported the decision to initiate treatment.
TREATMENT
There is no consensus about the pharmacologic and nonpharmacologic treatment of MCI. Because the MCI syndrome is considered the earliest manifestation of AD (in those patients with underlying AD pathology as the cause of their MCI), and cholinesterase inhibitors (ChEIs) are used to treat patients with AD, several studies examined whether ChEIs can improve cognition in patients with MCI and prevent the conversion from MCI to AD. The use of donepezil in subjects with MCI during a 48-week period showed modest improvements,72,73 and three large-scale studies showed that ChEIs failed to prevent conversion from MCI to AD.74–76 A 3-year double-blind study that examined the effects of 2000 IU of vitamin E daily or 10 mg of donepezil daily on conversion from MCI to AD found that vitamin E had no benefit in patients with MCI. Although donepezil was associated with a lower rate of progression to AD during the first year of treatment, the rate of progression to AD was similar among the treated and placebo arms at the end of the study.74
ChEIs have shown benefits in subjects with MCI and symptoms of depression.77 However, the use of ChEIs in subjects with major depression with or without cognitive deficits should be carefully weighed. Recent studies conducted in elderly patients with late-life major depression showed that donepezil improved cognition but increased the rates of major depression (44% with donepezil versus 12% without it) and did not prevent progression to dementia compared to placebo.78
Studies that examined whether anti-inflammatory therapies prevent AD in MCI (ie, rofecoxib) or in mixed populations that included normal and MCI subjects (ie, celecoxib or naproxen) showed no benefits.79,80 Similarly, Gingko biloba showed no benefits preventing dementia in mixed populations.81,82 Observational studies have shown that cognitive83 and physical activity decreased the risk of dementia and MCI,84,85 and physically active subjects had higher brain volumes later in life.84 However, the effects of physical activity or cognitive interventions on cognition still have to be tested in large-scale studies. Although the literature has suggested that current AD therapies are not effective in preventing the conversion from MCI to dementia, these treatments should be discussed with patients since placebo-controlled studies with ChEIs in MCI have shown a modest symptomatic response. It is important to point out that all of the prevention trials using ChEIs were conducted in subjects with amnestic MCI, and there are no studies on the other clinical forms of MCI.
KEY POINTS
Patients with mild cognitive impairment are at risk of developing dementia, especially Alzheimer disease.
The mild cognitive impairment syndrome is not restricted to memory deficits, and these patients can present with a much broader cognitive syndrome, which may not include memory impairments.
The prevalence of mild cognitive impairment in the general elderly population ranges from 2% to more than 20%.
The mild cognitive impairment syndrome with memory-only deficits is less prevalent than mild cognitive impairment with a much broader cognitive syndrome in the general population.
The mild cognitive impairment syndrome, as an expression of an incipient neurodegenerative disorder that may lead to dementia, is extremely heterogeneous and may coexist with systemic, neurologic, or psychiatric disorders that can cause cognitive deficits.
Approximately 20% of patients with diagnosis of mild cognitive impairment return to normal cognition on follow-up examination.
Patients with mild cognitive impairment can present with mild deficits in instrumental activities of daily living.
Increased CSF phosphorylated tau and decreased Aβ-42 protein levels increase the short-term risk of conversion to Alzheimer disease in mild cognitive impairment patients.
Decreased metabolism in temporal-parietal regions, including the precuneus (detected with fluorodeoxyglucose-PET), and increased amyloid deposition (detected with amyloid ligands) increase the risk of conversion to Alzheimer disease.
Biomarker studies should be interpreted with caution. Although they indicate that Alzheimer disease pathology is present, they do not provide information about when the patient will progress to an Alzheimer disease clinical dementia.
There are no therapies that can prevent the conversion from mild cognitive impairment to Alzheimer disease. However, studies conducted with cholinesterase inhibitors have shown a modest cognitive improvement in subjects with mild cognitive impairment treated with these medications compared to placebo.
ACKNOWLEDGMENTS
This article was supported, in part, by grants AG01533 and AG20098 from the National Institute on Aging.
