Summary:
The authors describe the case of a patient with two particularly rare contiguous tumors, myofibroblastoma and osteosarcoma, in the same breast. Rare does not mean untreatable, and the chance of recovery is no less than with more common tumors. However, rare tumors do present a significant problem for pathologists due to diagnostic difficulties, and so an exact prognosis is not always possible.
Keywords: Synchronous breast tumors, Myofibroblastoma, Osteosarcoma, Mastectomy
Introduction
The incidence of unilateral or bilateral synchronous breast tumors varies from 1 to 11% (1, 2). Whether they originate from a single or from independent primary tumors cannot be established with certainty. A multicenter, multifactorial origin, especially for unilateral tumors, is suggested in the case of non-invasive tumors which are touching but discrete. This is even more likely with synchronous tumors with different histotypes and/or which are particularly rare (3, 4). We describe a case of two synchronous breast tumors involving uncommon histotypes.
Case report
An 82-year-old woman presented with a large, ulcerated, bleeding mass in the right breast. Her past medical history included type II diabetes, arterial hypertension and COPD. She had no family history of breast cancer.
The patient was unable to give precise details as to when and how the lump first formed. She had first noticed bleeding from the ulceration about 10 days earlier. This initially resembled an exudation but some hours earlier had become more copious, with bright red blood and clots.
