Summary:
The giant cell tumor of tendon sheath (GCTTS) is the most common benign neoplasm in the hand after the ganglion cyst. Several hypotheses were formulated about the etiological factors of these tumors, but still there is not a common opinion on etiology, prognostic factors and recurrence rate.
This article presents a review of literature of the last 15 years about GCTTS to assess the demographic, clinical and histological profile. We compared the information obtained from literature with our experience of 64 cases between 2000 and 2012. Our study showed similar results to those reported in literature, except for the recurrence rate: only 3 cases (4.7%) of 64 patients reported recurrence (versus about 15% on average in literature). Among the various possible factors that predispose to recurrence, it is necessary that the surgeon ensures complete excision of the tumor and removal of any residual satellite nodules.
Although the marginal excision is the treatment of choice, it is often difficult to perform due to for the location and the strict adherence of the tumor to the tendon or neurovascular bundles.
We used in all cases a magnifying loupe to help a careful research of satellite lesions and to respect surrounding structures.
Keywords: Hand, Synovial neoplasm, Tenosynovial giant cell tumor, Tendon sheath, Tumor, Surgery
Introduction
Giant cell tumor of the tendon sheath (GCTTS) is the second most common tumor of the hand after ganglion cysts (1,2). It is a slowly growing, usually painless benign lesion of soft tissues. The tumor affects individuals between the age of 30 and 50 years old and is found more often in women than men (3–6). Despite its benign character, local recurrence after excision has been reported in up to 45% of cases (7); there isn’t still a defined treatment protocol and local excision with or without radiotherapy is the treatment of choice to date (1,2,7–13).
We made a retrospective study of literature of the last 15 years and evaluated the demographic, clinical and histological aspects of the GCTTS of the hand and compared the results with our experience in a series of 64 cases from 2000 to 2012 to assess the factors that mostly contribute to incidence and recurrence of this tumor.
Patients and methods
We searched for published articles regarding the GCTTS from 1998 using the PubMed search engine. The keywords used were as follows: “giant cell tumor, tumor tendon, hand tumor”; all retrieved papers were analysed and their reference list were also screened if relevant. For each report, information was gathered on characteristics of the trial and study population, location and multicentricity of lesion, kind and severity of symptoms. We also recorded the applied treatment modality, histopathological examination of the excised tumor and recurrence rate.
A retrospective study was conducted in our Department of Plastic and Reconstructive Surgery and all data were collected from medical records of 64 GCTTS patients within this Department from 2000 to 2012. Medical record included the age, gender, tumor location, presentation and size, clinical features, treatment modality, histopathological report and neurovascular or tendon involvement.
All cases were operated under tourniquet control, using a magnifying loupe. Special care was taken to excise the tumor in total, retaining the capsule, if present, with margin of normal tissue. The operating field is searched for presence of satellite lesions.
The histopathological diagnosis and immunohistochemical studies were conducted by the Department of Pathology within the same Hospital. Follow-up ranged from 2–153 months.
No patient within this study had been treated with chemotherapy or radiation prior to treatment at our institution, and no additional adjuvant treatments were performed.
Results
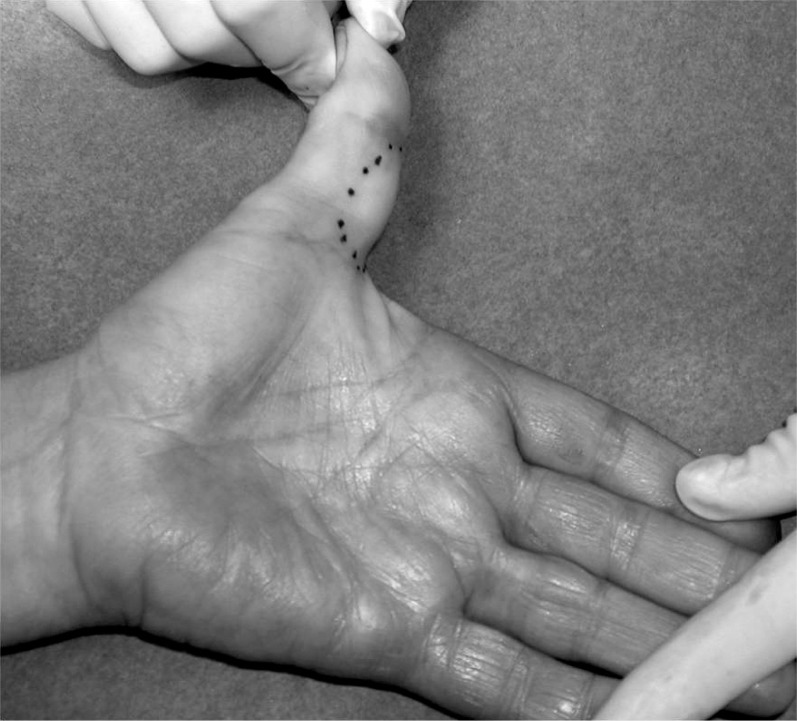
Age of patients ranged from 15 to 77 years (mean age 45 years) and GCTTS is found more often in the fourth and fifth decade of life. Out of 64 patients, 40 were females and 24 males, with a male to female ratio 1:1,66. The most frequent location of the tumor was the long finger in 23,5% (n=15). The other lesions were found over the thumb in 20,3% of the patients (n=13) (Fig. 1,2), index finger in 20,3% (n=13), over the hand in 20,3% of patients (n=13), ring finger in 7,8% (n=5) and little finger in 7,8% (n=5) (Table 1).
