Summary:
Introduction.
Primary cardiac tumors are uncommon in cardiac surgery. To investigate the clinical presentation, surgical results and long-term follow-up we retrospectively analyzed our experience in the treatment of primary cardiac tumors.
Patients and methods.
Ninety-one patients with primary cardiac tumors underwent surgery in our department in the last 20 years. Fifthy-one patients were female, the mean age was 62,2 years. Sixty-three had myxomas, 22 had papillary fibroelastoma, 4 had malignant neoformations and 2 had other benign tumors.
Results
All myxomas, fibroelastomas and angiomyolipoma were radically removed. Only a palliative treatment was possible in malignant disease. In-hospital mortality was 1.2%. The mean follow-up time was 78.5 months. Three patients had recurrence of myxoma, all patients with malignant disease dead during the follow-up.
Discussion.
Primary benign cardiac tumors can be treated with low morbidity and mortality. The follow-up demonstrates that radical surgery is curative in case of benign tumors. The prognosis of malignant tumors is still poor. Palliative procedures have small impact on survival in these patients.
Keywords: Lung cancer, Advanced stage, Older patients, Extended resection
Introduction
Primary tumors of the heart are rare. In literature operations for heart tumors represents the 0,3% of all cardiac surgical procedures (1). Cardiac tumors are classified clinically and histologically in benign and malignant ones. Moreover often the heart can be involved as secondary localization of other carcinomas (2).
With new imaging the diagnosis of cardiac neoformations has been easier and faster. Surgical intervention in case of benign tumors offers a potential for cure. In case of malignant tumors surgery is useful to obtain a correct diagnosis and a palliation of the symptoms but rarely is curative.
We report our experience in the surgical treatment of primary cardiac tumors.
Patients and methods
Between January 1990 to May 2010, 91 patients with primary cardiac tumors underwent surgery in our department. Secondary cardiac tumors and pericardial neoformations were excluded from the study (37 patients in the same period). In all patients the diagnosis was made by echocardiography (Fig. 1) and computed tomography or magnetic resonance. Fifty-one patients were female (56%) and 40 were male (44%); the mean age was 62.2±13.8 years (range 22–85). Seven patients had a previous cardiac operation. Myxomas represent 69.2% (63 patients) of the heart tumors. Papillary fibroelastomas represent the 24.2% (22 patients). Two patients had other benign tumors, one angiomyolipoma and one hamartoma of mature cardiac myocytes (HMCM). In four cases the diagnosis was a malignant neoformation (4.4%): two angiosarcoma, one leiomyosarcoma, and one fibrosarcoma.
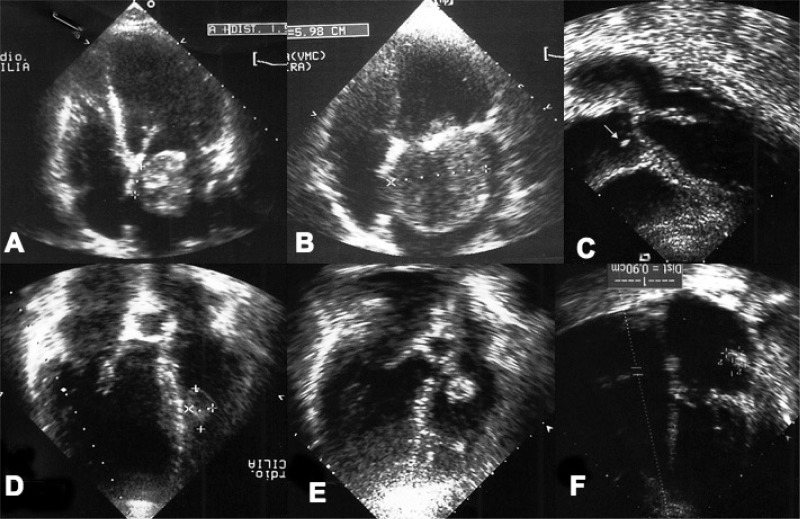
Fig. 1.
A, B) Preoperative echocardiography showing clearly the mechanism of obstruction of the mitral valve. C) Papillary fibroelastoma of the right aortic leaflet (white arrow) in a patient with previous embolic acute myocardial infarction. D) Papillary fibroelastoma of the right interventricular septum in a patient with malignant arrhythmia. E) Papillary fibroelastoma of the tricuspid valve. F) Recurrent myxoma of the left atrial wall after 29 months from the first surgery.
