Abstract
In Drosophila melanogaster, much of our understanding of sexually dimorphic neuronal development and function comes from the study of male behavior, leaving female behavior less well understood. Here, we identify a post-embryonic population of Insulin-like peptide 7 (Ilp7)-expressing neurons in the posterior ventral nerve cord that innervate the reproductive tracts and exhibit a female bias in their function. They form two distinct dorsal and ventral subsets in females, but only a single dorsal subset in males, signifying a rare example of a female-specific neuronal subset. Female post-embryonic Ilp7 neurons are glutamatergic motoneurons innervating the oviduct and are required for female fertility. In males, they are serotonergic/glutamatergic neuromodulatory neurons innervating the seminal vesicle but are not required for male fertility. In both sexes, these neurons express the sex-differentially spliced fruitless-P1 transcript but not doublesex. The male fruitless-P1 isoform (fruM) was necessary and sufficient for serotonin expression in the shared dorsal Ilp7 subset, but although it was necessary for eliminating female-specific Ilp7 neurons in males, it was not sufficient for their elimination in females. By contrast, sex-specific RNA-splicing by female-specific transformer is necessary for female-type Ilp7 neurons in females and is sufficient for their induction in males. Thus, the emergence of female-biased post-embryonic Ilp7 neurons is mediated in a subset-specific manner by a tra- and fru-dependent mechanism in the shared dorsal subset, and a tra-dependent, fru-independent mechanism in the female-specific subset. These studies provide an important counterpoint to studies of the development and function of male-biased neuronal dimorphism in Drosophila.
Keywords: Female behavior, Motoneuron, Neuronal identity, Neuronal lineage
INTRODUCTION
Behavioral differences between males and females often arise from sexually dimorphic neurons and circuits (Cooke et al., 1998; Wade and Arnold, 2004; Villella and Hall, 2008; Paus, 2010). Stereotyped male behaviors in Drosophila melanogaster provide the basis for our current understanding of the genetic mechanisms and neural substrates that generate sexually dimorphic behaviors (Yamamoto, 2007; Villella and Hall, 2008). The sex determination cascade generates dimorphic neuronal populations largely through the sex-specific RNA splicing of the transcription factor genes doublesex (dsx) and fruitless (fru) (Salz and Erickson, 2010; Dauwalder, 2011). In males, dsx and a fru transcript driven from its P1 promoter (fru-P1) undergo default RNA splicing into coding dsxM and fruM isoforms. Females express the RNA splicing factor transformer (tra), which drives female-specific splicing of dsx and fru-P1 into a coding dsxF isoform and a non-coding fruF isoform. Both Fru and Dsx are expressed in a largely overlapping set of ∼2000 neurons that play crucial roles in sexually dimorphic behaviors (Cachero et al., 2010; Rideout et al., 2010; Robinett et al., 2010; Yu et al., 2010), in which Fru and Dsx direct sexual dimorphic neuronal gene expression and functional properties, as well as differences in branching and connectivity (Yamamoto, 2007; Villella and Hall, 2008; Dauwalder, 2011). Curiously, only males are reported to have numerically expanded neuronal populations or unique populations not found in females (Yamamoto, 2007; Cachero et al., 2010; Rideout et al., 2010; Yu et al., 2010; Kimura, 2011).
Much of our understanding of the genetic and neural substrates of sexually dimorphic behavior comes from analysis of males, with comparatively less work having been performed on female behavior (Ferveur, 2010). Egg laying in females is under tight neuronal control and its regulatory circuitry is one of the best understood female behaviors (Middleton et al., 2006; Rodríguez-Valentín et al., 2006; Yapici et al., 2008; Yang et al., 2009; Rezával et al., 2012). After eggs exit the ovary, they are propelled through the oviduct by somatic-like muscles that ring the oviduct (Hudson et al., 2008). Peristaltic contraction/relaxation activity of these muscles is directed by unidentified excitatory glutamatergic motoneurons and inhibitory octopaminergic neurons (Middleton et al., 2006; Rodríguez-Valentín et al., 2006; Kapelnikov et al., 2008). Insulin-like peptide 7 (Ilp7)-expressing neurons are also reported to innervate the oviduct, and their electrical silencing blocks egg laying (Yang et al., 2008); yet, as Ilp7 mutants have no egg-laying phenotype (Grönke et al., 2010), the function of these neurons is uncertain.
Here, we identify a post-embryonic population of Ilp7-expressing neurons in the posterior adult ventral nerve cord that innervates the female oviduct and the male seminal vesicles. This population exhibits a functionally biased role in females as well as a rare phenomenon in Drosophila: a female-specific subset of CNS neurons. Examination of the role of the sex determination cascade in the dimorphisms displayed by these neurons indicates that a postmitotic tra- and fruM-dependent mechanism accounts for the dimorphisms of the shared population of Ilp7 neurons, but that a postmitotic tra-dependent and fru- and dsx-independent mechanism is responsible for generating the female-specific neuronal subset in females.
MATERIALS AND METHODS
Fly stocks
Flies were maintained on standard cornmeal food at 70% humidity at 18°C, 25°C or 29°C. Strains from Bloomington Drosophila Stock Center were: UAS-nEGFP; UAS-mCD8::GFPLL5; UAS-hid; UAS-reaper; elavGAL4-C155; UAS-Dicer2; tubP-GAL80TS; w1118 (control strain). Strains obtained as gifts were: Nkx6-GAL4 (Broihier et al., 2004), VGlutOK371-GAL4 (Mahr and Aberle, 2006), Tdc2-GAL4 (Cole et al., 2005) and tubP >Gal80 >;UAS-CD8::GFP;hs-Flp, MKRS (Gordon and Scott, 2009), Act >STOP >nlacZ; UAS-Flp (Struhl and Basler, 1993), MHC-CD8-GFP-Shaker (Zito et al., 1999), UAS-TraF, UAS-TradsRNAi (Chan and Kravitz, 2007), fruitless-P1-GAL4 (Stockinger et al., 2005), fruF, fruM, fru4-40 (fruDf) (Demir and Dickson, 2005), dsx-GAL4 (Rideout et al., 2010). UAS-dsRNAi strains were: UAS-Ilp7dsRNAi-KK105024 (Vienna Drosophila RNAi Center), UAS-VGluTdsRNAi-JF02689; UAS-TβHdsRNAi-JF02746 (TRiP). To generate Ilp7-GAL4, we PCR amplified -1040 to +660 (Ilp7 start codon) relative to the Ilp7 transcriptional start site from Oregon R. This was placed upstream of GAL4 within the pC3G4 vector. Fly transformation was performed by Best Gene Inc. To generate Flp-out mosaics in Ilp7 neurons, flies were heat-shocked once (40°C, 55 minutes) as 72-hour pupae or as pharate adults. This produced GAL80 Flp-outs in one to four Ilp7 neurons in 80% of flies.
Immunohistochemistry
Primary antibodies used were: rabbit anti-Ilp7 (1:1000; E. Hafen, ETH, Zurich, Switzerland); guinea pig anti-Fork head (1:1000; H. Jäckle, Max Planck Institute, Göttingen, Germany); guinea pig anti-Odd skipped (1:200; J. Reinitz, University of Chicago, IL, USA); rat anti-TβH (1:50; M. Monastirioti, FORTH, Greece); rat anti-Doublesex (1:100; M. Arbeitman, USC, CA, USA); mouse anti-Abd-A (1:400; clone D; I. Duncan, Washington University, St Louis, MO, USA); chicken anti-β-Gal (1:1000; ab9361; Abcam); rabbit anti-VGlut, anti-GluRIIC and anti-GluRIIB (1:1000; A. DiAntonio, Washington University, St Louis, MO, USA). rabbit anti-5-HT (1:1000; S5545; Sigma); goat anti-HRP-Cy5 (1:100; Jackson ImmunoResearch); rabbit anti-pMad (1:100; 41D10; Cell Signaling Technology). The Developmental Studies Hybridoma Bank provided anti-Dachshund (1:10; clone dac2-3), anti-BrdU (1:10; clone G3G4), anti-Bruchpilot (1:50; clone nc82), anti-Discs large (1:50; clone 4F3), anti-Abd-B (1:20; clone 1A2E9) and anti-DGluR-IIA (1:1000; clone 8B4D2). Standard protocols were used (Eade and Allan, 2009), except as follows. For serotonin, samples were fixed in 4% paraformaldehyde with 7.5% picric acid for 1 hour. For VGluT and GluRs, we fixed for 5 minutes in Bouin’s fixative. For bromodeoxyuridine (BrdU) detection, mid L3 to late L3 larvae were fed 1 mg/ml BrdU (B5002-1G, Sigma) in yeast paste. After standard fixation, adult ventral nerve cords (VNCs) were treated with 2 N HCl (20 minutes) prior to standard immunohistochemistry for anti-BrdU. Secondary antibodies used were: donkey anti-mouse, anti-chicken, anti-rabbit, anti-guinea pig and anti-rat IgG (H+L) conjugated to DyLight 488, Cy3 or Cy5 (1:100, Jackson ImmunoResearch). All images acquired on an Olympus FV1000 confocal microscope. Images were processed using Fluoview FV1000 and Adobe Photoshop CS5.
Egg-lay assays
Egg-lay assays were performed on yeast paste-supplemented grape juice/agar plates at 25°C and 70% humidity. Flies were not exposed to CO2 when plates were switched. For the 6-hour egg-lay assay, groups of males and females were mated at a ratio of one female to three males. The egg-lay assays were performed with only three females per plate. Eggs were counted and divided by three to give the number of eggs laid per female. For female fertility assays, groups of males and females were mated at a ratio of one female to three males. For male fertility assays, groups of males and females were mated at a ratio of one female to one male. Egg-lay assays and analysis was performed as for the 6-hour egg-lay assay. The total number of viable progeny per 6-hour plate was counted (i.e. not divided by three).