Fig. 1.
Giant cell tumor of tendon sheath of thumb, preoperative aspect.
Table 1.
ANATOMIC LOCATION OF GCTTS.
| Location | Patients, n | Rate, % |
|---|---|---|
| Thumb | 13 | 20,3 |
| Index finger | 13 | 20,3 |
| Long finger | 15 | 23,5 |
| Ring finger | 5 | 7,8 |
| Little finger | 5 | 7,8 |
| Hand | 13 | 20,3 |
|
| ||
| Total | 64 | 100 |
Our recurrence rate was 4,7% (n=3).
Macroscopically the average size of tumor was 1,35 cm (min= 0,3 cm, max= 5 cm). Microscopically all tumors contained multinucleated giant cells, histiocytes and haemosiderin deposits. Five cases were single nodule within a thin capsule.
In all cases we used magnifying loupe or operating microscope.
In 3 patients (4,7%) we founded bone erosion, in 7 cases (10,9%) tendon involvement with flexors/extensors ratio 4:3. In these cases we made a complete excision and bone curettage.
Seven patients (10,9%) presented the involvement of neurovascular bundle; in five cases it was possible to respect the vascular axis, in the other two patients the axes were rebuilt with vein grafting.
In the three recurrence cases surgical excision was difficult due to the relationship with the neurovascular structures and the layout of the surrounding soft tissues that did not allow easy exposure of the structures involved.
Discussion
Histologically GCTTS is composed of multinucleated giant cells, histiocytes polyhedral, fibrotic material and hemosiderin deposits (12,14,15); histological aspects such as the cellularity and mitosis does not seem to affect the prognosis of cancer (5,9,16).
Jaffe was the first, in 1941, who described GCTTS as a tenosynovitis, a nonneoplastic reaction (17).
This conclusion was supported by Vogrincic et al (1997), who detected polyclonal cells in the lesion, utilizing a polymerase chain reaction based assay for methylation of the X-linked human gene (18). Cytogenetic studies, however, suggested otherwise; for example simple structural and numeric aberrations, as well as a variety of balanced chromosomal aberrations have been discovered (19). In particular, clonal structural aberrations affecting the 1p11 to 1p13 region (20) and trisomies (21)of chromosomes 5 and 7 were commonly found. Using fluorescent in situ hybridization probes, Nilsson et al detected recurrent breakpoints localized to 1p13, often partnered with 2q35 (19,20). On these results, they suggested activation of a growth promoting gene through balanced translocation as the pathogenic mechanism. However, because similar translocations had been found in hemorrhagic and rheumatoid synovitis, there were still doubts about a neoplastic origin (3).
Macrophage colony stimulating factor (CSF1) is a secreted cytokine/hematopoietic growth factor that plays an essential role in the proliferation, differentiation, and survival of monocytes, macrophages, and related cells. It is localized to the 1p13 breakpoint and appears to have a major oncogenic role in GCTTS (22). Using a variety of molecular techniques, investigators from Stanford University detected high levels of receptor (CSFR1) expression in most of the cells in GCTTS, so it is possible that the neoplastic cells are driven by an autocrine mechanism (22). From these results, they concluded that GCTTS was indeed a neoplasm (19). However, the neoplastic cells constitute a minor component within the tumor, accounting for only 2–16% of the cells. Most cells are non-neoplastic, inflammatory cells recruited and activated by CSF1 produced by neoplastic cells, a phenomenon they called tumor landscaping (19). Probably neoplastic cells eluded detection in the X-linked human androgen receptor gene clonality assay in Vogrincic study, because they are so sparse (18,23).
