Summary:
Secretory carcinoma of the breast is a rare tumor initially described in children but occurring equally in adult population. This unusual breast cancer subtype has a generally favorable prognosis, although several cases have been described in adults with increased aggressiveness and a risk of metastases. However, surgery is still considered the most appropriate treatment for this pathology. We describe the case of a 50 – year-old woman who has undergone a breast conservative surgery for a little tumor, preoperatively diagnosticated by a fine needle aspiration biopsy (FNAB) as a well differentiated infiltrating carcinoma.
Keywords: Juvenile/secretory breast cancer, Triple-negative carcinoma, Chemoresistance
Introduction
Secretory carcinoma is the commonest type of breast cancer in children and it was first reported as “juvenile breast carcinoma” by McDivitt in 1966; subsequent studies have shown that also adults can be affected by this disease and the original term has been replaced by the more appropriate “secretory breast cancer” (SBC) in 1980s (2/3 of approximately of 100 published cases of SBC have been in adult population). It accounts for less of 0.15% of all infiltrating breast cancers and this disease affects both males and females (1).
The typical clinical presentation of SBC is a slow-growing, painless, well-circumscribed, mobile, palpable mass occurring anywhere in the breast but it may be non-palpable and be detected as a radiologic lesion. SBC ultrasound appearance shows a solitary, microlobulated, hypoecoic mass resembling a benign lesion such as a fibradenoma or other well-circumscribed carcinomas. Tumor size ranges from 0.5 cm to 16 cm but is usually between 1.5 and 3 cm and microscopically arranged in microcystic, ductal and solid pattern, associated with low grade ductal carcinoma in situ (DCIS)and foci of invasion in the surrounding tissue (2); tumor cells are characterized by a granular eosinophilic cytoplasm and show abundant intra- and extracellular secretion of material containing sulfated mucopolysaccharides and mucin, PAS-positive. SBC are generally negative for estrogen and progesterone receptors, have a low proliferation index and usually do not present HER-2/neu gene amplification (triple negative breast carcinoma); these features, added to the expression of cell markers like cytokeratins 5/6, 14 and 17, contribute to delineate a basal-like immunohistochemical profile (3). Some recent studies have demonstrated that SBC has a specific molecular and genetic marker, characterized by the presence of a chromosomal translocation t(12;15) that results in the expression of a chimeric tyrosine kinase, encoded by the ETV6-NTRK3 fusion gene (4,5).
Anyway the clinical course of SBC is characterized by a tendency for late local recurrence and prolonged survival, even with lymph node metastases and death from metastatic secretory carcinoma is extremely rare (6). According to the literature about this rare subtype, only 5 cases of secretory carcinoma with distant metastases were reported, in association with large tumor size and multiple lymph node involvement.
Case report
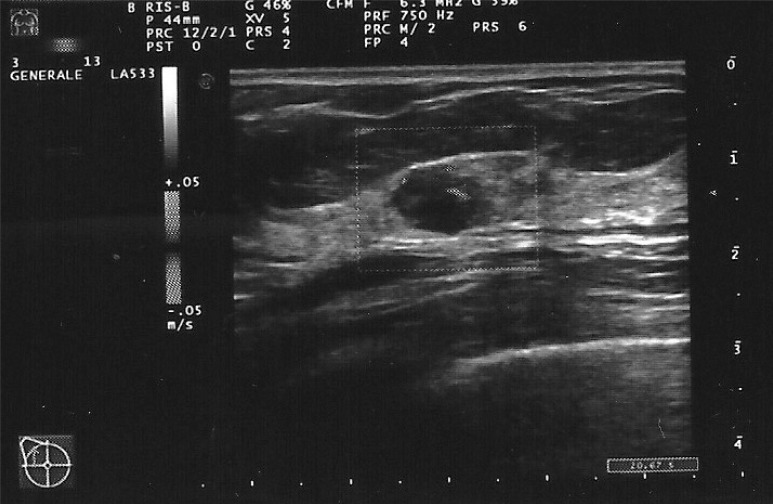
A 50 years old woman was admitted to our Breast Surgery Unit with an ultrasound diagnosis (Figure 1) of a 15 mm hypoecoic, circumscribed, microlobulated lump, well-vascularized at Doppler examination, located in the upper-outer quadrant of the right breast and discovered during a screening-check two months before. At the same time of the ultrasonography examination, a FNAB (fine needle aspiration biopsy) of the lesion was performed and revealed the presence of a mixed, infiltrating carcinoma consisting of a well differentiated, invasive ductal form, associated with a tubular carcinoma; tumor cells were negative for estrogen and progesterone receptors whereas positive for cytokeratin 5.