Statistics
GraphPad Prism 5 was used for all analysis and data presentation. Data are presented as mean±s.d. unless otherwise noted. All data underwent D’Agostino and Pearson, Shapiro-Wilk Normality tests. Normally distributed data were compared using a parametric unpaired t-test. Non-normally distributed data were compared using a non-parametric Mann-Whitney test. Statistical results are shown to the exact P-value down to P<0.0001.
RESULTS
Adult females have a unique subset of posterior Ilp7 neurons not present in males
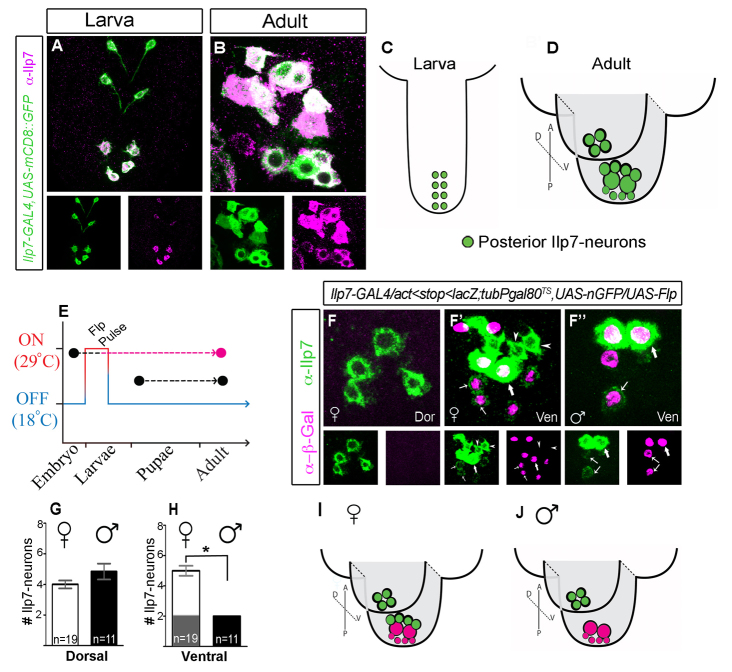
We generated a transgenic Ilp7-GAL4 reporter line (Materials and methods) that faithfully reports Ilp7 expression at all ages (detailed in supplementary material Fig. S1). In larvae, the posterior Ilp7 subset comprises eight dMP2 neurons in abdominal segments A6 to A9 that innervate the hindgut (Miguel-Aliaga et al., 2008; Cognigni et al., 2011) (Fig. 1A,C). They are born and differentiate in the embryo, thus we term them ‘embryonic’ Ilp7 neurons. The posterior Ilp7 neuronal population had not been examined in detail. Our analysis showed that it reorganizes into distinct dorsal and ventral Ilp7-expressing neuronal clusters by adulthood (Fig. 1B,D).
Fig. 1.
Female-specific posterior Ilp7 neurons in adults. (A,B) Ilp7-GAL4,UAS-mCD8::GFP and anti-Ilp7 overlap in posterior abdominal VNC Ilp7 neurons in female larvae (A) and adults (B). (C,D) Representation of ventral and dorsal clusters of posterior Ilp7 neurons in larvae (C) and adults (D). (E-J) Fate-tracking of embryonic Ilp7 neurons in adults. (E) Schematic of the transient Flp-induction protocol. (F-F′) In both sexes, β-Gal immunoreactivity was absent in dorsal (Dor) Ilp7 neurons (shown for females). (F′,F′) In ventral (Ven) Ilp7 neurons, β-Gal was seen in two large Ilp7 neurons (thick arrows) with high Ilp7 expression and in four to six small Ilp7 neurons with very low Ilp7 levels (thin arrows). Females had additional β-Gal-negative Ilp7 neurons (arrowheads) in the ventral cluster that were not seen in males. Arrows/arrowheads indicate representative neurons of each Ilp7 subset. (G,H) Numbers of posterior Ilp7 neurons in adults (mean±s.e.m.), excluding the small embryonic Ilp7 neurons (thin arrows in F′,F′). (G) Both sexes have similar numbers in the dorsal cluster (female, 4.0±0.8; male, 4.8±2.2). (H) The ventral cluster has two embryonic Ilp7 neurons in both sexes (gray region), and also female-specific, non-embryonic Ilp7 neurons (white region) (female, 5.0±1.1; male, 2.0±0.0). *P=0.003. (I,J) Summary of female and male VNC Ilp7 subsets. Green circles indicate non-embryonic Ilp7 neurons; pink circles indicate embryonic Ilp7 neurons.
Are embryonic Ilp7 neurons retained within the posterior Ilp7 population in adults? To test this, we fate-tracked embryonic Ilp7 neurons into adulthood by permanently marking them in young larvae, and examining marker expression in adults. This was achieved by temporally delimited Flp-in of a permanent lacZ reporter during larval stages L1 and L2, using animals of genotype [Act5C <stop <nLacZ, UAS-Flp/Ilp7-GAL4; tubP-GAL80TS, UAS-nEGFP]. Ilp7-GAL4 was used to target UAS-Flp recombinase expression to Ilp7 neurons. Delimitation of Flp expression to an L1/L2 window was achieved using temperature-sensitive GAL80TS, which blocks GAL4 activity at 18°C but permits it at 29°C (McGuire, 2004). Animals were kept at 18°C throughout life, except for during L1/L2, when they were placed at 29°C. The resulting transient Flp expression allowed Flp-in of lacZ to be expressed permanently from a ubiquitous promoter (Struhl and Basler, 1993).
After confirming that lacZ Flp-in robustly and selectively marked all embryonic Ilp7 neurons (supplementary material Fig. S2A), we examined anti-β-galactosidase and anti-Ilp7 overlap in adults. β-Galactosidase (β-Gal) immunoreactivity was absent from dorsal cluster Ilp7 neurons (Fig. 1F). Instead, β-Gal marked subsets of Ilp7 neurons within the ventral cluster: in two large cells (∼13 μm in diameter) with intense Ilp7 immunoreactivity and also in four to six small cells (∼9 μm in diameter) that had extremely low Ilp7 immunoreactivity, which was often undetectable (Fig. 1F′,F′). In males, these neurons accounted for the entire ventral subset (Fig. 1F′). Unexpectedly, females always had an additional three or four Ilp7 neurons in the ventral cluster that were not marked by β-Gal. (Fig. 1F′). These female-specific Ilp7 neurons were non-embryonic and were ∼9 μm in diameter with consistently moderate to high Ilp7 levels. These data are quantified in Fig. 1G,H and summarized in 1I,J.
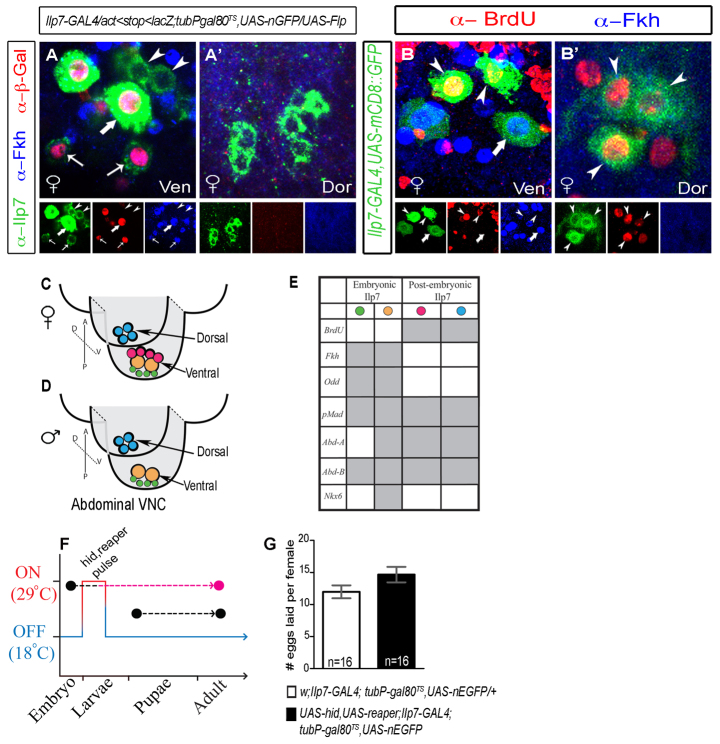
We wished to identify useful discriminatory markers between embryonic and non-embryonic subsets. Thus, in adult Ilp7 neurons, we tested the expression of transcription factors reported to be expressed by Ilp7 neurons in the embryo (supplementary material Fig. S2) (Miguel-Aliaga and Thor, 2004; Miguel-Aliaga et al., 2008). Notably, we found that Fork head (Fkh) and Odd-skipped (Odd) were expressed in all β-Gal-positive (embryonic) Ilp7 neurons but were absent in all non-embryonic β-Gal-negative Ilp7 neurons (Fkh shown in Fig. 2A). We use Fkh immunoreactivity hereafter as a marker to discriminate between embryonic and non-embryonic Ilp7 neurons.
Fig. 2.