Cupp et al (2007) subsequently found a subset of cells with high CSF1 expression but the absence of 1p13 translocation, suggesting an alternate mechanism in some tumors (23). Finally CSFR1 is a group II receptor tyrosine kinase that shows structural homology with KIT. For this reason probably a tyrosine kinase receptor inhibitor, such as Imatinib, could be useful to treat GCTTS (24).
In contrast with the indeterminate etiology, the clinical features and the diagnostic modalities of this tumor have been well described in the literature. According to Fotidias et al (2011) the giant cell tumor of the tendon sheath affects more often women, with a male to female ratio 1:1,47 and the mean age ranged from 30 to 50 years (25). The most frequent tumor location is the index finger (29,7%) (25). Other tumor sites are the thumb (12,9%), the long (24,6%), the ring (16,8%) and little (16%) fingers (2,7,9,10,12). The vast majority of patients presents with a painless swelling (84,3%) (2,7–9,12).Sensory disturbances of the digits are recorded in 4,57% of cases (1,2,7–9,12). The average duration of symptoms ranges from 6 to 30 months (range, 1 to 120 month) (1,2,7–13). Only 5% of the patients has a definite history of soft tissue trauma at the time of initial presentation (1,2,7–13).
Sonography can detect whether tumor is solid or cystic, and to note if there are satellite lesions. It also describes the relationship of the lesion to the surrounding structures (26). Information regarding the extent of contact with underlying tendon and the percentage of circumferential involvement is possible with sonography (26). Byers classified GCTTS into localized nodular type (common in hand) and diffuse type (common in joints) (9,10). Al-Qattan proposed a new classification for GCTTS, where he classified Type-I as single tumor which is round or multilobulated, and Type-II, where there are two or more distinct tumors which are not joined (9).
Concerning the recurrence there is a large statistical heterogeneity in the literature. In more recent studies, on average, 14.8% of patients developed recurrence (2,7–13,27). Various factors have been described predictive of recurrence, including pressure erosion on radiographs, location at the interphalangeal joint, presence of degenerative joint disease and incompletely excision. Reilly et al (1999) and Grover et al (1998) noticed that bone erosion, as confirmed in plain X-rays, might be a reason for recurrence (8,27).However, Kitagawa (2004) did not support this theory, he advocated the bone involvement was due to simple erosion, caused by the pressure effect of the tumor, and was not a true invasion (11). Lowyck (2006) did not find significant correlation of recurrence with pressure erosions, or degenerative joint disease, neither with the location at the distal interphalangeal joint (28).
However, the site of the tumor has been associated with recurrence rate by many other Authors (8,9,27). Reilly et al observed that recurrence of giant cell tumor was much higher at the thumb interphalangeal (IP) joint and digital distal interphalangeal (DIP) joints (8,9,27). This finding might be attributed to the inherent difficulty of adequately excising the tumor distally at the IP and DIP joint levels, where the neurovascular structures are quite close to tumor margins and the surrounding soft tissue envelope is not ideal (2,9,11). Williams et al (2010) reported that the high risk group was defined as tumor involvement of the extensor tendon, flexor tendon or joint capsule (13).
Type-II tumors have been associated with a higher recurrence rate compared to Type-I giant cell tumors, probably due to an undetected satellite lesion and subsequent incomplete excision, therefore it cannot be always considered as a true recurrence (10,25,27). The lower recurrence rate in prospective studies might reflect the surgeon’s concern of identifying tumor margins and subsequently achieving a good result. In addition there may simply not be enough follow-up in these prospective studies to show the true recurrence value.
A lower rate of recurrence should be expected when magnifying glasses or microscope are used at the time of mass resection (10); Ikeda had only one recurrence in 18 patients with GCTTS after microscopic excision of the lesion (10).
Kotwal et al recommended postoperative radiotherapy of 20 Gy in divided daily doses of 2 Gy in case of possible incomplete excision, presence of mitotic figures (9) and bone involvement (7). In their study, recurrence rate by following the protocol was 0% (0 out of 14 patients) (25). Ng in 2010 proposed the use of fine needle aspiration cytology (FNAC) as a primary diagnostic aid and helps in preoperative planning to prevent recurrence (29).