Figure 1.
Power-Doppler breast ultrasonography shows the hypoecoic, microlobulated and well-vascularized lesion.
At physical examination the palpable right breast mass was painless, with well-defined margins and increased in density, extended for more than 1 cm and shifting on the underlying layers and under the overlying too. There was no nipple discharge or inversion, nor clinical evidence of axillary lymph node involvement. Her familial anamnesis was positive for a case of breast cancer and she underwent monolateral hystero-oophorectomy at the age of 46.
Therefore, on the basis of these findings, a surgical procedure of right breast quadrantectomy with omolateral axillary sentinel lymph node (SLN) biopsy was performed. Intraoperative pathological evaluation of SLN indicated that it was negative and no complete axillary dissection was then carried out. SLN was further postoperatively examined with hematoxylin and eosin (H&E) as well as immunohistochemical techniques and it was not involved by metastases, in accordance with preoperatively frozen section (FS) analysis. The macroscopic examination of breast operative specimens revealed the presence of a nodule measuring 1 cm in its largest diameter. Microscopic findings detected a well-differentiated secretory breast carcinoma, characterized by central desmoplasia and peripheral mononuclear cells infiltrate; there were also seen cystic spaces, apocrine metaplasia and microcalcification in the context of the removed breast tissue. Immunohistochemistry was also performed: tumor cells were negative for estrogen receptors, p53, C-erbB-2/neu although weakly positive for progesterone receptors (4% positive cells); 10% was the value of MIB1-labeling index and the SBC was diagnosed as stage IA disease,G1 (pT1pN0 M0).
There was no indication for chemotherapy because of the non-responsiveness of this cancer in addition to the absence of node-positive disease; an adjuvant radiotherapy schedule was then planned.
Follow-up
A postoperative abdomen ultrasonography and a bone scintigraphy were performed to exclude any metastatic diffusion. After five weeks of radiation therapy, patient’s follow-up time has been started, with annual clinical and diagnostic imaging controls.
Discussion and conclusions
There is still no consensus of opinion as to how SBC should be treated, because there aren’t enough reports in literature (7). However there are specific features that can guide us in the management of this pathology. Secretory breast carcinoma is better detected by ultrasonography as a mass with a round or oval or tubular shape, with relatively well-circumscribed or partially microlobulated margins, and with an hypoecoic or an isoechoic internal echo texture (8). It’s typically slow-growing and it can mimic benign lesions, such as a fibroadenoma and the differential diagnosis include a wide range of benign processes or malignant lesions (i.e. cystic hypersecretory hyperplasia, juvenile papillomatosis with apocrine metaplasia or mucinous carcinoma, apocrine carcinoma and cystic hypersecretory carcinoma). Invasive secretory carcinoma can be differentiated from most of the entities listed above, by demonstrating the absence of myoepitelial cell layer; so the diagnosis is straightforward either with a fine needle biopsy or core biopsy that can demonstrate its specific histological pattern.