Post-embryonic Ilp7 neurons are sufficient for female fertility and can be distinguished from embryonic Ilp7 neurons by lack of Forkhead expression. (A-E) Expression of transcription factors in posterior Ilp7 neurons in adults. (A,A′) Transient Flp-induction-marked embryonic Ilp7 neurons with β-Gal. Forkhead (Fkh) was only expressed in embryonic Ilp7 neurons (arrows). (B,B′) BrdU incorporation into Ilp7 neurons in mid-late L3 larvae was only seen in Fkh-negative Ilp7 neurons (arrowheads) in ventral (Ven) and dorsal (Dor) clusters. Arrows/arrowheads indicate representative neurons of each Ilp7 subset. (C-E) Summary of transcription factor profile in embryonic and post-embryonic posterior Ilp7 neurons in adults (supporting data in supplementary material Fig. S2). Green circles indicate small embryonic Ilp7 neurons; yellow circles indicate large embryonic Ilp7 neurons; pink circles indicate the female-specific post-embryonic Ilp7 neurons; blue circles indicate the common post-embryonic Ilp7 neurons. (F,G) Selective killing of embryonic Ilp7 neurons does not disrupt female fertility. (F) Schematic of transient cell death gene expression in embryonic Ilp7 neurons (hid, reaper pulse). (G) Quantification of the number of eggs laid per female during a 6-hour assay following a 24-hour mating (mean±s.e.m.). Female fertility was not significantly different after killing embryonic Ilp7 neurons (black column), compared with control (white column) (control, 9.4±3.3; experimental, 11.8±5.7). n, number of egg-lay assays.
Post-embryonic Ilp7 neurons innervate the reproductive tracts but are only necessary for female fertility
The additional posterior Ilp7 neurons that appear after larval development might be born through post-embryonic neurogenesis in larvae (Truman, 1990), or are perhaps developmentally frozen to express Ilp7 only after metamorphosis (Veverytsa and Allan, 2012). To discriminate between these possibilities, larvae were fed BrdU between mid L3 and pupariation; BrdU incorporation into Ilp7 neurons was examined at adult day A1 (Fig. 2B,B′). In females, we detected BrdU in Ilp7 neurons that did not express Fkh, including the dorsal Ilp7 neurons shared by both sexes and all female-specific ventral Ilp7 neurons. Thus, the non-embryonic Ilp7 neurons are generated by post-embryonic neurogenesis in late L3 larvae (hereafter termed post-embryonic Ilp7 neurons). The position and transcription factor profile of each Ilp7 neuronal subset is summarized in Fig. 2C-E.
Electrical silencing of all Ilp7 neurons in adults was shown to block egg laying in females (Yang et al., 2008). However, our identification of an unanticipated post-embryonic Ilp7-expressing neuronal population raised the question of which Ilp7 neuronal subset is required for egg laying. To test this, we adapted the temporally delimited Flp protocol (Fig. 1E) to temporally delimit the cell death of embryonic Ilp7 neurons. In animals of genotype [UAS-hid, UAS-reaper/+; Ilp7-GAL4; tubP-GAL80TS, UAS-nEGFP], we used GAL80TS to delimit expression of the cell death genes hid (Wrinkled - FlyBase) and reaper into Ilp7 neurons only in L1 and L2 larvae (Fig. 2F). This killed all embryonic Ilp7 neurons but left post-embryonic Ilp7 neurons intact (supplementary material Fig. S3). In spite of this, female egg laying was not affected (Fig. 2G); thus, post-embryonic Ilp7 neurons are sufficient for egg laying.
Post-embryonic Ilp7 neurons selectively innervate the reproductive tracts
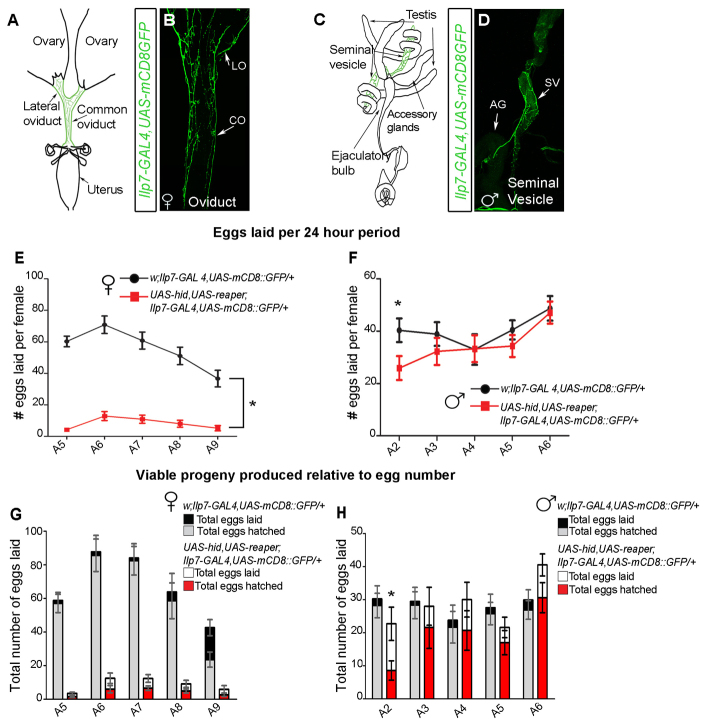
Ilp7 immunoreactivity has been reported at the oviduct, as has the requirement of Ilp7 neuronal activity for female fertility (Yang et al., 2008), but it was not clear which Ilp7 neurons innervate the oviduct nor whether Ilp7 neurons innervate and regulate male reproductive tract function. In Ilp7-GAL4, UAS-mCD8::GFP adults, we found that Ilp7 innervation of the reproductive tracts was restricted to developmentally analogous tissues: the oviduct in females (Fig. 3A,B) and the seminal vesicles in males (Fig. 3C,D) (Bryant, 1978; Kozopas et al., 1998; Sánchez et al., 2001). We also observed hindgut innervation in both sexes, as previously shown (Cognigni et al., 2011).
Fig. 3.
Post-embryonic Ilp7 neurons are required only for female fertility. (A-D) Ilp7-GAL4,UAS-mCD8::GFP-expressing neurons project to the female lateral (LO) and common (CO) oviducts and the male seminal vesicle (SV). AG, accessory gland. (E,F) Control and Ilp7-KO females were mated to control males for 24 hours, then males were removed. Thereafter, we counted egg numbers laid per female per 24-hour period, for 5 days (supplementary material Table S1). (E) Ilp7-KO females (red) exhibited severely reduced egg laying compared with controls (black). (F) Control (black) or Ilp7-KO (red) males were mated to control virgin females for 24 hours. Then, mated females were removed and males were provided new virgin females for another 24 hours. This was repeated for 5 days (A2-A5). After females were removed, we counted egg numbers per female over 24 hours. Females mated to Ilp7-KO and control males laid similar egg numbers; only females on assay day 1 had reduced egg numbers. (G,H) Using the mating protocols described for E,F, we counted the total number of viable larvae produced per plate (not per female) within 6-hour assay periods, over 5 days (supplementary material Table S2). (G) Control females produced a high percentage of larvae. The decline in larvae by A9 reflects a lack of mating for 5 days. Ilp7-KO females produced low egg numbers and only ∼40% of eggs produced larvae at each time point (H) Ilp7-KO and control males produced similar percentages of viable larvae at most ages, except for during the first day of the assay. Graphs show mean±s.e.m. for egg number per female. n, number of egg-lay assays.
Is Ilp7 innervation of the male seminal vesicle required for male fertility, as it appears to be for female fertility (Yang et al., 2008)? We compared the effects of killing Ilp7 neurons on male and female fertility, using Ilp7-GAL4 to drive the cell death genes UAS-hid and UAS-reaper (Ilp7-KO) (Zhou et al., 1997; Veverytsa and Allan, 2012). To test male fertility, we mated 1-day-old (A1) Ilp7-KO and control males to new groups of virgin control females each day for 5 days. After each 24-hour mating period, females were removed and placed on an egg-lay plate for 6 hours and then on a second plate for 18 hours (three females per plate). The numbers of eggs laid, per female, per 24 hours was quantified from both plates. The total number of viable larvae produced was counted on the 6-hour plate and compared with the total egg number on that plate. We found that Ilp7-KO and control males fertilized females to the same extent, as egg production and larval viability were not different on most days (Fig. 3F,H). Only on the first day of mating did Ilp7-KO males have reduced fertility. This suggests that newly eclosed, 1-day-old Ilp7-KO males exhibit a slight delay in achieving full reproductive capacity, but this is quickly resolved to full fertility by 2 days after eclosion.
To test Ilp7-KO female fertility, we mated adult day A4 Ilp7-KO or control females to control males for a 24-hour period. Males were then removed from the females and their egg production (per female) and larval viability (per group of three females) was tracked over the next 5 days. Ilp7-KO females exhibited severely reduced egg laying throughout the 5-day test period (Fig. 3E). These females also had distended abdomens and eggs were always found jammed in the lateral oviduct (supplementary material Fig. S4A-A′). Of the small number of eggs laid by Ilp7-KO females, only 40% produced viable larvae, compared with 90% of control females (Fig. 3G).