The patient was in poor clinical condition with peripheral edemas. Her right breast was deformed by the presence of a lump, about 10 cm maximum diameter, mainly affecting the medial quadrants and extending to below the areola and nipple, which were both slightly retracted. The skin of the lower inner quadrant was affected by a 2-cm ulcer from which blood issued, with clots. On palpation the lump was hard and woody, with irregular margins. It extended to the outer quadrants. It adhered strongly to the skin but not to the muscle layer. There was no clinically visible sign of axillary lymphadenopathy.
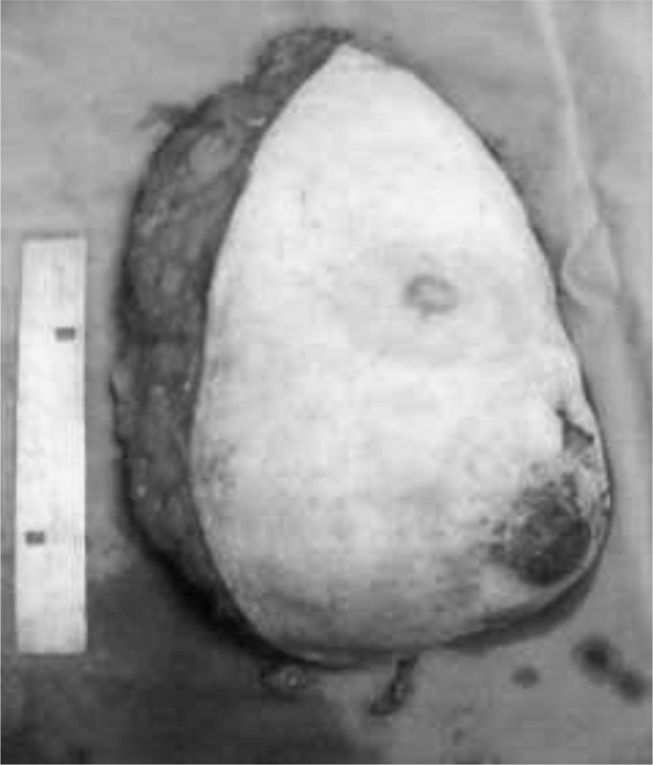
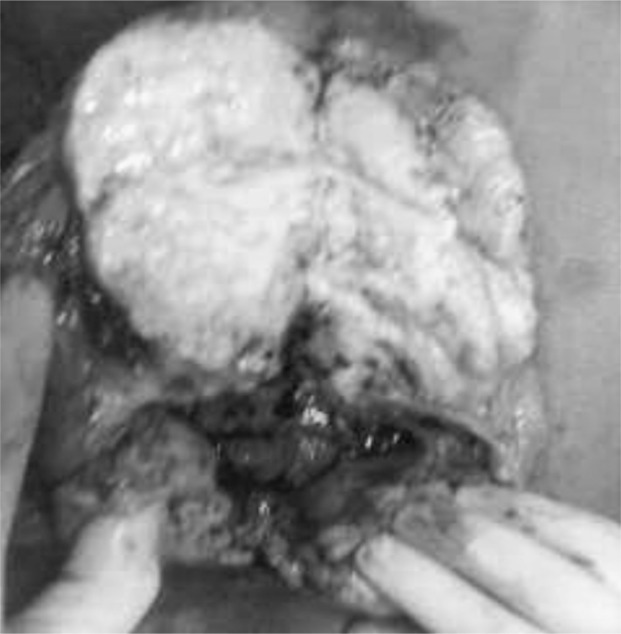
Given the bleeding and the patient’s general condition, it was decided to perform a right mastectomy. Macroscopic examination of the excised tissue revealed complete excision of the mammary gland (Figure 1), with the fascia of the pectoralis major intact. On cutting, the breast was found to be almost fully occupied by two contiguous tumors, roughly oval in shape. The larger was a whitish mother-of-pearl color with a compact structure, while the smaller was brown, with large areas of liquefaction containing necrotic debris (Figure 2). Histological examination revealed a growth consisting of two contiguous tumors:
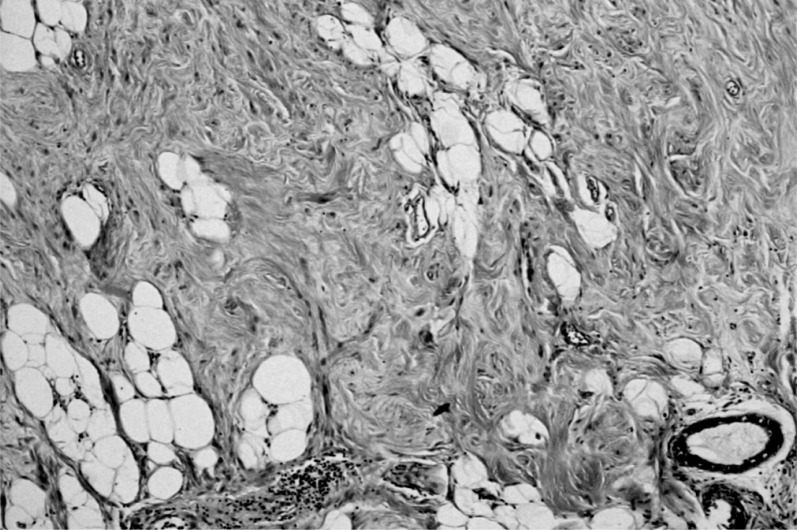
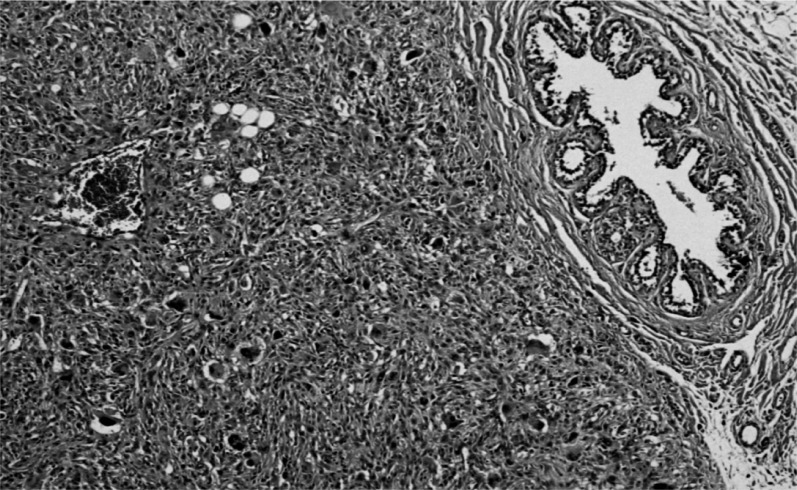
- the larger was characterized by monomorphic proliferation of spindle cells, arranged in short or long bundles separated by thick collagen bands (Figure 3), enclosing the adipose tissue lobules and breast structures (Figure 4). These features suggested a myofibroblastoma.