Although SBC is well circumscribed macroscopically, there may be foci of invasion in the surrounding breast tissue and associated ductal carcinoma in situ, which can be responsible for local recurrence after incomplete excision. Secretory breast carcinoma behavior seems to be less aggressive in children than in adults and a good prognosis is associated with a tumor size of less than 2 cm and a mass with circumscribed margins (9). Surgery is still considered the most proper strategy to treat this disease. In children local excision with sentinel lymph node biopsy or complete axillary dissection is preferred; in adults conservative surgery either simple or modified radical mastectomy can be performed, depending on the tumor size and on the lymphonodal status (2). However, involvement of more than 3 nodes may indicate the risk of systemic spread to distant sites (i.e.lung, liver, bone and scalp) and a poor outcome (9).
Since systemic metastases are extremely rare, there is no evidence to suggest that adjuvant therapy would be beneficial for such patients and SBC appears to be non-responsive to various chemotherapy protocols. This chemoresistance is probably due to the acquiring of mutations during the slow growth of this carcinoma and it’s important to underline the necessity for a long-term follow–up of the patients (10). Latest findings show that secretory breast cancer has an immunohistochemical and genetic feature that can differentiate it from the more common ductal carcinoma of the breast. The detection of a frequent expression of ETV6-NTRK3 fusion gene could implement current diagnostic and therapeutic strategies such as with future targeted therapies.
References
- 1.Horovitz DP, Sharma CS, Connolly E, Gidea –Addeo D, Deutsch I. Secretory carcinoma of the breast:Results from survival, epidemiology and end results database. The Breast. 2012;21:350–53. doi: 10.1016/j.breast.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Costa NM, Rodrigues H, Pereira H, Pardal F, Matos E. Secretory Breast Carcinoma-case report and review of the medical literature. The Breast. 2004;13:353–55. doi: 10.1016/j.breast.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Vasudev P, Onuma K. Secretory breast carcinoma:unique, triple negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch Pathol Lab Med. 2011;12:1606–10. doi: 10.5858/arpa.2010-0351-RS. [DOI] [PubMed] [Google Scholar]
- 4.Laè M, Frèneaux P, Sastre-Garau X, Chouchane O, Sigal-Zafrani B, Vincent-Salomon A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Modern Pathology. 2009;22:291–298. doi: 10.1038/modpathol.2008.184. [DOI] [PubMed] [Google Scholar]
- 5.Chiosea SI, Griffiyh C, Assaad A, Seethala RR. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol. 2012;36:343–50. doi: 10.1097/PAS.0b013e318242a5b0. [DOI] [PubMed] [Google Scholar]
- 6.Kavalakat AJ, Covilakam RK, Culas TB. Secretory carcinoma of the breast in a 17-year-old male. World Journal of Surgical Oncology. 2004;2:17. doi: 10.1186/1477-7819-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arce C, Cortes-Padilla D, Huntsman DG, Miller MA, Duennas-Gonzales A, Alvarado A, Perez V, Gallardo-Rincòn D, Lara-Medina F. Secretory carcinoma of the breast containing the ETV6-NTRK3 fusion gene in a male: case report and review of the literature. World Journal of Surgical Oncology. 2005;3:35. doi: 10.1186/1477-7819-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Secretory carcinoma of the breast: sonographic features. J Ultrasound Med. 2008;27:947–54. doi: 10.7863/jum.2008.27.6.947. [DOI] [PubMed] [Google Scholar]
- 9.Tixier H, Picard A, Guiu S, Coudert B, Loustalot C, Depret O, Arnold l, Cuisenier J. Long-term recurrence of secretory breast carcinoma with metastatic sentinel lymph nodes. Arch Gynecol Obstet. 2011;238:77–8. doi: 10.1007/s00404-010-1669-9. [DOI] [PubMed] [Google Scholar]
- 10.Herz H, Cooke B, Goldstein D. Metastatic secretory breast cancer. Non–responsiveness to chemotherapy. Case report and review of the literature. Annals of Oncology. 2000;11:1343–1347. doi: 10.1023/a:1008387800525. [DOI] [PubMed] [Google Scholar]