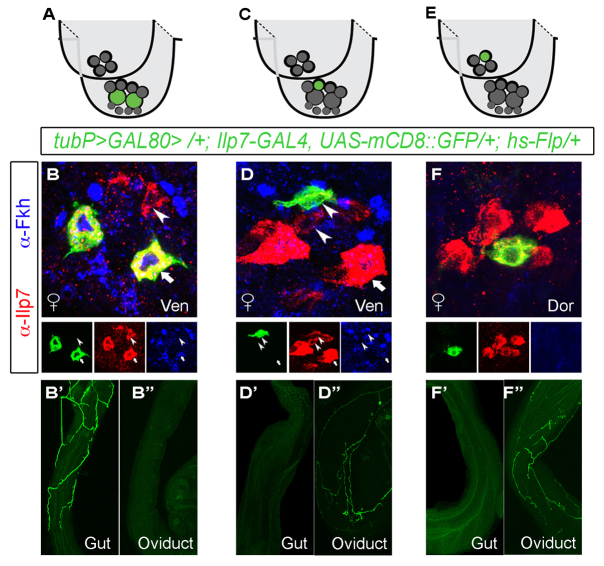
The sufficiency of post-embryonic Ilp7 neurons for egg laying (see Fig. 1G) and the hindgut innervation of embryonic Ilp7 neurons in larvae (Cognigni et al., 2011) led us to test whether the post-embryonic Ilp7 neurons selectively innervate the oviduct. We took a genetic mosaic strategy (Gordon, 2009) to visualize individual Ilp7 neurons in flies of genotype [tubP >GAL80 >/+; Ilp7-GAL4, UAS-mCD8::GFP/+; hs-Flp/+]. Transient heat-shock of a Flp transgene causes stochastic, mosaic excision of FRT (>)-flanked GAL80 from cells, which permits cell-autonomous UAS-mCD8::GFP expression in any neuron that expresses GAL4. Based on soma position and Fkh expression, we assigned labeled Ilp7 neurons a subset identity and examined their projections. The two large ventral-cluster embryonic Ilp7 neurons exclusively innervated the hindgut and rectum but not the oviduct (n=6 mosaic animals; Fig. 4A-B′) whereas all labeled post-embryonic Ilp7 neurons exclusively innervated the oviduct (n=11 mosaic animals; Fig. 4C-F′).
Fig. 4.
Post-embryonic Ilp7 neurons selectively innervate reproductive tracts. Using stochastic GAL80 Flp-out to generate mosaic Ilp7-GAL4,UAS-mCD8::GFP expression, we imaged individual neurons from each Ilp7-subset. (A,C,E) Cartoons depict the mosaically labeled neurons in the images below. Green circles indicate the neurons in which GAL80 was excised for the images shown; gray circles indicate all other Ilp7 neurons. (B,D,E) Images of labeled neurons within ventral (Ven) and dorsal (Dor) subsets. Fkh is expressed in embryonic Ilp7 neurons (arrows), but not post-embryonic Ilp7 neurons (arrowheads). (B-B′) The large embryonic Ilp7 neurons innervate the gut only. (D-D′) Female-specific post-embryonic Ilp7 neurons innervate the oviduct only. (F-F′) Post-embryonic Ilp7 neurons of the dorsal cluster innervate the oviduct only in females. Arrows/arrowheads indicate representative neurons of each Ilp7 subset.
Post-embryonic Ilp7 neuronal phenotype is sexually dimorphic
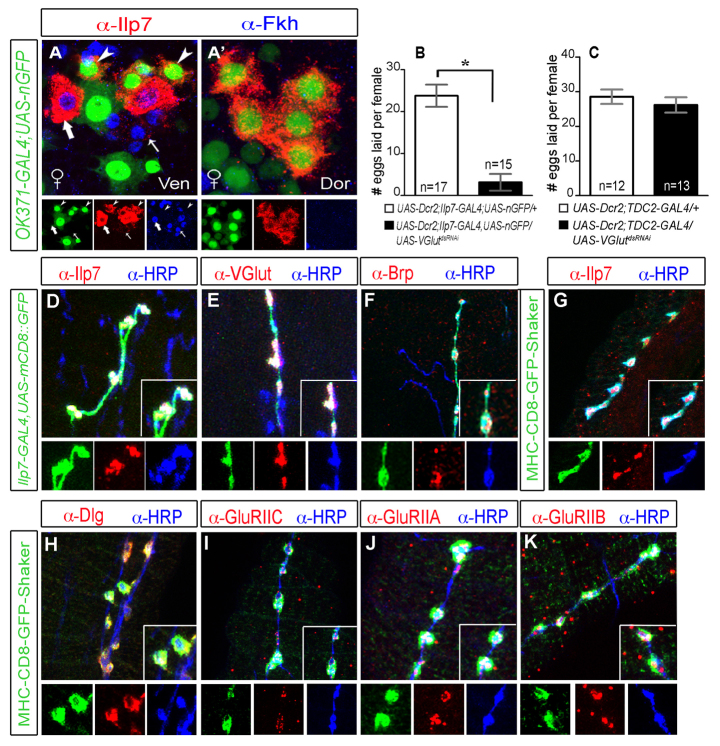
Targeted mutants of the Ilp7 gene do not exhibit female fertility defects (Grönke et al., 2010), so why does elimination of Ilp7 neurons produce a profound female fertility defect? We confirmed that Ilp7 peptide plays no essential role in egg laying, by expressing UAS-ILP7dsRNAi from Ilp7-GAL4 and showed that females laid similar numbers of eggs to controls (supplementary material Fig. S5A,B). We reasoned then that Ilp7 neurons use another essential mode of neurotransmission. Octopaminergic innervation of the oviduct is essential for egg laying (Monastirioti, 2003; Rodríguez-Valentín et al., 2006). We tested whether Ilp7 neurons are octopaminergic. Ilp7 neurons did not express reporters for the octopamine enzymes tyrosine decarboxylase 2 (TDC2-GAL4) or tyrosine β-hydroxylase (anti-TβH) (supplementary material Fig. S5C-D′). Moreover, TβH knockdown (UAS-TβHdsRNAi) blocked egg laying when expressed in octopaminergic neurons (using TDC2-GAL4) but not when expressed in Ilp7 neurons (Ilp7-GAL4) (supplementary material Fig. S5F,G). To identify alternative modes of neurotransmission, we screened through neurotransmitter markers and found that all post-embryonic Ilp7 neurons expressed OK371-GAL4 (Fig. 5A,A′), an enhancer-trap that reports Vesicular glutamate transporter (VGlut) gene expression (Mahr and Aberle, 2006). This was intriguing in light of reports of glutamatergic motoneuron innervation of the oviduct; type I-like neuromuscular junctions are present on the oviduct, which contracts vigorously in response to exogenous glutamate (Middleton et al., 2006; Rodríguez-Valentín et al., 2006; Kapelnikov et al., 2008).
Fig. 5.
Female post-embryonic Ilp7 neurons are glutamatergic motoneurons that terminate at type I-like NMJs on oviduct muscle. (A,A′) OK371-GAL4,UAS-nEGFP (VGlut reporter) was expressed in Fkh-negative post-embryonic Ilp7 neurons (arrowheads) but not Fkh-positive embryonic Ilp7 neurons (arrows). Arrows/arrowheads indicate representative neurons of each Ilp7 subset. (B,C) Number of eggs laid per female (mean±s.e.m.) over 6 hours. n, number of egg-lay assays. (B) VGluTdsRNAi expression in Ilp7 neurons reduced egg laying (control 23.8±10.9; experimental 3.2±7.7; *P<0.0001). (C) VGluTdsRNAi expression in octopaminergic neurons (TDC2-GAL4) did not affect egg laying (control 28.5±7.2; experimental 26.2±7.9). (D-K) Images of Ilp7 neuronal synapses on the oviduct. (D-F) Ilp7-GAL4,UAS-mCD8::GFP-labeled axons and synaptic boutons co-labeled with anti-HRP (general neuronal marker) and anti-Ilp7 (D), anti-VGlut (E) and anti-Bruchpilot (Brp) (F). (G) CD8-GFP-Shaker (type I NMJ marker) is localized to Ilp7 synaptic boutons. (H-K) The following NMJ markers are localized to CD8-GFP-Shaker-labeled Ilp7 NMJs: anti-Dlg (type I NMJ marker) (H), GluRIIC (I), GluRIIA (J) and GluRIIB (K).
In Ilp7-GAL4, UAS-mCD8::GFP females, Ilp7 neurons terminated on the radial muscles of the oviduct with boutons immunoreactive for VGlut and Bruchpilot, a marker for presynaptic active zones (Wagh et al., 2006) (Fig. 5E,F). Moreover, these boutons were apposed to synaptic accumulations of CD8-GFP-Shaker and Discs large (Dlg) (Fig. 5G,H), which together are unique markers for type I neuromuscular junctions (NMJs) of somatic muscle (Guan et al., 1996; Parnas et al., 2001). Indeed, all CD8-GFP-Shaker and Dlg synaptic accumulations were innervated by Ilp7 neurons (supplementary material Fig. S6A). We examined the localization of glutamate receptor (GluR) subunits to these oviduct NMJs and further confirmed that Ilp7 neurons terminate at type I NMJs. GluRs form heterotetrameric complexes and the subunits GluRIIC (also GluRIII), GluRIIA and GluRIIB are all localized to type I NMJs (DiAntonio, 2006). At the oviduct, GluRIIC, GluRIIA and GluRIIB all cluster exclusively at Ilp7 boutons within Dlg and CD8-GFP-Shaker synaptic accumulations (Fig. 5H-K). Is glutamatergic transmission of Ilp7 neurons essential for oviduct function? We expressed UAS-VGluTdsRNAi in Ilp7 neurons and confirmed that VGlut was efficiently knocked down (supplementary material Fig. S6B,B′). These females had a severe reduction in egg laying (Fig. 5B), and an ‘egg-jam’ phenotype in the lateral oviduct (supplementary material Fig. S6B′), which phenocopies Ilp7-KO females (supplementary material Fig. S4A′). As a control, we expressed UAS-VGluTdsRNAi in octopaminergic neurons (TDC2-GAL4) and found that this did not disrupt egg laying (Fig. 5C).