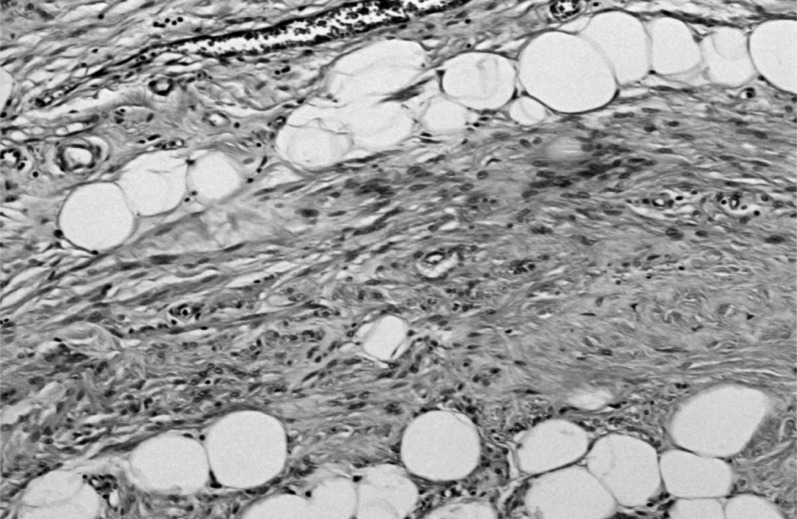
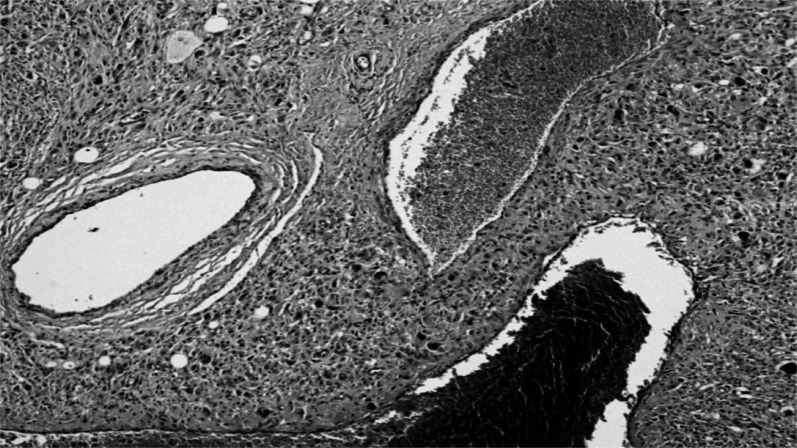
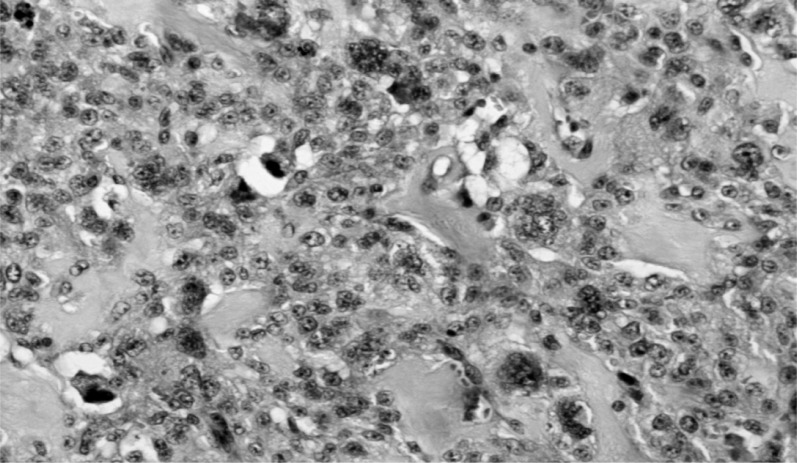
-The smaller was a pleomorphic tumor with a high density of predominantly spindle cells but with numerous multinucleated giant cells (Figure 5). It was highly vascularized but with extensive non-vascularized areas (Figure 6) and with multiple osteoid foci and numerous mitoses; some areas showed aspects of bone differentiation with formation of osteoid intercellular material (Figure 7).