In 10 patients (10.9%), including, 5 myxomas, 3 papillary fibroelastomas, 1 angiomyolipoma and 1 fibrosarcoma, the diagnosis was incidental.
The clinical presentation of myxomas was characterized by palpitation due to sopraventricular arrhythmias in 24 patients (38.1%), congestive heart failure due to obstruction of mitral inflow in 7 patients (11.1%) (Fig. 1 A,B), syncope in 6 patients (9.5%), embolic stroke in 11 patients (17.5%), coronary artery embolization with acute myocardial infarction in 2 patients (3.2%), pulmonary embolism in one patient (3.2%). Six patients (9.5%) had a combination of different symptoms. The mean time from onset of symptoms to diagnosis was 4.1±8.3 months. The clinical presentation of papillary fibroelastomas was malignant ventricular arrhythmias (right ventricular localization of the mass) in 2 patient (9.1%), embolic stroke in 8 patient (36.4%), transient ischemic attack in 4 patients (18.2%), coronary embolization with acute myocardial infarction in 6 patients (27.3%). The average time from symptoms onset to diagnosis was 3.2±8.1 months. The diagnosis of cardiac cavernous hemangioma was made incidentally in a 34-years-old man. The clinical presentation of HMCM was exertional, dyspnea, palpitation, dry-cough and chest-tightness in a 35-years-old female. The clinical presentation of malignant tumors were pericardial effusion with cardiac tamponade in 2 patients, and congestive heart failure with malignant arrhythmias in 1 patient. The mean age was 41±6.3 years (range 23–58) for patients with malignant histology, three were male. The average time from symptoms onset to diagnosis was 2.4±5.2 months. The preoperative characteristics are reported in Table 1.
Table 1.
PREOPERATIVE CHARACTERISTICS OF THE PATIENTS.
| Data | Myxoma | Papillary fibroelastoma | Other Benign tumors | Malignant tumors |
|---|---|---|---|---|
| Male, n (%) | 21 (33.3%) | 8 (36.4%) | - | 3 (75%) |
| Female, n (%) | 42 (67.7%) | 14 (63.6%) | 2 (100%) | 1 (25%) |
| Age, yrs (mean ± SD, range) | 63.6±12.3 34–85 |
49.8±19.2 22–71 |
34.5±0.2 34,35 |
41±6.3 23–58 |
| Atrial fibrillation, n (%) | 24 (38.1%) | - | 1 (50%) | - |
| Ventricular arrhythmia, n (%) | - | 2 (9.1%) | - | 1 (25%) |
| Heart failure, n (%) | 7 (11.1%) | - | 1 (50%) | - |
| Syncope, n (%) | 6 (9.5%) | - | - | - |
| Stroke, n (%) | 11 (17.5%) | 8 (36.4%) | - | - |
| TIA, n (%) | - | 4 (18.2%) | - | - |
| Coronary embolism, n (%) | 2 (3.2%) | 6 (27.3%) | - | - |
| Pulmonary embolism, n (%) | 2 (3.2%) | - | - | - |
| Pericardial effusions, n (%) | - | - | - | 2 (50%) |
| Asymptomatic, n (%) | 5 (7.9%) | 3 (13.6%) | 1 (50%) | 1 (25%) |
| Reoperation, n (%) | 7 (11.1%) | - | - | - |
| Associated valvulopathy, n (%) | 5 (7.9%) | 3 (13.6%) | - | - |
| Coronaropathy, n (%) | 5 (7.9%) | 4 (18.2%) | - | - |
| NYHA class, mean ± SD | 2.8±1.2 | 2.1±0.8 | - | 3.3±0.5 |
| CCS class, mean ± SD | 2.2±0.3 | 3.2±0.9 | - | - |
| Other previous tumors, n (%) | 26 (41.3) | 3 (13.6%) | 2 (100%) | 3 (60%) |
Legend: TIA: transient ischemic attack.
The surgical approach was through median sternotomy and cardiopulmonary bypass, using aortic and bicaval cannulation in case of neoformation localized in the left or right atrium or ventricle. Upper j-shaped ministernotomy was used in case of aortic valve neoformation. Mini-thoracotomy was used in 18 cases of atria neoformations. All the surgical specimens were sent to the surgical pathologist for histological evaluation.