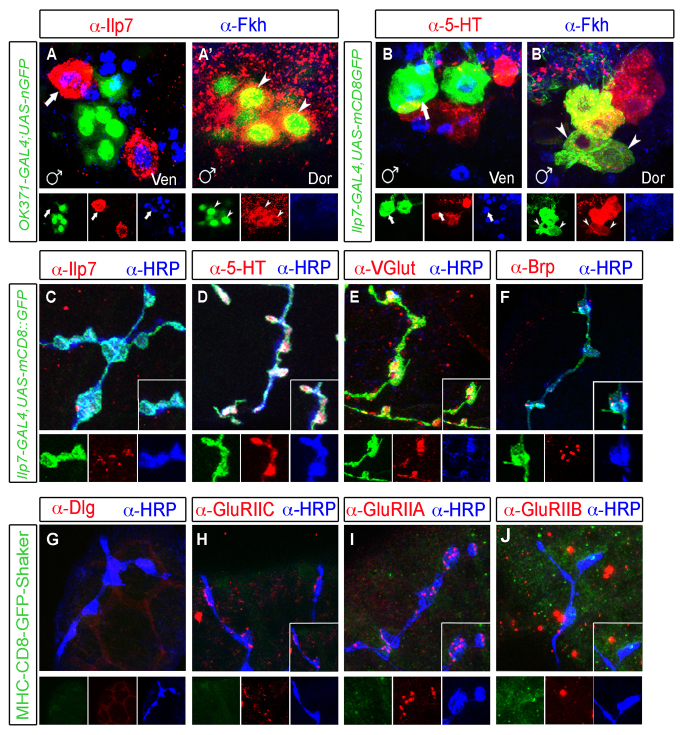
A layer of muscle surrounds the male seminal vesicle (Bairati, 1967; Kozopas et al., 1998) that is innervated by serotonergic input from posterior abdominal VNC neurons (Lee et al., 2001; Billeter and Goodwin, 2004). We tested the neurotransmitter identity of male postmitotic Ilp7 neurons, and found that they expressed both the vesicular glutamate transporter and also serotonin (Fig. 6A-B′). Serotonin is expressed in a posterior neuronal cluster in males, the SAbg, which innervates numerous male reproductive tract structures (Lee et al., 2001; Billeter and Goodwin, 2004). We found that Ilp7- and serotonin-expressing neurons were a small subset of this serotonergic cluster (Fig. 6B′), and our data would suggest that this subset exclusively innervates the seminal vesicle. We examined Ilp7 neuron innervation of the seminal vesicle. In Ilp7-GAL4, UAS-mCD8::GFP males, we found that the seminal vesicle was only innervated by Ilp7 neurons, as determined by counterstaining with the pan-neuronal membrane marker anti-HRP. We then confirmed that all Ilp7 projections were strongly immunoreactive for serotonin (Fig. 6D) and for VGlut (Fig. 6E), and also found that boutons were immunoreactive for Brp (Fig. 6F). We note here that female Ilp7 neurons did not express serotonin (supplementary material Fig. S6C,C′).
Fig. 6.
Male post-embryonic Ilp7 neurons are serotonergic and glutamatergic and innervate the seminal vesicle with type II-like NMJs. (A,A′) In males, OK371-GAL4,UAS-nEGFP (VGlut reporter) was not expressed in Fkh-positive embryonic Ilp7 neurons of the ventral (Ven, arrows) cluster but was expressed in Fkh-negative post-embryonic Ilp7 neurons of the dorsal (Dor) cluster. (B,B′) Serotonin (5-HT) is expressed by post-embryonic (B′) but not embryonic (B) Ilp7 neurons. (C-J) Images of Ilp7 neuronal synapses on the seminal vesicle. Ilp7-GAL4,UAS-mCD8::GFP-labeled axons and synaptic boutons co-labeled with anti-HRP (general neuronal marker) and anti-Ilp7 (C), anti-serotonin (5-HT) (D), anti-VGluT (E) and anti-Bruchpilot (Brp) (F). (G) Neither CD8-GFP-Shaker nor Dlg (type I NMJ markers) were localized to Ilp7 synaptic boutons. (H-J) Low level GluRIIC (H) and GluRIIA (I) were observed at most Ilp7 synapses, but GluRIIB expression was not (J). Arrows/arrowheads indicate representative neurons of each Ilp7 subset.
Although male post-embryonic Ilp7 neurons express glutamatergic markers, we did not expect them to function as motoneurons, owing to their limited functional role in fertility and their co-expression of serotonin. In confirmation, synaptic accumulation of Dlg and CD8-GFP-Shaker was absent from the seminal vesicle and Ilp7 boutons (Fig. 6G). Moreover, we found only weak and infrequent postsynaptic GluRIIC and GluRIIA immunoreactivity apposed to Ilp7 boutons, and no GluRIIB immunoreactivity was observed (Fig. 6H-J). These findings match the marker expression profile of neuromodulatory type-II-like NMJs at somatic muscle, which utilize glutamate and octopamine as co-transmitters, but have no accumulation of Dlg, CD8-GFP-Shaker or GluRIIB, and have faint and infrequent GluR receptor clustering of GluRIIA and GluRIIC (Zito et al., 1997; Monastirioti, 2003; Marrus et al., 2004; Prokop, 2006). This analysis demonstrates sexual dimorphism in the transmitter phenotype and in NMJs of Ilp7 neurons innervating the reproductive tracts of males and females, with a functional bias to females.
Genetic regulation of Ilp7 neuron dimorphism
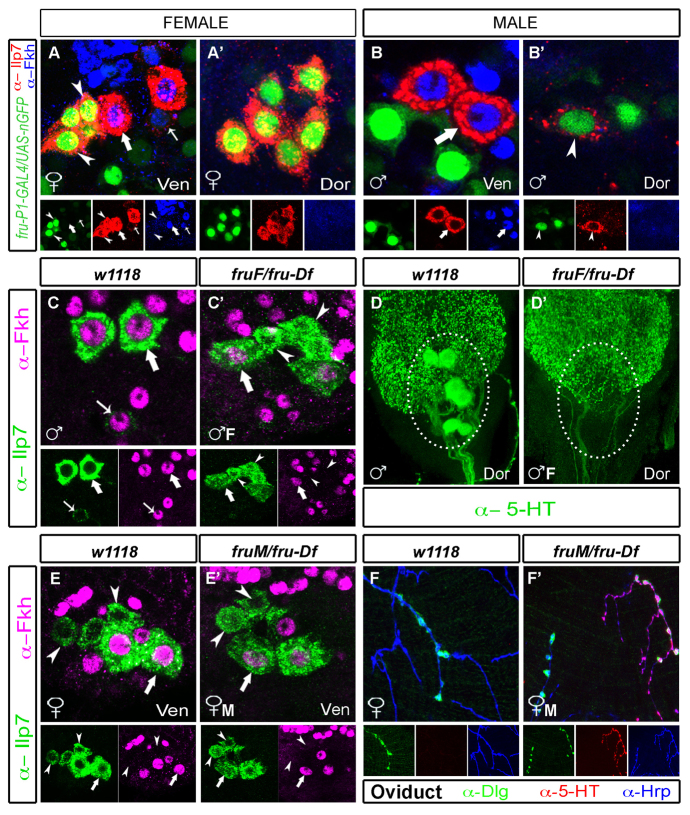
We tested the role of the sex determination cascade in generating the observed Ilp7 neuronal dimorphisms. The output of this cascade is principally mediated through sex-specific splicing of fru-P1 and dsx. In males, they are ‘default’ spliced into coding dsxM and fruM transcripts. In females, the presence of tra splices fru-P1 into a non-coding fruF isoform and dsx into a coding dsxF isoform. This cascade generates male-specific neurons, but female-specific neuronal subsets have not been investigated (Ferveur, 2010; Kimura, 2011). We first examined fru-P1 and dsx expression in Ilp7 neurons, using a GAL4 reporter for the fru-P1 promoter (which drives in both sexes) and Doublesex immunoreactivity (recognizing a common domain in DsxM and DsxF). We found that all post-embryonic Ilp7 neurons were fru-P1 positive but Dsx negative in adults. Embryonic Ilp7 neurons did not express fru-P1 or Dsx (Fig. 7A-B′; supplementary material Fig. S7A-B′). Dsx may be transiently expressed at an earlier stage but, as shown in supplementary material Fig. S7C-D′, we could not detect Dsx expression at any time in the lineage of post-embryonic Ilp7 neurons, even by lineage tracing using dsx-GAL4 to Flp-in permanent lacZ expression. Thus, dsx is probably not expressed in the post-embryonic Ilp7 neuron lineage.
Fig. 7.
FruM is necessary and sufficient for dorsal Ilp7 serotonergic fate, and is necessary, but not sufficient, for loss of female-specific Ilp7 neurons. (A-B′) In both sexes, fru-P1-GAL4, UAS-nEGFP is expressed in all post-embryonic Ilp7 neurons (arrowheads; Fkh negative) but not in embryonic Ilp7 neurons (thick and thin arrows; Fkh positive). (C-D′) In fruF/fru-Df males, in which fruM is absent (♂F), post-embryonic female-specific Ilp7 neurons are generated in the ventral cluster (C′, arrowheads, Fkh negative, Ilp7 positive) adjacent to embryonic Ilp7 neurons (arrows, Fkh positive). Also, posterior serotonin expression is lost (D′). (E-F′) In fruM/fru-Df females (♀M), post-embryonic female-specific Ilp7 neurons are not lost (E′, arrowheads) but serotonin expression is observed in dorsal (but not ventral) post-embryonic Ilp7 neurons (supplementary material Fig. S8B). Here, we show ectopic serotonin expression at Ilp7 projections on the oviduct. (F,F′) In controls, female Ilp7 neurons lack serotonin expression at the oviduct (F), but serotonin is expressed by about half of the Ilp7 neurons apposing Dlg-marked NMJs in fruM/fruDF females (probably dorsal subset Ilp7 neurons) (F′). Arrows/arrowheads indicate representative neurons of each Ilp7-subset.