Immunohistochemistry revealed:
- for the myofibroblastoma: CD34 pos, EgR pos, PgR pos, Her2 neg, MIB 1 <2%;
- for the pleomorphic tumor: cytokeratin neg, vimentin pos, focal AML pos, desmin neg, focal CD99 pos, c-Kit neg, MIB 1 pos>50%, EgR neg, PgR neg, Her2 neg.
Figure 1.
Surgical tissue: bleeding ulceration visible in QII.
Figure 2.
Surgical tissue: presence of two contiguous lesions.
Figure 3.
Monomorphic proliferation of spindle cells, organized in bundles, separated by thick collagen bands.
Figure 4.
Detail of fibroblastic appearance.
Figure 5.
Area of spindle cell proliferation, with multinucleated giant cells.
Figure 6.
The entire tumor has large areas of non-vascularization.
Figure 7.
Aspects of bone differentiation and formation of intercellular osteoid tissue.
These findings excluded metaplastic carcinoma (high-grade tumor negative for cytokeratins) and enabled the case to be identified as a collision tumor consisting of a myofibroblastoma and pleomorphic osteosarcoma or a dedifferentiated myofibroblastoma. The estrogen and progesterone receptor profile, cell proliferation index and HER-2 expression were also evaluated in both growths:
- myofibroblastoma: ER 70%; PGR 80%; MIB 125%; HER-2 0;
- osteosarcoma: ER neg.; PGR neg.; MIB 1 >50%; HER-2 neg.
The patient subsequently underwent full body CT and bone scintigraphy, which were negative for metastasis. Clinical and instrumental follow-up over the following four years revealed no signs of recurrence or metastasis.
Discussion
It may be useful at this point to recall the modern pathologic classification of breast tumors:
epithelial tumors, arising from glandular epithelial cells;
fibro-epithelial tumors, containing mesenchymal or stromal material as well as the epithelial component;
mesenchymal tumors, tumoral or pseudotumoral forms, that are found in soft (skin and subcutaneous) tissues as well as the breast;
rare tumors with various histotypes that are difficult to classify, have a low incidence and have an unusual clinical presentation (5).
Primary mesenchymal breast tumors may include numerous histopathologic variants: malignant phyllodes tumor, fibrosarcoma, liposarcoma, osteogenic sarcoma, chondrosarcoma, leio- or rhabdomyosarcoma, malignant fibrous histiocytoma, angiosarcoma, and myofibroblastoma. These variants account for no more than 1% of all breast tumors.
The clinical features of myofibroblastoma and osteosarcoma, as found in our patient, are reported below.
Myofibroblastoma
This rare mesenchymal tumor was first described by Wargotz in 1987 (6). It is most common in adults aged 35–67 and is mainly found in males (8:2). It usually occurs as a single, unilateral tumor (7, 8).
Its etiology is still unknown. It is mainly observed in patients with gynecomastia or who have been treated with anti-androgens, leading to the theory that it may be related to the patient’s hormone profile. This is supported by the fact that even though the response to hormone therapy affects all mesenchymal tissue, other sites affected by myofibroblastoma comprise the inguinal area, the muscles of the abdominal wall and the posterior wall of the vagina, with above all an apparent predominance along the milk line (9).
It presents macroscopically as a mobile, non-encapsulated but well-circumscribed mass of from 2 to 13 cm (mean 5.8 cm) in the mammary parenchyma. It is thus clinically similar to a fibroadenoma. Its consistency is taut and elastic, almost rubbery, with a smooth or lobulated outer surface whose color may be white, pink or brown. On cutting, the surface may be irregular or nodular, without any sign of cystic degeneration, necrosis or hemorrhage (10).