Results
Myxomas and fibroelastomas have been completed resected in all patients. The 4.2x3.3x2.7cm HMCM was located along the posterior-inferior and medium-basal segment of the left ventricle bulking in the left ventricular cavity just under the mitral annulus and involving the base of the posterior papillary muscle (Fig. 2A,B). The tumor was partially removed resolving the mechanical obstruction of the left ventricular inflow.
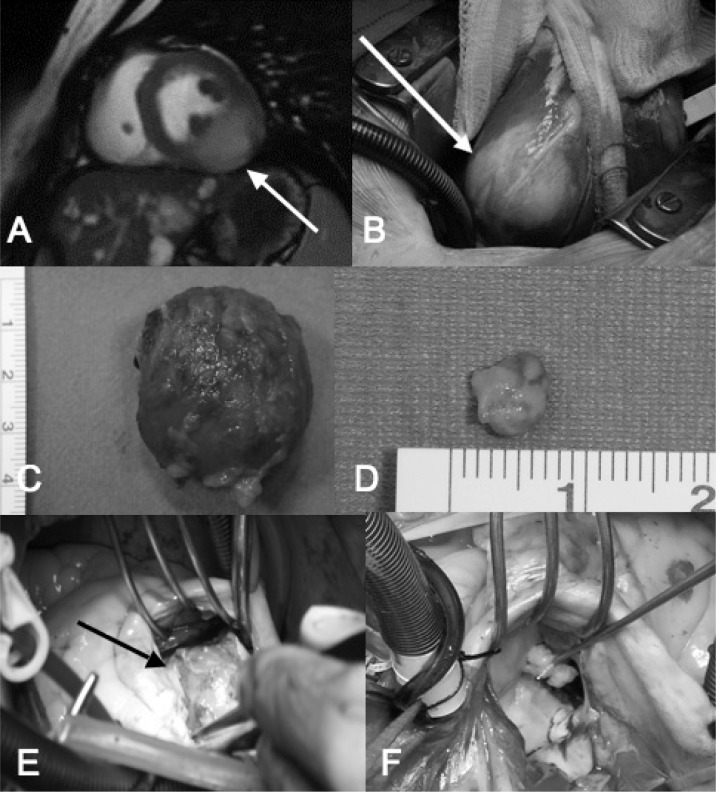
Fig. 2 - A–F).
A) Cardiac magnetic-resonance T2-weighted of the patient with HMCM, showing a mildly hyperintense mass on the inferior wall if the left ventricle involving the base of the posterior-medial papillary muscle (arrow). B) Intraoperative view of HCMC, on the postero-inferior-basal surface became evident a whitish and translucent area not clearly demarcated from the normal myocardial tissue(arrow). C) Operative specimen of a typical cardiac myxoma. D) Operative specimen of a tipical papillary fibroelastoma. E) Angiomyolipoma of the interventricular septum. The neoplasm originated in the thickness of the septum and became evident only after the incision of endocardium. F) Intraoperative view of a papillary fibroelastoma originating from second order chorda of the anterior leaflet of the mitral valve.
Myxomas (Fig. 2C) were located in the left interatrial septum in 47 patients (74.6%), in the left free atrial wall in 8 patients (12.7%), in the anterior mitral leaflets in one patient (1.6%), in the mitral annulus in one patient (1.6%), in the right atrium in 6 patients (9.5%). The tumor size ranged from 3x3.2x3.1 to 10.3x8x7.4 cm, the weight ranged from 9.4 to 136g (mean 47g). Papillary fibroelastoma (Fig. 2D) were located in the aortic valve leaflets (see also Fig. 1C) in 12 patients (54.5%), with multiple neoplasms involving all the aortic leaflets in one case, and in the right ventricle in 3 patients (13.6%) (Fig. 1D), in the mitral leaflet in 3 patients (13.6%), in the second order mitral chord in one patient (4.5%) (Fig. 2F), in the tricuspid leaflet in 3 patients (13.6%) (see also Fig. 1E). The fibroelastoma size raged from 3x2x3 to 19x20x15mm. The 4.2x4.1x2.6cm angiomyolipoma originates in the thickness of the interatrial septum (Fig. 2E). It was completely removed with a huge portion of the interatrial septum that was replaced with a goretex patch.
The four malignant neoformations were extensively infiltrating cardiac muscle and pericardium. The fibrosarcoma starting from the right atrium and invading the pericardium and the right ventricle. Both angiosarcomas originated from the right ventricle. The leiomyosarcoma originated probably from the left side of intervenetricular septum. Only palliative or diagnostic procedures have been performed in case of malignant disease. The cardiac localization of the tumors is summarized in Table 2.