We examined fru function in the generation of female-type and male-type Ilp7 neurons. Using constitutive fruM or fruF-expressing alleles (Demir and Dickson, 2005), we examined the fate of post-embryonic Ilp7 neurons in hemizygous fru-F males (fruF/fru-Df, which do not express FruM) and also in hemizygous fruM females (fruM/fru-Df, which express FruM protein). In adult fruF-males, post-embryonic Ilp7 neurons were feminized; we observed the generation of the ventral subset of female-specific Ilp7 neurons (three or four Ilp7-positive/Fkh-negative neurons) adjacent to the embryonic Ilp7 neurons (Fig. 7C,C′). The shared dorsal cluster of Ilp7 neurons is retained (as in females) but serotonin expression is lost from this region (Fig. 7D,D′), as was observed in fru-null males (Lee and Hall, 2001; Lee et al., 2001; Billeter et al., 2006). Thus, fruM is necessary in males for serotonin expression in dorsal Ilp7 neurons and for the loss of the female-specific Ilp7 neurons.
By contrast, post-embryonic Ilp7 neurons were not entirely masculinized in fruM females. Notably, the ventral cluster of female-specific Ilp7 neurons was not affected by fruM expression (Fig. 7E,E′). Do these female-specific Ilp7 neurons now express serotonin? It was not possible to co-immunostain for Ilp7 and serotonin; however, serotonin is normally expressed in very few neurons in the vicinity of female-specific Ilp7 neurons, and we found in fruM females that there was no apparent increase in the number of serotonin-expressing neurons in the region (supplementary material Fig. S8A,B). Examination of the shared dorsal cluster, however, showed that these post-embryonic Ilp7 neurons were masculinized in fruM females. These fruM females had gained a population of posterior serotonin-expressing neurons similar to that previously reported in females expressing UAS-fruM isoforms (supplementary material Fig. S8A′,B′) (Billeter et al., 2006). We examined the oviducts of fruM females to confirm that dorsal Ilp7 neurons were indeed masculinized to express serotonin. As expected, a subset of Dlg-stained NMJs was indeed apposed to serotonin-expressing axons (Fig. 7F,F′). In these oviducts, we find that approximately half of all Ilp7 projections are serotonin positive, probably reflecting innervation by serotonergic dorsal Ilp7 neurons and serotonin-negative ventral Ilp7 neurons. Thus, although fruM is necessary and sufficient for serotonin expression in dorsal cluster Ilp7 neurons and necessary for loss of female-specific neurons in males, it is not sufficient for the loss of ventral female-specific Ilp7 neurons in females.
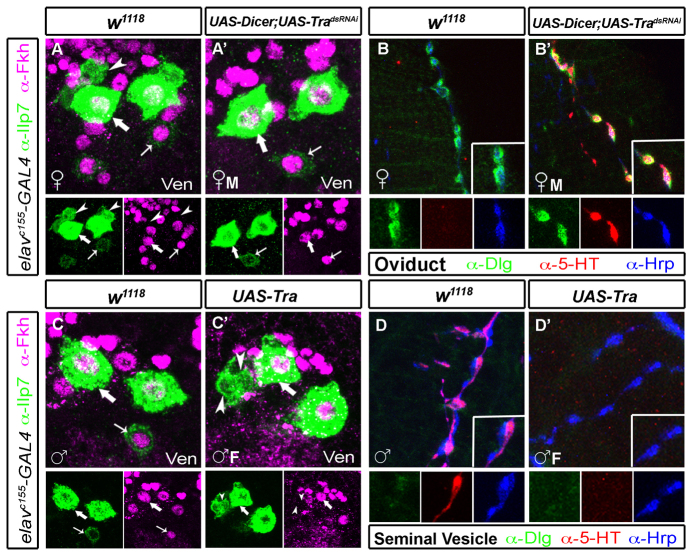
The apparent lack of effect of fruM, and presumably of dsx, in the generation of female-specific neurons in females led us to test whether transformer plays a role, which could affect sex-specific gene expression/function beyond that accounted for by dsx or fru (Finley et al., 1997; Goldman and Arbeitman, 2007). We manipulated transformer expression pan-neuronally in postmitotic neurons, using elav-GAL4 to express dsRNAi to tra (UAS-tradsRNAi) in females, or to express tra (UAS-tra) in males. In tradsRNAi females (in which fruM would be expressed post-mitotically in female Ilp7 neurons), we observed a total loss of the female-specific, post-embryonic Ilp7 neurons in the ventral cluster (Fig. 8A,A′) whereas embryonic Ilp7 neurons were unaffected. We also observed ectopic serotonin in a posterior cluster of dorsal neurons (supplementary material Fig. S8C,C′). To confirm that dorsal Ilp7 neurons now expressed serotonin, we examined serotonin immunoreactivity at the oviduct. Indeed, serotonin was expressed by axons terminating at Dlg-stained neuromuscular junctions (Fig. 8B,B′). Notably, there was a large reduction in the number of Dlg-marked NMJs at the oviduct and all innervation of Dlg-positive NMJs was serotonergic. We suggest that this phenotype results from a loss of innervation by the ventral post-embryonic Ilp7 neurons, although the mechanism is unknown; possible mechanisms include mis-targeting, programmed cell death or an inability to induce postsynaptic Dlg accumulation.
Fig. 8.
transformer is necessary and sufficient for feminizing post-embryonic Ilp7 neurons. (A-B′) Pan-neuronal, postmitotic UAS-tradsRNAi-masculinized Ilp7 neurons in females. (A,A′) In controls, female-specific Ilp7 neurons (arrowheads, Ilp7 positive/Fkh negative) are present in the ventral (Ven) cluster (A). These are not seen in UAS-tradsRNAi-females (♀M) (A′). (B,B′) In UAS-tradsRNAi-females, Ilp7 neuronal innervation to the oviduct is much reduced, and serotonin is expressed in all Ilp7 processes apposed to Dlg-positive NMJs. Oviducts of control females do not have serotonin in the oviduct (B). (C-D′) Pan-neuronal, postmitotic UAS-Tra-feminized Ilp7 neurons in males. (C,C′) UAS-Tra males (♂F) have female-specific post-embryonic Ilp7 neurons in the ventral (Ven) cluster (arrowheads, Ilp7 positive/Fkh negative) that are not seen in controls. (D,D′) Dorsal cluster post-embryonic Ilp7 neurons are feminized and lose serotonin expression (supplementary material Fig. S8D,D′). Here, we show that UAS-Tra males retain innervation of the seminal vesicle (D′) but have lost serotonin expression in those neurons. Arrows/arrowheads indicate representative neurons of each Ilp7 subset.
Feminization of male neurons by postmitotic, pan-neuronal expression of UAS-tra led to the opposite phenotype. Female-specific Ilp7 neurons were observed in the ventral subset, adjacent to the embryonic Ilp7 neurons (Fig. 8C,C′). Moreover, serotonin immunoreactivity was lost in the posterior VNC and at the seminal vesicle, even though the seminal vesicle retains its innervation (Fig. 8D,D′; supplementary material Fig. S8D,D′). We used Ilp7-GAL4 to drive UAS-tradsRNAi in females and UAS-tra in males, but found no change in the Ilp7 neuronal population in these animals (data not shown). That total re-sexualizing of Ilp7 neurons is possible post-mitotically but not after Ilp7 expression commences indicates that the ‘decision’ to become female-type or male-type Ilp7 neurons is irreversibly made in young postmitotic Ilp7 neurons prior to expression of Ilp7 itself.
DISCUSSION
Functional bias of female post-embryonic Ilp7 neurons
Using a standard set of genetic and immunological tools, we demonstrate that female post-embryonic Ilp7 neurons are the sole glutamatergic motoneuron input that terminates at fast excitatory type I-like NMJs on the oviduct, whereas their male counterparts terminate at neuromodulatory type II-like NMJs on the seminal vesicle (Jia et al., 1993; Prokop, 2006). Glutamatergic neurotransmission is required for contraction of oviduct muscle, which comprises super-contractile radial muscle fibers (Middleton et al., 2006; Rodríguez-Valentín et al., 2006). By contrast, the male seminal vesicle is lined by thin striated muscle that receives only Ilp7/serotonergic innervation and has no NMJs characteristic of fast excitatory transmission. Male seminal vesicle contractility has not been examined, but peristaltic activity of the adjacent ejaculatory duct is under serotonergic modulation; however, innervation is not essential for this (Susic-Jung et al., 2012). Together with our data here, it appears that innervation of the seminal vesicle is not a requirement for the passage of sperm.
Our study leaves unresolved the role of Ilp7 at the oviduct. To our knowledge, insulin-like peptide (ILP) expression in motoneurons has only been described in Caenorhabditis elegans (Pierce et al., 2001; Sieburth et al., 2005). The nervous system is a primary locus for ILP expression in C. elegans (Pierce et al., 2001), but any specific motoneuron role for ILPs is unknown. In Drosophila, Ilp7 mutants have no overt phenotype in viability, development, lifespan, fecundity or response to starvation (Grönke et al., 2010), and we detected no egg-laying phenotype after knockdown of Ilp7. Ilp7 functions in the selection of appropriate substrates for egg laying (Yang et al., 2008); however, the circuitry and function of Ilp7 underlying this behavior are unknown.