The most common form is often confused with fibroadenoma on US scanning. On mammography, it appear as a homogenous, lobulated nodule with well-circumscribed margins and no microcalcifications. Microscopically, the classic form is devoid of ducts and lobules, consisting of uniform bipolar (myoepithelial and myofibroblast) spindle cells with an oval or elongated nucleus, containing finely dispersed chromatin and arranged in fascicles of spindle cells interspersed with thick collagen bundles, often with a zigzag appearance, and adipose tissue (11). There may be rare multinucleated giant cells and mitotic elements. In a minority of cases, there may be adipose cells or cartilaginous differentiation. These tumors are poorly vascularised with small, often hyalinized vessels, with perivascular lymphocytic infiltrates.
There are diverse variants of myofibroblastoma with very particular pathological features (12, 13):
- forms in which collagen predominates, containing irregular gaps, like cracks, between the tumor cells and the stroma;
- epithelioid variant, with polygonal cells whose histological appearance may suggest invasive lobular carcinoma;
- densely cellular form, characterized by intense proliferation of spindle-shaped myofibroblasts and scarce collagen bands. This variant tends to reveal infiltrating edges under the microscope;
- infiltrating variant, with groups of rapidly growing cells and a tendency to infiltrate the blood vessels.
All these variants can be problematic for differential diagnosis against other tumors such as sarcoma and metaplastic carcinoma which, however, present greater cellularity and frequent mitosis (14). Differential diagnosis against fibromatosis is also difficult. This stellate, invasive tumor can be distinguished from myofibroblastoma by the presence of round or fusiform myoid cells organized in broad bundles rather than small clusters, with ample collagen and a significant inflammatory component, which may also be perilesional. Only immunohistochemistry is capable of differentiating myofibroblastoma (desmin, CD34 and actin-positive) from myofibroblastic cirrhotic carcinomas.
Osteosarcoma or osteogenic sarcoma
This generally originates in the bones, but may rarely originate from the soft tissues. Primary breast osteosarcoma accounts for less than 1% of all breast tumors and 12.5% of breast sarcomas (15). It is a highly malignant tumor with anaplastic pleomorphic cells that may be epithelioid, plasmacytoid, fusiform, ovoid, small and round, light, or fusiform giant mono- or multinucleated cells. These cells produce osteoid, associated with variable amounts of fibrous cartilage tissue (16).
The histogenesis of this tumor is still not fully clear. The most credible theory is that it originates from pluripotent mesenchymal breast stromal cells or is due to the transformation of an existing tumor, such as a fibroadenoma or phyllodes tumor (17, 18). Clinically, it presents as a palpable, hard, non-painful mass, often of a considerable size. It is often indistinguishable from a breast fibroadenoma (19, 20). It has never been associated with clinical signs of lymph node metastasis. Its appearance on mammography can vary considerably. In most cases, it appears as a large mass with relatively well-defined margins and lobulated edges, often containing focal or diffuse calcifications, that are generally coarse and dense (21–23). However, cases with irregular margins are not uncommon.
The presence of osteoid tissue in a breast tumor is not in itself indicative of osteosarcoma, as it may also be found in both benign and malignant epithelial or mesenchymal growths such as fibroadenoma, phyllodes tumor and metaplastic carcinoma. However, scintigraphy will reveal an intense focal hotspot of the radionuclide 99mTc-diphosphonate, a specific marker for osteoid tissue, and therefore may strongly indicate a soft tissue bone cancer. It is thus useful in the instrumental diagnosis of breast osteosarcoma (24, 25).
Given the low sensitivity of diagnostic tests, the exact identification of a primary breast sarcoma is possible only through histological examination, enabling the exclusion of osteogenic sarcoma deriving from the underlying bone structures (20). On immunohistochemistry, the tumor cells are generally positive on vimentin staining but negative for the epithelial markers MNF116 and CAM5.2 (cytokeratin), for the markers S-100, AE1/AE3, HER-2, desmin and actin and for progesterone and estrogen receptors (PGR and ER).