Conclusions
In conclusion, surgery seems to be the main factor influencing the rate of recurrence. The role of intrinsic biology of the lesion and postoperative irradiation in decreasing rate of recurrence is still unknown and controversial. Incomplete excision and leaving behind satellite nodules is considered as the most important factor deciding recurrence pattern.
Thanks to an adequate surgical exposure, meticulous dissection and use of magnification loupe we obtained a low recurrence rate, underling the role of surgery as the first prognostic factor of GCTTS.
More well-designed studies with a large number of cases are still necessary to determine a proper protocol for this tumor.
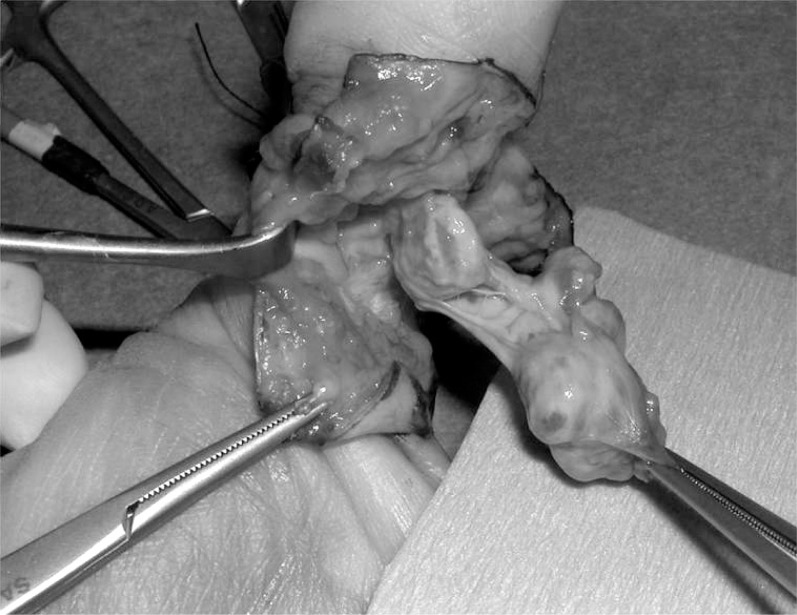
Fig. 2.
Giant cell tumor of tendon sheath of thumb, intraoperative aspect.
References
- 1.Darwish FM, Haddad WH. Giant cell tumour of tendon sheath: experience with 52 cases. Singapore Med J. 2008;49(11):879–882. [PubMed] [Google Scholar]
- 2.Uriburu IJF, Levy VD. Intraosseous growth of giant cell tumors of the tendon sheath (localized nodular tenosynovitis) of the digits: report of 15 cases. J Hand Surg Am. 1998;23:732–736. doi: 10.1016/S0363-5023(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Suresh SS, Zaki H. Giant cell tumor of tendon sheath: case series and review of literature. J Hand Microsurg. 2010;2(2):67–71. doi: 10.1007/s12593-010-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg B, Kotwal PP. Giant cell tumour of the tendon sheath of the hand. J Orthop Surg (Honk Kong) 2011;19(2):218–220. doi: 10.1177/230949901101900218. [DOI] [PubMed] [Google Scholar]
- 5.Monaghan H, Salter DM, Al-Nafussi A. Giant cell tumour of tendon sheath (localized nodular tenosynovitis): clinicopathological features of 71 cases. J Clin Pathol. 2001;54:404–407. doi: 10.1136/jcp.54.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams EL, Yoder EM, Kasdan ML. Giant cell tumor of the tendon sheath: experience with 65 cases. Eplasty. 2012;12:e50. [PMC free article] [PubMed] [Google Scholar]
- 7.Kotwal PP, Gupta V, Malhotra R. Giant cell tumour of the tendon sheath- is radiotherapy indicated to prevent recurrence after surgery? J Bone Joint Surg. 2000;82B:571–573. doi: 10.1302/0301-620x.82b4.10328. [DOI] [PubMed] [Google Scholar]
- 8.Reilly KE, Stern PJ, Dale JA. Recurrent giant cell tumors of the tendon sheath. J Hand Surg Am. 1999;24:1298–1302. doi: 10.1053/jhsu.1999.1298. [DOI] [PubMed] [Google Scholar]
- 9.Al-Qattan M. Giant cell tumors of tendon sheath: classification and recurrence rate. J Hand Surg. 2001;26B:72–75. doi: 10.1054/jhsb.2000.0522. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Osamura N, Tomita K. Giant cell tumour in the tendon sheath of the hand: importance of the type of lesion. Scand J Plast Reconstr Surg Hand Surg. 2007;41:138–142. doi: 10.1080/02844310601159766. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa Y, Ito H, Yokoyama M, et al. The effect of cellular proliferative activity on recurrence and local tumour extent of localized giant cell tumour of tendon sheath. J Hand Surg Br. 2004;29:604–607. doi: 10.1016/j.jhsb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Ozalp T, Yercan H, Kurt C, et al. Giant cell tumor of tendon sheath of the hand. Acta Orthop Traumatol Turc. 2004;38:120–124. [PubMed] [Google Scholar]