Sexual dimorphism of post-embryonic Ilp7 neurons
There are approximately ten male-specific serotonergic neurons in the posterior dorsal VNC, termed SAbg, that innervate the seminal vesicle, accessory glands and ejaculatory duct of the male reproductive tract (Lee and Hall, 2001; Lee et al., 2001; Billeter et al., 2006). Our results now show that a subset of these neurons (approximately four) co-express Ilp7 and serotonin, and selectively innervate the seminal vesicle. Comparing our data with that of previous reports, we can now propose that the generation of dimorphic SAbg neurons is different for the Ilp7/serotonergic subset and the other SAbg neurons. In males, the expression of serotonin in all SAbg neurons requires fruM (Lee et al., 2001), but UAS-fruM only generates a reduced subset of approximately four SAbg neurons in females (Billeter et al., 2006). We show here that these are Ilp7 neurons, as they innervate oviduct NMJs in fruM females. Why does fruM expression in females only generate serotonin in the Ilp7 subset of SAbg neurons? The answer lies in the control of neuroblast lineage progression by dsx. Many SAbg neurons are lost in dsx-null males, and a subset is gained in dsx-null females (Billeter et al., 2006). Underlying this is the induction of female-specific programmed cell death of posterior neuroblast lineages in females by DsxF, and their survival and lineage progression in males due to DsxM (Taylor and Truman, 1992; Billeter et al., 2006; Birkholz et al., 2013). The function of fruM is thereafter limited to activating serotonin expression in the remaining neurons (Billeter et al., 2006). We propose that the absence of dsx expression in the lineages of post-embryonic Ilp7 neurons might spare them from DsxF-induced programmed cell death in larvae, so that they survive to become oviduct motoneurons in females.
The postmitotic activity of tra fully accounts for all dimorphisms observed in post-embryonic Ilp7 neurons. Aside from regulation of neuroblast progression, postmitotic mechanisms also contribute to the generation of male-specific neurons, including the P1 and TN1 clusters that function in male courtship behavior, and also the motoneurons that innervate the male-specific muscle of Lawrence (moL) (Rideout et al., 2010; Kimura, 2011). Female-specific loss of P1 neurons is solely mediated by dsxF acting in a pro-apoptotic manner (Kimura et al., 2008). Female-specific loss of TN1 neurons requires a pro-apoptotic role for dsxF, but this can be partially counteracted by co-expression of pro-survival dsxM (Sanders and Arbeitman, 2008). Female loss of the moL motoneuron is due to a necessary and sufficient role for fruM in promoting motoneuron survival (Usui-Aoki et al., 2000; Billeter et al., 2006). In the context of these studies, we were interested to uncover how a female-specific set of neurons emerges. Our initial hypothesis, based on the role of tra, the expression of fru-P1 and the absence of dsx, held that the presence of ventral female-specific Ilp7 neurons is a default state that was masculinized by fruM. However, although fruM in males is necessary for the loss of female-specific Ilp7 neurons, it is not sufficient to eliminate them in females.
These data suggest the existence of a tra-dependent factor(s) that functions selectively in females and is sufficient for the generation of female-specific Ilp7 neurons, independently of fru and dsx. Three additional genes that act in the sex determination cascade function in female sexual differentiation: intersex (Garrett-Engele et al., 2002; Siegal and Baker, 2005), hermaphrodite (Li and Baker, 1998) and dissatisfaction (Finley et al., 1998), the latter of which has been demonstrated to function in a tra-dependent, dsx-independent manner in at least one neuronal population (Finley et al., 1997). Moreover, genomic approaches to identifying sex-differentially expressed genes have identified numerous genes for which sex-specific expression is tra-dependent but neither dsx- nor fru-dependent (Goldman and Arbeitman, 2007). Ongoing work will address any potential role for these genes in the generation of female-specific Ilp7 neurons. Also, it will be interesting to determine the ‘fate’ of female-specific Ilp7 neurons in males. In this light, it is interesting that tra manipulation fully re-assigned the sexual identity of Ilp7 neurons when manipulated from elav-GAL4 but not from Ilp7-GAL4. This finding suggests that the underlying pathway makes a permanent ‘decision’ soon after the neuron exits the cell cycle but before Ilp7 is expressed. Such studies would provide an intriguing counterpoint to mechanisms that generate male-biased neuronal populations in males.
Female-specific circuits and female-specific neuronal populations as models for neuronal sexual dimorphism
Egg laying is a well-characterized, stereotypical, sex-specific behavior. Efferent populations regulating this behavior include octopamine- (Monastirioti, 2003), Ilp7- (Yang et al., 2008) and dsx-expressing neurons (Rideout et al., 2010). Regulatory circuits into these reproductive tract efferents are likely to be complex. One candidate population is the pickpocket- and doublesex-expressing reproductive tract sensory neurons. Upon mating, these neurons relay a signal to the suboesophageal ganglion and the posterior abdominal VNC, to decrease receptiveness to male courtship and increase egg laying (Häsemeyer et al., 2009; Yang et al., 2009; Rezával et al., 2012). The projection of these sensory neurons into the posterior VNC is particularly intriguing, because octopaminergic, Ilp7-, and other fru- and/or dsx-positive neurons ramify their dendritic fields in this region. Work in this field will no doubt provide more details of the circuitry between such neurons, to which our identification of the oviduct motoneurons contributes significantly. Ongoing studies of the development and function of neuronal circuits regulating female-specific behavior will provide an important counterpoint to such studies in males and will lead to a more full understanding of how sex-specific circuits are built and function.
Acknowledgments
We are very grateful to the following for critical reagents: M. Arbeitman (University of Southern California, USA), A. DiAntonio (Washington University St Louis, USA), B. Dickson (Institute of Molecular Pathology, Austria), I. Duncan (Washington University St Louis, USA), S. Goodwin (Oxford, UK), M. Gordon (UBC, Canada), E. Hafen (ETH, Switzerland), H. Jäckle (Max Planck Institute, Germany), E. Kravitz (Harvard Medical School, USA), I. Miguel-Aliaga (Imperial College London, UK), M. Monastirioti (FORTH, Greece), F. Pignoni (Upstate Medical University, NY, USA), J. Reinitz (U.Chicago, USA), J. Skeath (Washington University St Louis, USA), the Developmental Studies Hybridoma Bank, Bloomington Drosophila Stock Center and Flybase. We are grateful to Drs Michael Gordon and Timothy O’Connor and the Allan lab (UBC) for support and critique.
Footnotes
Funding
The authors received funding for this work from the EJLB Foundation; the Canadian Institutes of Health Research (CIHR); the Canadian Foundation for Innovation; the British Columbia Knowledge Development Fund; and the Michael Smith Foundation for Health Research. D.W.A. was a CIHR New Investigator and a Michael Smith Foundation for Health Research Career Scholar. M.C.C. was a recipient of a University of British Columbia College for Interdisciplinary Studies 4 year Fellowship. J.C.Y.T. was a recipient of a CIHR Banting and Best Studentship Award.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
M.C. and D.W.A. conceived of all experiments and wrote the manuscript. M.C. performed all experiments and data analysis. J.C.Y.T. generated the Ilp7-GAL4 transgenic strain.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.094714/-/DC1
References
- Bairati A. (1967). [The structure and ultrastructure of the male genital apparatus of the Drosophila melanogaster Meig. 1. The testis]. Z. Zellforsch. Mikrosk. Anat. 76, 56–99 [PubMed] [Google Scholar]
- Billeter J. C., Goodwin S. F. (2004). Characterization of Drosophila fruitless-gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. J. Comp. Neurol. 475, 270–287 [DOI] [PubMed] [Google Scholar]
- Billeter J. C., Villella A., Allendorfer J. B., Dornan A. J., Richardson M., Gailey D. A., Goodwin S. F. (2006). Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 16, 1063–1076 [DOI] [PubMed] [Google Scholar]
- Birkholz O., Rickert C., Berger C., Urbach R., Technau G. M. (2013). Neuroblast pattern and identity in the Drosophila tail region and role of doublesex in the survival of sex-specific precursors. Development 140, 1830–1842 [DOI] [PubMed] [Google Scholar]
- Broihier H. T., Kuzin A., Zhu Y., Odenwald W., Skeath J. B. (2004). Drosophila homeodomain protein Nkx6 coordinates motoneuron subtype identity and axonogenesis. Development 131, 5233–5242 [DOI] [PubMed] [Google Scholar]
- Bryant P. J. (1978) Pattern Formation in Imaginal Discs. London: Academic Press; [Google Scholar]
- Cachero S., Ostrovsky A. D., Yu J. Y., Dickson B. J., Jefferis G. S. (2010). Sexual dimorphism in the fly brain. Curr. Biol. 20, 1589–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P., Bailey A. P., Miguel-Aliaga I. (2011). Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13, 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. H., Carney G. E., McClung C. A., Willard S. S., Taylor B. J., Hirsh J. (2005). Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 280, 14948–14955 [DOI] [PubMed] [Google Scholar]
- Cooke B., Hegstrom C. D., Villeneuve L. S., Breedlove S. M. (1998). Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 19, 323–362 [DOI] [PubMed] [Google Scholar]
- Dauwalder B. (2011). The roles of fruitless and doublesex in the control of male courtship. Int. Rev. Neurobiol. 99, 87–105 [DOI] [PubMed] [Google Scholar]
- Demir E., Dickson B. J. (2005). fruitless splicing specifies male courtship behavior in Drosophila. Cell 121, 785–794 [DOI] [PubMed] [Google Scholar]
- DiAntonio A. (2006). Glutamate receptors at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75, 165–179 [DOI] [PubMed] [Google Scholar]