This is generally an aggressive tumor which often spreads through the circulation. For this reason, axillary dissection may not be indicated. Furthermore, due to the high risk of recurrence removal of the entire breast is recommended, with regular follow-up to enable early detection of any locoregional recurrences.
Conclusion
Rare tumors, while individually having a low incidence, collectively account for a considerable fraction of all breast tumors. “Rare” is used to describe tumors whose frequency ranges from 1 to 10%. These should be distinguished from unusual tumors, with a frequency of less than 1%, and exceptional tumors, of which there are only a few literature reports. Although these forms can be very complex, it should be stressed that rare does not mean incurable.
On the whole, rare tumors are a major problem for both clinicians and, above all, pathologists, given the difficulty in forming a precise diagnosis from the possible classifications and the not uncommon impossibility of arriving at a clear prognosis. Pathologic classification is even more problematic in the exceptional cases in which two or more forms are found in the same breast.
All this should further stimulate the commitment of multidisciplinary groups to study the clinical, biological and genomic aspects of these rare tumors in ever more depth in order to develop adequate diagnostic and therapeutic protocols.
References
- 1.Onoe S, Tsuda H, Akashi-Tanaka S, Hasebe T, Iwamoto E, Hojo T, Kinoshita T. Synchronous unilateral triple breast cancers composed of invasive ductal carcinoma, invasive lobular carcinoma, and Paget's disease. Breast cancer. 2010:8. doi: 10.1007/s12282-010-0245-2. [DOI] [PubMed] [Google Scholar]
- 2.Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, Dickman PW, Hall P. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;20(27):4210–6. doi: 10.1200/JCO.2006.10.5056. 25, [DOI] [PubMed] [Google Scholar]
- 3.Nichol AM, Yerushalmi R, Tyldesley S, Lesperance M, Bajdik CD, Speers C, Gelmon KA, Olivotto IA. A case-match study comparing unilateral with synchronous bilateral breast cancer outcomes. J Clin Oncol. 2011;29(36):4763–8. doi: 10.1200/JCO.2011.35.0165. [DOI] [PubMed] [Google Scholar]
- 4.Schmid SM, Pfefferkorn C, Myrick ME, Viehl CT, Obermann E, Schötzau A, Güth U. Prognosis of early-stage synchronous bilateral invasive breast cancer. Eur J Surg Oncol. 2011;37(7):623–8. doi: 10.1016/j.ejso.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Irshad A, Ackerman SJ, Pope TL, Moses CK, Rumboldt T, Panzegrau B. Rare breast lesions:correlation of imaging and histologig features with WHO classification. Radiographics. 2008;28(5):1399–1414. doi: 10.1148/rg.285075743. [DOI] [PubMed] [Google Scholar]
- 6.Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol. 1987;11(7):493–502. doi: 10.1097/00000478-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Yoo CC, Pui JC, Torosian MH. Myofibroblastoma associated with bilateral gynecomastia: report and literature review. Oncol Rep. 1998;5(3):731–3. doi: 10.3892/or.5.3.731. [DOI] [PubMed] [Google Scholar]
- 8.Hamele-Bena D, Cranor ML, Sciotto C, Erlandson R, Rosen PP. Uncommon presentation of mammary myofibroblastoma. Mod Pathol. 1996;9(7):786–90. [PubMed] [Google Scholar]