Table 2.
TUMORS LOCALIZATION.
| Localizations | Myxoma | Papillary fibroelastoma | Other benign tumors | Malignant tumors |
|---|---|---|---|---|
| Inter-atrial septum, n (%) | 47 (74.6%) | - | 1(50%) | - |
| Left-atrial wall, n (%) | 8 (12.7%) | - | - | - |
| Mitral leaflets, n (%) | 1 (1.6%) | 3 (13.6%) | - | - |
| Mitral chorda, n (%) | - | 1 (4.5%) | - | - |
| Tricuspid leaflets, n (%) | - | 3 (13.6%) | - | - |
| Mitral annulus, n (%) | 1 (1.6%) | - | - | - |
| Right atrium, n (%) | 6 (9.5%) | - | - | 1 (25%) |
| Aortic valve, n (%) | - | 12 (54.5%) | - | - |
| Right ventricle, n (%) | - | 3 (13.6%) | - | 2 (50%) |
| Left ventricle, n (%) | - | - | 1 (50%) | 1 (25%) |
| Pericardial infiltration, n (%) | - | - | - | 4 (100%) |
Most of the myxoma were sessile and attached to the atrial endocardium. In 43 patients (68.3%) the tumors were excised with an associated patch repair. Mitral valve replacement was necessary in one patient with myxoma involvement of mitral leaflet. Aortic valve replacement was necessary in one patient with multiple fibroelastomas of aortic leaflets. Aortic valve free edge reinforcement with goretex (GORE-TEX®, Flagstaff, Arizona, 86004, USA) was necessary in 6 patients with fibroelastomas. Associated procedures included coronary artery by-pass grafting in 9 patients, 1 Bentall procedure, 3 ablation for atrial fibrillation, 4 mitral valve repairs, 3 tricuspid valve repair with annuloplasty and one tricuspid bicuspidalization.
The mean-extracorporeal circulation time was 51.7±25.8 minutes, the mean-clamping time 37.7±21.1 minutes.
The hospital mortality was 1/91(1.2%): a 72 years-old patient with left atrial mixoma died 12-days after the operation because of sepsis. Postoperative complications occurred in 12 patients: 2 transient ischemic attack (2.2%), 2 acute renal failure (2.2%), 1 low out-put syndrome (1.1%), 3 respiratory failure (3.3%), 4 re-thoracotomy for bleeding (4.4%), 2 pace-maker implantation (2.2%). The mean follow-up time was 78.46±67.7 months (range 6–226 months), 100% complete. Three patients with left atrial myxoma had recurrence of myxoma and they were reoperated after 29, 46 and 58 months respectively (Fig. 1F). Two patients who had aortic valve repair for papillary fibroelastoma have been reoperated after 67 and 51 months respectively because of development of severe aortic regurgitation due to failure of previous valve repair. One patient who had a mitral valve repair due to papillary fibroelastoma of anterior mitral chords has been re-operated after 112 months because of severe mitral regurgitation.
Ten patients died during the follow-up period (11%), 3 because of car accident, 4 because of extra-cardiac neoplasm, 1 because of myocardial infarction after 46 months from the operation and two for respiratory infection. Twenty-four patients had at least an episode of detected atrial fibrillation during the follow-up period (19 myxomas, 4 fibroelastoma, 1 angiomyolipoma) after a mean-time from the operation of 18±34months. Thirteen of these patients (54.2%) had atrial fibrillation as presentation symptom of neoplasm. Nine of these 24 patients (37.5%) have developed chronic atrial fibrillation treated with anti-arrhythmic drugs and anticoagulant therapy. Four of these patients (16.7%) underwent associated procedure (1 bentall, 2 mitral valve repair, 1 ablation for chronic atrial fibrillation). No embolic complications have been documented. After one-year follow-up the patient with HMCM is asymptomatic and in good clinical condition, without increase of the residual tumor diameter.
The patient with leiomyosarcoma died 9 months after the operation. The patient with fibrosarcoma died 26 months after the operation. The patients with angiosarcoma died after 5 and 7 months respectively.