- 13.Williams J, Hodari A, Janevski P, Siddiqui A. Recurrence of giant cell tumors in the hand: a prospective study. J Hand Surg Am. 2010;35:451–456. doi: 10.1016/j.jhsa.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Messoudi A, Fnini S, Labsaili N, et al. Giant cell tumors of the tendon sheath of the hand: 32 cases. Chir Main. 2007;26:165–169. doi: 10.1016/j.main.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu PT. Radiological reasoning: acutely painful swollen finger. Am J Roentgenol. 2007;188:A13–S17. doi: 10.2214/ajr.188.3_supplement.0s13. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues C, Desai S, Chinoy R. Giant cell tumor of the tendon sheath: a retrospective study of 28 cases. J Surg Oncol. 1998;68:100–103. doi: 10.1002/(sici)1096-9098(199806)68:2<100::aid-jso5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe H, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch Pathol. 1941;31:731–765. [Google Scholar]
- 18.Vogrincic GS, O’Connell JX, Gilks CB. Giant cell tumor of tendon sheath is a polyclonal cellular proliferation. Hum Pathol. 1997;28(7):815–819. doi: 10.1016/s0046-8177(97)90155-6. [DOI] [PubMed] [Google Scholar]
- 19.David R, Lucas Tenosynovial Giant Cell Tumor: Case Report and Review. Archives of Pathology & Laboratory Medicine. 2012 Aug;136(8):901–906. doi: 10.5858/arpa.2012-0165-CR. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson M, Höglund M, Panagopoulos I, et al. Molecular cytogenetic mapping of recurrent chromosomal breakpoints in tenosynovial giant cell tumors. Virchows Arch. 2002;441(5):475–480. doi: 10.1007/s00428-002-0640-y. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher JA, Henkle C, Atkins L, Rosenberg AE, Morton CC. Trisomy 5 and trisomy 7 are nonrandom aberrations in pigmented villonodulare synovitis: confirmation of trisomy 7 in uncultered cells. Genes Chromosomes Cancer. 1992;4(3):264–266. doi: 10.1002/gcc.2870040312. [DOI] [PubMed] [Google Scholar]
- 22.West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a trans location in a minority of tumor cells. Proc Natl Acad Sci USA. 2006;103(3):690–695. doi: 10.1073/pnas.0507321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cupp JS, Miller MA, Montgomery KD, et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol. 2007;31(6):970–976. doi: 10.1097/PAS.0b013e31802b86f8. [DOI] [PubMed] [Google Scholar]
- 24.Cassier PA, Gelderblom H, Stacchiotti S, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118(6):1649–1655. doi: 10.1002/cncr.26409. [DOI] [PubMed] [Google Scholar]
- 25.Fotidias E, Papadopoulos A, Svarnas T, et al. Giant cell tumour of tendon sheath of the digits. A systematic review. Am Ass Hand Surg. 2011;6:244–249. doi: 10.1007/s11552-011-9341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton WD, Patel V, Teefey SA, et al. Giant cell tumors of the tendon sheath: an analysis of sonographic findings. Am J Roentgenol. 2004;188:A13–S17. doi: 10.2214/ajr.183.2.1830337. [DOI] [PubMed] [Google Scholar]
- 27.Grover R, Grobbelaar AO, Richman PI, et al. Measurement of invasive potential provides an accurate prognostic marker for giant cell tumor of tendon sheath. J Hand Surg. 1998;23B:728–731. doi: 10.1016/s0266-7681(98)80084-3. [DOI] [PubMed] [Google Scholar]
- 28.Lowyck H, De Smet L. Recurrence rate of giant cell tumors of the tendon sheath. Eur J Plast Surg. 2006;28:385–388. [Google Scholar]
- 29.Ng VY, Thomas K, Crist M, et al. Fine needle aspiration for clinical triage of extremity soft tissue masses. Clin Orthop Rel Res. 2010;468:1120–1128. doi: 10.1007/s11999-009-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]