REFERENCES
- 1. Kaszniak AW, Poon LW, Riege W. Assessing memory deficits: an information-processing approach. In: Poon LW, ed Handbook for clinical memory assessment of older adults. Washington, DC: American Psychological Association, 1986 [Google Scholar]
- 2. Rubin EH, Storandt M, Kinscherf DA, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol 1998; 55 (3): 395– 401 [DOI] [PubMed] [Google Scholar]
- 3. Bowen J, Teri L, Kukull W, et al. Progression to dementia in patients with isolated memory loss. Lancet 1997; 349 (9054): 763– 765 [DOI] [PubMed] [Google Scholar]
- 4. Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001; 58 (3): 397– 405 [DOI] [PubMed] [Google Scholar]
- 5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 1999; 56 (3): 303– 308 [DOI] [PubMed] [Google Scholar]
- 6. Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognitive Study: part 1. Arch Neurol 2003; 60 (10): 1385– 1389 [DOI] [PubMed] [Google Scholar]
- 7. Tierney MC, Szalai JP, Snow WG, et al. Prediction of probable Alzheimer’s disease in memory-impaired patients: a prospective longitudinal study. Neurology 1996; 46 (3): 661– 665 [DOI] [PubMed] [Google Scholar]
- 8. Hanninen T, Hallikainen M, Koivisto K, et al. A follow-up study of age-associated memory impairment: neuropsychological predictors of dementia. J Am Geriatr Soc 1995; 43 (9): 1007– 1015 [DOI] [PubMed] [Google Scholar]
- 9. Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 2004; 63 (1): 115– 121 [DOI] [PubMed] [Google Scholar]
- 10. Lopez OL, Becker JT, Chang YF, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology 2012; 79 (15): 1599– 1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blackford RC, LaRue A. Criteria for diagnosing age associated memory impairment: proposed improvements from the field. Dev Neuropsychol 1989; 5 (4): 295– 306 [Google Scholar]
- 12. Crook TH, Bartus RT, Ferris SH, et al. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change. Report of a National Institute of Mental Health Work Group. Dev Neuropsychol 1986; 2 (4): 261– 276 [Google Scholar]
- 13. Graham JE, Rockwood K, Beattie EL. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349 (9068): 1793– 1796 [DOI] [PubMed] [Google Scholar]
- 14. Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86: 257– 260 [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen RC, Smith GE, Waring SC, et al. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 1997; 9 (suppl 1): 65– 69 [DOI] [PubMed] [Google Scholar]
- 16. Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr 1994; 6 (1): 63– 68 [PubMed] [Google Scholar]
- 17.American Psychiatric Association. DSM-IV: diagnostic and statistic manual of mental disorders, 4th ed Washington, DC: American Psychiatric Association, 1994 [Google Scholar]
- 18. Lopez OL, Kuller LH, Becker JT, et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study. Arch Neurol 2007; 64 (3): 416– 420 [DOI] [PubMed] [Google Scholar]
- 19. Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology 1991; 41 (7): 1006– 1009 [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization The ICD-10 classification of mental and behavioral disorders: diagnostic criteria for research. Geneva, Switzerland: World Health Organization, 1993 [Google Scholar]
- 21. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256 (3): 183– 194 [DOI] [PubMed] [Google Scholar]
- 22. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256 (3): 240– 246 [DOI] [PubMed] [Google Scholar]
- 23. O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003; 2 (2): 89– 98 [DOI] [PubMed] [Google Scholar]
- 24. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7 (3): 270– 279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker JT, Lopez OL, Wess J. Material-specific memory loss in probable Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1992; 55 (12): 1177– 1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol 1995; 52 (6): 612– 619 [DOI] [PubMed] [Google Scholar]
- 27. Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. Br J Psychiatry 1997; 171: 373– 376 [DOI] [PubMed] [Google Scholar]
- 28. Jorm AF, Christensen H, Korten AE, et al. Do cognitive complaints either predict future cognitive decline of reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychol Med 1997; 27 (1): 91– 98 [DOI] [PubMed] [Google Scholar]
- 29. Smith GE, Petersen RC, Ivnik RJ, et al. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging 1996; 11 (2): 272– 279 [DOI] [PubMed] [Google Scholar]
- 30. Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010; 67 (4): 414– 422 [DOI] [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12 (3): 189– 198 [DOI] [PubMed] [Google Scholar]
- 32. Howieson DB, Dame A, Camicioli R, et al. Cognitive markers preceding Alzheimer’s dementia in the healthy oldest old. J Am Geriatr Soc 1997; 45 (5): 584– 589 [DOI] [PubMed] [Google Scholar]
- 33. Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996; 46 (1): 121– 125 [DOI] [PubMed] [Google Scholar]
- 34. Hanninen MA, Hallikainen M, Koivisto K, et al. Decline of frontal lobe functions in subjects with age-associated memory impairment. Neurology 1997; 48 (1): 148– 153 [DOI] [PubMed] [Google Scholar]
- 35. Chen P, Ratcliff G, Belle SH, et al. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology 2000; 55 (12): 1847– 1853 [DOI] [PubMed] [Google Scholar]
- 36. Snitz BE, Saxton J, Lopez OL, et al. Identifying mild cognitive impairment at baseline in the Ginkgo Evaluation of Memory (GEM) study. Aging Ment Health 2009; 13 (2): 171– 182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000; 157 (12): 1949– 1954 [DOI] [PubMed] [Google Scholar]
- 38. DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 2001; 58 (4): 643– 647 [DOI] [PubMed] [Google Scholar]
- 39. Chetelat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology 2003; 60 (8): 1374– 1377 [DOI] [PubMed] [Google Scholar]
- 40. Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 2010; 133 (11): 3336– 3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006; 5 (3): 228– 234 [DOI] [PubMed] [Google Scholar]