Discussion and conclusions
In 1559 was certified for the first time the existence of cardiac neoplasm (3). A classification system of primary cardiac tumors similar to what we use today has been introduced by Yater in 1931 (4), the first ante-mortem diagnosis was made by Barnes in 1934 (5). The first surgical excision was performed by Bahnson and Newman in 1952. In 1954 Crafoord removed an atrial myxoma by using extracorporeal circulation (6). Nowadays in a high volume cardiac surgery department primary cardiac tumors are an uncommon but not rare entities. Between January 1990 to May 2010 we performed in our department 22093 cardiac operations, 91 of these were for primary cardiac tumors, representing the 0.4% of all operations. Autopsy studies reported a prevalence of primary cardiac tumors ranging from 0.001 to 0.3% (1). It has been estimated that surgery for cardiac tumors represent about 0.3% of all open-heart procedures (7,8). In details during the first decade of our experience we diagnosed 22 primary cardiac tumors representing 0.2% (22/9189 operations) of total surgical activity. In the meantime in the second decade of our experience we diagnosed 69 primary cardiac tumors representing the 0.5% (69/12904 operations) of total surgical activity. Moreover in the last years with the advent of new diagnostic tools and improved availability the diagnosis of cardiac tumors has been easier and more frequent (9). Primary cardiac tumors are classified into benign or malignant. Benign tumors are more common than malignant. In our experience 96% of tumors were benign, myxomas were the more common (69%) followed by papillary fibroelastomas (24.2%). Nevertheless cardiac tumors may be considered benign histologically but surely not clinically. Indeed in our experience 33 patients (36.3%) had serious embolic events, 24% in the myxoma group and 82% in the fibroelastoma group. We found that in case of myxoma the patients who suffered from an embolic events, the histological evaluation showed in the 89.6% of the cases a weight major than 25g with multiple area of hemorrhagic necrosis inside the mass. We think that during growth of the myxomas is achieved a critical dimension beyond which the tumor becomes necrotic because of the discrepancy between mass and nutrients, increasing the risk of fragmentation and embolism. Severe congestive heart failure due to obstruction of mitral or tricuspid inflow may be the presentation of atrial myxomas as happens in 7 of our cases. The mean mass of obstructive tumors were major than 47g with a mean dimension of 4.5×4×3.8cm.
In six patients the papillary fibroelastoma of aortic valve has been responsible of an acute myocardial infarction due to coronary embolism documented during selective angiography. Eight patients suffered from an embolic stroke. Malignant ventricular arrhythmia was the clinical presentation of two fibroelastomas of right ventricle. In our patients the clinical presentation of fibroelastomas has emerged more malignant with a higher incidence of severe embolic events or malignant arrhythmia than myxomas. Therefore in our opinion any of these tumors should be removed by urgent indication supported by low surgical mortality (6,9,10). Moreover it has been demonstrated that less invasive approaches could be used safety (11).
An interesting issue is the risk of recurrence in particular in case of myxomas. Some authors recommend a wide excision with patch repair (10,11). In our series of myxomas, the patch repair was used in 43 patients (68.3%). During the follow-up period we had three recurrence of myxoma in patients that underwent first resection without patch repair (16.7% 3/18patients without patch repair) after 29 and 46 months respectively. The familial histories of these patients were negative for cardiac myxomas and Carney syndrome (12). Recurrence can be due to inadequate resection, intraoperative implantation, embolization or multicentric growth (13). Then the cause of relapse, in our patients, is probably an incomplete removal of the tumor at the level of the base of implantation. Indeed the tumors were located very closely with the previous incision. Therefore in the last ten years we use systematically the patch repair and we have not seen further recurrence. In our experience we have never seen recurrent fibroelastomas, although the removal of these usually is less radical because of the need to avoid valve replacement.
The necessity to replace the heart valves for cardiac tumors is low. Centofanti et al. reported less than 3% of valve replacement in 91 patients (10). We were forced to replace the valve only in two patients (2.2%), an aortic valve replacement in a 39 years-old patients with multiple fibroelstoma and a mitral valve replacement in a 43 years-old patient with a papillary fibroelastoma involving the anterior mitral valve chords.