- Eade K. T., Allan D. W. (2009). Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling. J. Neurosci. 29, 3852–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J. F. (2010). Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Curr. Opin. Neurobiol. 20, 764–769 [DOI] [PubMed] [Google Scholar]
- Finley K. D., Taylor B. J., Milstein M., McKeown M. (1997). dissatisfaction, a gene involved in sex-specific behavior and neural development of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94, 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley K. D., Edeen P. T., Foss M., Gross E., Ghbeish N., Palmer R. H., Taylor B. J., McKeown M. (1998). Dissatisfaction encodes a tailless-like nuclear receptor expressed in a subset of CNS neurons controlling Drosophila sexual behavior. Neuron 21, 1363–1374 [DOI] [PubMed] [Google Scholar]
- Garrett-Engele C. M., Siegal M. L., Manoli D. S., Williams B. C., Li H., Baker B. S. (2002). intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development 129, 4661–4675 [DOI] [PubMed] [Google Scholar]
- Goldman T. D., Arbeitman M. N. (2007). Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 3, e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M. D., Scott K. (2009). Motor control in a Drosophila taste circuit. Neuron 61, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S., Clarke D. F., Broughton S., Andrews T. D., Partridge L. (2010). Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B., Hartmann B., Kho Y. H., Gorczyca M., Budnik V. (1996). The Drosophila tumor suppressor gene, dlg, is involved in structural plasticity at a glutamatergic synapse. Curr. Biol. 6, 695–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsemeyer M., Yapici N., Heberlein U., Dickson B. J. (2009). Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518 [DOI] [PubMed] [Google Scholar]
- Hudson A. M., Petrella L. N., Tanaka A. J., Cooley L. (2008). Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Dev. Biol. 314, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X. X., Gorczyca M., Budnik V. (1993). Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J. Neurobiol. 24, 1025–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelnikov A., Rivlin P. K., Hoy R. R., Heifetz Y. (2008). Tissue remodeling: a mating-induced differentiation program for the Drosophila oviduct. BMC Dev. Biol. 8, 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. (2011). Role of cell death in the formation of sexual dimorphism in the Drosophila central nervous system. Dev. Growth Differ. 53, 236–244 [DOI] [PubMed] [Google Scholar]
- Kimura K., Hachiya T., Koganezawa M., Tazawa T., Yamamoto D. (2008). Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759–769 [DOI] [PubMed] [Google Scholar]
- Kozopas K. M., Samos C. H., Nusse R. (1998). DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 12, 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Hall J. C. (2001). Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J. Neurosci. 21, 513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Villella A., Taylor B. J., Hall J. C. (2001). New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J. Neurobiol. 47, 121–149 [DOI] [PubMed] [Google Scholar]
- Li H., Baker B. S. (1998). hermaphrodite and doublesex function both dependently and independently to control various aspects of sexual differentiation in Drosophila. Development 125, 2641–2651 [DOI] [PubMed] [Google Scholar]
- Mahr A. A., Aberle H. (2006). The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6, 299–309 [DOI] [PubMed] [Google Scholar]
- Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A. (2004). Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24, 1406–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Mao Z., Davis R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 [DOI] [PubMed] [Google Scholar]
- Middleton C. A., Nongthomba U., Parry K., Sweeney S. T., Sparrow J. C., Elliott C. J. (2006). Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 4, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Aliaga I., Thor S. (2004). Segment-specific prevention of pioneer neuron apoptosis by cell-autonomous, postmitotic Hox gene activity. Development 131, 6093–6105 [DOI] [PubMed] [Google Scholar]
- Miguel-Aliaga I., Thor S., Gould A. P. (2008). Postmitotic specification of Drosophila insulinergic neurons from pioneer neurons. PLoS Biol. 6, e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M. (2003). Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 264, 38–49 [DOI] [PubMed] [Google Scholar]
- Parnas D., Haghighi A. P., Fetter R. D., Kim S. W., Goodman C. S. (2001). Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron 32, 415–424 [DOI] [PubMed] [Google Scholar]
- Paus T. (2010). Sex differences in the human brain: a developmental perspective. Prog. Brain Res. 186, 13–28 [DOI] [PubMed] [Google Scholar]
- Pierce S. B., Costa M., Wisotzkey R., Devadhar S., Homburger S. A., Buchman A. R., Ferguson K. C., Heller J., Platt D. M., Pasquinelli A. A., et al. (2001). Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 15, 672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. (2006). Organization of the efferent system and structure of neuromuscular junctions in Drosophila. Int. Rev. Neurobiol. 75, 71–90 [DOI] [PubMed] [Google Scholar]
- Rezával C., Pavlou H. J., Dornan A. J., Chan Y. B., Kravitz E. A., Goodwin S. F. (2012). Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22, 1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout E. J., Dornan A. J., Neville M. C., Eadie S., Goodwin S. F. (2010). Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci. 13, 458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett C. C., Vaughan A. G., Knapp J. M., Baker B. S. (2010). Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Valentín R., López-González I., Jorquera R., Labarca P., Zurita M., Reynaud E. (2006). Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 209, 183–198 [DOI] [PubMed] [Google Scholar]
- Salz H. K., Erickson J. W. (2010). Sex determination in Drosophila: The view from the top. Fly (Austin) 4, 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez L., Gorfinkiel N., Guerrero I. (2001). Sex determination genes control the development of the Drosophila genital disc, modulating the response to Hedgehog, Wingless and Decapentaplegic signals. Development 128, 1033–1043 [DOI] [PubMed] [Google Scholar]
- Sanders L. E., Arbeitman M. N. (2008). Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol. 320, 378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth D., Ch’ng Q., Dybbs M., Tavazoie M., Kennedy S., Wang D., Dupuy D., Rual J. F., Hill D. E., Vidal M., et al. (2005). Systematic analysis of genes required for synapse structure and function. Nature 436, 510–517 [DOI] [PubMed] [Google Scholar]
- Siegal M. L., Baker B. S. (2005). Functional conservation and divergence of intersex, a gene required for female differentiation in Drosophila melanogaster. Dev. Genes Evol. 215, 1–12 [DOI] [PubMed] [Google Scholar]
- Stockinger P., Kvitsiani D., Rotkopf S., Tirián L., Dickson B. J. (2005). Neural circuitry that governs Drosophila male courtship behavior. Cell 121, 795–807 [DOI] [PubMed] [Google Scholar]
- Struhl G., Basler K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 [DOI] [PubMed] [Google Scholar]
- Susic-Jung L., Hornbruch-Freitag C., Kuckwa J., Rexer K. H., Lammel U., Renkawitz-Pohl R. (2012). Multinucleated smooth muscles and mononucleated as well as multinucleated striated muscles develop during establishment of the male reproductive organs of Drosophila melanogaster. Dev. Biol. 370, 86–97 [DOI] [PubMed] [Google Scholar]
- Taylor B. J., Truman J. W. (1992). Commitment of abdominal neuroblasts in Drosophila to a male or female fate is dependent on genes of the sex-determining hierarchy. Development 114, 625–642 [DOI] [PubMed] [Google Scholar]
- Truman J. W. (1990). Metamorphosis of the central nervous system of Drosophila. J. Neurobiol. 21, 1072–1084 [DOI] [PubMed] [Google Scholar]
- Usui-Aoki K., Ito H., Ui-Tei K., Takahashi K., Lukacsovich T., Awano W., Nakata H., Piao Z. F., Nilsson E. E., Tomida J., et al. (2000). Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat. Cell Biol. 2, 500–506 [DOI] [PubMed] [Google Scholar]
- Veverytsa L., Allan D. W. (2012). Temporally tuned neuronal differentiation supports the functional remodeling of a neuronal network in Drosophila. Proc. Natl. Acad. Sci. USA 109, E748–E756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villella A., Hall J. C. (2008). Neurogenetics of courtship and mating in Drosophila. Adv. Genet. 62, 67–184 [DOI] [PubMed] [Google Scholar]
- Wade J., Arnold A. P. (2004). Sexual differentiation of the zebra finch song system. Ann. N. Y. Acad. Sci. 1016, 540–559 [DOI] [PubMed] [Google Scholar]
- Wagh D. A., Rasse T. M., Asan E., Hofbauer A., Schwenkert I., Dürrbeck H., Buchner S., Dabauvalle M. C., Schmidt M., Qin G., et al. (2006). Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833–844 [DOI] [PubMed] [Google Scholar]
- Yamamoto D. (2007). The neural and genetic substrates of sexual behavior in Drosophila. Adv. Genet. 59, 39–66 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Belawat P., Hafen E., Jan L. Y., Jan Y. N. (2008). Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Rumpf S., Xiang Y., Gordon M. D., Song W., Jan L. Y., Jan Y. N. (2009). Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N., Kim Y. J., Ribeiro C., Dickson B. J. (2008). A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37 [DOI] [PubMed] [Google Scholar]
- Yu J. Y., Kanai M. I., Demir E., Jefferis G. S., Dickson B. J. (2010). Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20, 1602–1614 [DOI] [PubMed] [Google Scholar]
- Zhou L., Schnitzler A., Agapite J., Schwartz L. M., Steller H., Nambu J. R. (1997). Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc. Natl. Acad. Sci. USA 94, 5131–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K., Fetter R. D., Goodman C. S., Isacoff E. Y. (1997). Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron 19, 1007–1016 [DOI] [PubMed] [Google Scholar]