- 42. Wolk DA, Price JC, Saxton J, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009; 65 (5): 557– 568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol 2003; 60 (12): 1696– 1702 [DOI] [PubMed] [Google Scholar]
- 44. Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging 2010; 33 (7): 1203– 1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raji CA, Lee C, Lopez OL, et al. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol 2010; 31 (5): 847– 855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chetelat G, Desgranges B, de la Sayette V, et al. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport 2002; 13 (15): 1939– 1943 [DOI] [PubMed] [Google Scholar]
- 47. Laakso MP, Soininen H, Partanen K, et al. MRI of the hippocampus in Alzheimer’s disease: sensitivity, specificity, and analysis of the incorrectly classified subjects. Neurobiol Aging 1998; 19 (1): 23– 31 [DOI] [PubMed] [Google Scholar]
- 48. Csernansky JG, Wang L, Joshi S, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology 2000; 55 (11): 1636– 1643 [DOI] [PubMed] [Google Scholar]
- 49. Soininen HS, Partanen K, Pitkanen A, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology 1994; 44 (9): 1660– 1668 [DOI] [PubMed] [Google Scholar]
- 50. Bell-McGinty S, Lopez OL, Meltzer CC, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol 2005; 62 (9): 1393– 1397 [DOI] [PubMed] [Google Scholar]
- 51. de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol 1993; 14 (4): 897– 906 [PMC free article] [PubMed] [Google Scholar]
- 52. Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999; 52 (7): 1397– 1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol 2000; 47 (4): 430– 439 [PubMed] [Google Scholar]
- 54. Convit A, de Asis J, de Leon MJ, et al. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer’s disease. Neurobiol Aging 2000; 21 (1): 19– 26 [DOI] [PubMed] [Google Scholar]
- 55. Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 2004; 25 (3): 303– 310 [DOI] [PubMed] [Google Scholar]
- 56. Chetelat G, Baron JC. Early diagnosis of Alzheimer’s disease: contribution of structural neuroimaging. Neuroimage 2003; 18 (2): 525– 541 [DOI] [PubMed] [Google Scholar]
- 57. Karas GB, Scheltens P, Rombouts SARB, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage 2004; 23 (2): 708– 716 [DOI] [PubMed] [Google Scholar]
- 58. Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 1997; 48 (5): 1297– 1304 [DOI] [PubMed] [Google Scholar]
- 59. Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology 2005; 64 (9): 1520– 1524 [DOI] [PubMed] [Google Scholar]
- 60. Jack CR, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 2000; 55 (4): 484– 489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carmichael OT, Kuller LH, Lopez OL, et al. Cerebral ventricular changes associated with transitions between normal cognitive function, mild cognitive impairment, and dementia. Alzheimer Dis Assoc Disord 2007; 21 (1): 14– 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chetelat G, Desgranges B, de la Sayette V, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 2003; 126 (pt 9): 1955– 1967 [DOI] [PubMed] [Google Scholar]
- 63. Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)-FDG PET. J Nucl Med 2005; 46 (10): 1625– 1632 [PubMed] [Google Scholar]
- 64. McKelvey R, Bergman H, Stern J, et al. Lack of prognostic significance of SPECT abnormalities in non-demented elderly subjects with memory loss. Can J Neurol Sci 1999; 26 (1): 23– 28 [PubMed] [Google Scholar]
- 65. Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005; 65 (3): 405– 411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosano C, Aizenstein HJ, Cochran J, et al. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biol Psychiatry 2005; 57 (7): 761– 767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250 (3): 856– 866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011; 305 (3): 275– 283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 2009; 132 (pt 5): 1355– 1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci 2009; 29 (47): 14770– 14778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Oh H, Habeck C, Madison C, et al. Covarying alterations in Abeta deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum Brain Mapp 2012, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment. A randomized placebo-controlled trial. Neurology 2004; 63 (4): 651– 657 [DOI] [PubMed] [Google Scholar]
- 73. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI. A 48-week randomized, placebo-controlled trial. Neurology 2009; 72 (18): 1555– 1161 [DOI] [PubMed] [Google Scholar]
- 74. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352 (23): 2379– 2388 [DOI] [PubMed] [Google Scholar]
- 75. Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 2007; 6 (6): 501– 512 [DOI] [PubMed] [Google Scholar]
- 76. Winblad B, Gauthier S, Feldman H, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008; 70 (22): 2– 24–2035 [DOI] [PubMed] [Google Scholar]
- 77. Lu PH, Edland SD, Teng E, et al. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009; 72 (24): 2115– 2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reynolds CF, 3rd, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry 2011; 68 (1): 51– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aisen PS, Thal LJ, Ferris SH, et al. Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double-blind, trial. Curr Alzheimer Res 2008; 5 (1): 73– 82 [DOI] [PubMed] [Google Scholar]
- 80.ADAPT Research Group; Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007; 68 (21): 1800– 1808 [DOI] [PubMed] [Google Scholar]
- 81. DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial [erratum published in JAMA 2008;300(23):2730]. JAMA 2008; 300 (19): 2253– 2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vellas B, Coley N, Ousset PJ, et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol 2012; 11 (10): 851– 859 [DOI] [PubMed] [Google Scholar]
- 83. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003; 348 (25): 2508– 2516 [DOI] [PubMed] [Google Scholar]
- 84. Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 2010; 75 (16): 1415– 1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 2010; 67 (1): 80– 86 [DOI] [PMC free article] [PubMed] [Google Scholar]