In our experience the recurrence or new occurrence of atrial fibrillation (37.5%) during the follow-up is the major complication in particular in patients who had excision of the left atrial myxoma. During the follow-up period six patients were reoperated (6.6%). Three patients because of myxoma recurrence, two because of failure of the aortic valve repair and one because of failure of mitral valve repair. The HMCM and angiomyolipoma are a rare benign heart tumors (14,15). Despite the benign histology, the location and the size of these tumors may complicate the surgical removal. In our patient with HMCM the complete resection was not feasible due to its location. We therefore opted for a partial resection just to resolve the mechanical obstruction of the left ventricular inflow. This choice is justified by the indolent grow of HMCM. Sometimes a partial resection may have a curative intent. Cardiac transplantation could be indicated in case of severely symptomatic disease not removable because of difficulty in maintaining the ventricular geometry (14). Cardiac sarcomas represent the common primary malignant cardiac tumor with prevalence of angiosarcoma. Sarcomas are common in the third and fifth decades of life. Our patients had 2 angiosarcoma, 1 leiomyosarcomas, and 1 fibrosarcoma. The mean age was slightly above the average reported in literature (16).
In our limited experience regarding four patients with malignant cardiac tumors we report mortality during the follow up period of 100% after a mean of 11.8 months after the operation. We would like to emphasize that all of our patients had at the time of the operation an extensive myocardial and pericardial infiltration, and they underwent surgery only to allow a secure and definitive histological diagnosis to guide the medical therapy or to perform palliative procedures such as pleuro-pericardial windows or a mass reduction.
In our experience the surgical resection is the treatment of choice for primary benign cardiac tumors because is safe, curative and with low surgical mortality. After a radical operation the recurrence is rare in the long-term follow-up. We are in favor of an aggressive attitude to reduce the risk of clinical complication like embolism or cardiac failure. In contrast surgery was done only with a diagnostic and palliative intent in our patients with malignant disease. In our hand palliative procedures have small impact on survival in these patients.
Prognosis could be improved by an early diagnosis, better adjuvant therapy, and in selected cases by heart transplantation.
References
- 1.Kun Y, Yinglong L, Hongyue W, et al. Epidemiological and pathological characteristics of cardiac tumors: a clinical study of 242 cases. Interact Cardiovasc Thorac Surg. 2007;6:636–639. doi: 10.1510/icvts.2007.156554. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Valente M, Poletti A, et al. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg. 1997;12:730–737. doi: 10.1016/s1010-7940(97)00246-7. [DOI] [PubMed] [Google Scholar]
- 3.Columbus MR. De Re Anatomica, Liber XV. p. 269. Venice, N Bevilacque: 1559.
- 4.Yater WM. Tumors of the heart and pericardium: pathology, symptomatology, and report of nine cases. Arch Intern Med. 1931;48:267. [Google Scholar]
- 5.Barnes AR, Beaver DC, Snell AMP. Primary sarcoma of the heart:report of a case with eletrocardiographic and pathological studies. Am Heart J. 1934;9:480. [Google Scholar]
- 6.Kirklin JW, Barratt-Boyes BG. Cardiac Surgery. 2nd ed. New York: Churchill Livigstone; 1993. pp. 1636–46. [Google Scholar]
- 7.Roberts WC. Primary and secondary neoplasm of the heart. Am J Cardiol. 1997;80:671–682. doi: 10.1016/s0002-9149(97)00587-0. [DOI] [PubMed] [Google Scholar]
- 8.Reynan K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107–116. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 9.Araoz PA, Mulvagh SL, Tazelaar HD, et al. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20(5):1303–19. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 10.Centofanti P, Di Rosa E, Deorsola L, et al. Primary cardiac tumors:early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236–1241. doi: 10.1016/s0003-4975(99)00700-6. [DOI] [PubMed] [Google Scholar]
- 11.Bossert T, Gummert JF, Battellini R, et al. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg. 2005;4:311–315. doi: 10.1510/icvts.2004.103044. [DOI] [PubMed] [Google Scholar]
- 12.Carney JA, Gordon H, Carpentier PC, et al. The complex of myxomas, potty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64(4):270–83. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Read RC, White HJ, Murphy ML. The malignant potential of left atrial myxoma. J Thorac Cardiovasc Surg. 1974;68:857–68. [PubMed] [Google Scholar]
- 14.Dell’Amore A, Lanzanova G, Silenzi A, Lamarra M. Hamartoma of mature cardiac myocites: a case report and review of the literature. Heart Lung Circ. 2011;20(5):336–340. doi: 10.1016/j.hlc.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Trawis WD, Branbilla E, Muller-Hermelink HK, et al. WHO Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the lung, pleura, thymus and heart, 2004. IARC Press; Lyon: 2004. [Google Scholar]
- 16.Burke A. Primary malignant cardiac tumors. Semin Diagn Pathol. 2008;25(1):39–46. doi: 10.1053/j.semdp.2007.10.006. [DOI] [PubMed] [Google Scholar]