- 9.Salomao DR, Crotty TB, Nascimento AG. Myofibroblastoma and solitary fibrous tumor of the breast: Histopathological and immunohistochemical comparative study. Mod Pathol. 2001;10(1):49–54. doi: 10.1054/brst.2000.0188. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi A, Kayani N. Myofibroblastoma of breast. Indian J Pathol Microbiol. 2008;51(3):395–6. doi: 10.4103/0377-4929.42524. [DOI] [PubMed] [Google Scholar]
- 11.McMenamin ME, Fletcher CD. Mammari-type Myofibroblastoma of soft tissue: a tumor closely related to spindle cell lipoma. Am J Surg Pathol. 2001;25(6):399–402. doi: 10.1097/00000478-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt FC, AC Mera A. Fine needle aspiration cytology presentation of a cellular variant of the breast myofibroblastoma. Report of a case with immunohistochemical studies. Acta Cytol. 1998;42(3):721–4. doi: 10.1159/000331833. [DOI] [PubMed] [Google Scholar]
- 13.Magro G. Mammary myofibroblastoma:a tumor with a wide morphologic spectrum. Arch Pathol Lab Med. 2008;132(11):1813–20. doi: 10.5858/132.11.1813. [DOI] [PubMed] [Google Scholar]
- 14.Simsir A, Cangiarella S, Boppana S, Waisman J. Aspiration cytology of the collagenized variant of mammary myofibroblastoma: a case report with review of the literature. Diagn Cytopathol. 2001;24(6):399–402. doi: 10.1002/dc.1088. [DOI] [PubMed] [Google Scholar]
- 15.Bahrami A, Resetkova E, Ro JY, Ibañez JD, Ayala AG. Primary osteosarcoma of the breast: report of 2 cases. Arch Pathol Lab Med. 2007;131(5):792–5. doi: 10.5858/2007-131-792-POOTBR. [DOI] [PubMed] [Google Scholar]
- 16.Trihia H, Valavanis C, Markidou S, Condylis D, Poulianou E, Arapantoni-Dadioti P. Primary osteogenic sarcoma of the breast: cytomorphologic study of 3 cases with histologic correlation. Acta Cytol. 2007;51(3):443–50. doi: 10.1159/000325764. [DOI] [PubMed] [Google Scholar]
- 17.Khan S, Griffiths EA, S Ravi Shan N. Primary osteogenic sarcoma of the breast: A case report. Cases J. 2008;1:10. 148. doi: 10.1186/1757-1626-1-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22(8):925–33. doi: 10.1097/00000478-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ogundiran TO, Ademola SA, Oluwatosin OM, Akang EE, Adebamowo CA. Primary osteogenic sarcoma of the breast. World J of Surg Oncol. 2006;11(4):90–3. doi: 10.1186/1477-7819-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AL, Holwill SD, Thomas VA, Sacks NP, Given-Wilson R. Primary osteosarcoma of the breast: imaging and histological features. Clin Radiol. 1998;53(12):920–22. doi: 10.1016/s0009-9260(98)80224-9. [DOI] [PubMed] [Google Scholar]
- 21.Murakami S, Isozaki H, Shou T, Sakai K, Yamamoto Y, Oomori M, Toyoda H. Primary Osteosarcoma of the breast. Pathol Int. 2009;59(2):111–5. doi: 10.1111/j.1440-1827.2008.02338.x. [DOI] [PubMed] [Google Scholar]
- 22.Dragoumis D, Bimpa K, Assimaki A, Tsiftsoglou A. Primary osteogenic sarcoma of the breast. Singapore Med J. 2008;49(11):315–7. [PubMed] [Google Scholar]
- 23.Ribeiro-Silva A, Zambelli Ramalho LN, Zucoloto S. Phyllodes tumor with osteosarcomatous differentiation: a comparative immunohistochemical study between epithelial and mesenchymal cells. Tumori. 2006;92(4):340–6. doi: 10.1177/030089160609200414. [DOI] [PubMed] [Google Scholar]
- 24.Yang GJ, Li CL, Hao RR, Zou LF. Primary osteogenic sarcoma of breast detected on Tc-99m MIBI scintigraphy and TC-99m MDP skeletal scintigraphy. Ann Nucl Med. 2008;22(1):79–82. doi: 10.1007/s12149-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 25.Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004;91(2):237–41. doi: 10.1038/sj.bjc.6601920. [DOI] [PMC free article] [PubMed] [Google Scholar]