Summary:
Aim.
To evaluate the safety and efficacy of the minimally invasive surgical approach (laparoscopic drainage) of liver abscesses in selected cases.
Case report.
Male, 58 years old, from a rural area, presented with epigastric abdominal pain, fever, weight loss, loss of appetite, a palpable mass in the epigastrium and neutrophilic leukocytosis. CT revealed a complex multiloculated liver abscess in segments 2–3. Systemic antibiotic therapy alone was ineffective; percutaneous drainage was excluded due to the characteristics of the lesion.
Result
Given the complexity of the lesion, a laparoscopic approach was chosen involving complete drainage of the abscess, debridement and irrigation; the cavity was unroofed using electrocautery and samples were obtained for bacterial culture and drug testing. Two drains were left in the cavity for seven days. No complications were observed.
Discussion.
In accordance with the scientific literature, after thorough imaging we performed laparoscopic drainage of a large, complex liver abscess as a safe, effective alternative to open surgery when antibiotic therapy alone failed and percutaneous drainage was uncertain.
Conclusion
Not all liver abscesses can be treated with antibiotic therapy or percutaneous drainage. Laparoscopic drainage in association with systemic antibiotic therapy is a safe and effective minimally invasive approach that should be considered in selected patients.
Keywords: Liver abscesses, Percutaneous drainage, Laparoscopic drainage, Antibiotic therapy
Introduction
Liver abscesses are a common disease whose diagnosis and treatment are still problematic, although considerable progress has been made since the late 80s. More advanced imaging techniques, from ultrasound to multi-slice CT, enable the easy identification of even small lesions in their initial stages, while aggressive treatments have now given way to minimally invasive procedures such as US- or CT-guided percutaneous or laparoscopic drainage (1, 2).
Case report
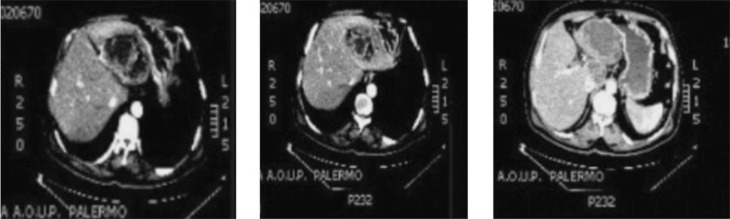
A 58-year old Caucasian man, weight 80 kg, from a rural area attended our department due the onset some days earlier of general malaise, septic fever, pressure pain in the epigastrium and right hypochondrium, appetite loss and premature satiety and nausea followed by food vomiting, which resulted in a considerable weight loss. On admission, an abdominal examination confirmed the tenderness in the upper abdomen and revealed a palpable, non-pulsing mass in the epigastrium and right hypochondrium, associated with liver enlargement. Laboratory tests revealed marked neutrophilic leukocytosis (WBC 18000/ l) with moderately raised liver markers but no cholestasis. Cancer and viral markers were negative. Empirical antibiotic treatment was begun with latest-generation Cephalosporin and Metronidazole. Liver US revealed a complex growth in S2–3, without establishing its nature (infected parasitic cyst?) (3). The patient then underwent contrast-enhanced multi-slice CT, which revealed two large (5 cm and 11 cm, respectively), multiloculated lesions in segment 1 (S1) and S2–S3, with a fluid-like or slightly higher density and containing some air. The walls and intra-lesion septa showed contrast enhancement, and an initial diagnosis of hepatic abscesses was proposed (Fig. 1). CT revealed the strong contiguity of this segmented, multi-chambered formation, max. diameter about 15 cm, with the surrounding areas, especially the lesser curvature of the stomach and the lesser omentum (hepatogastric ligament). However, this did look cleavable, as it had its own wall and showed no signs of fistulization.
Fig. 1.
CT images of the liver abscess: contrast enhanced intralesion septa.
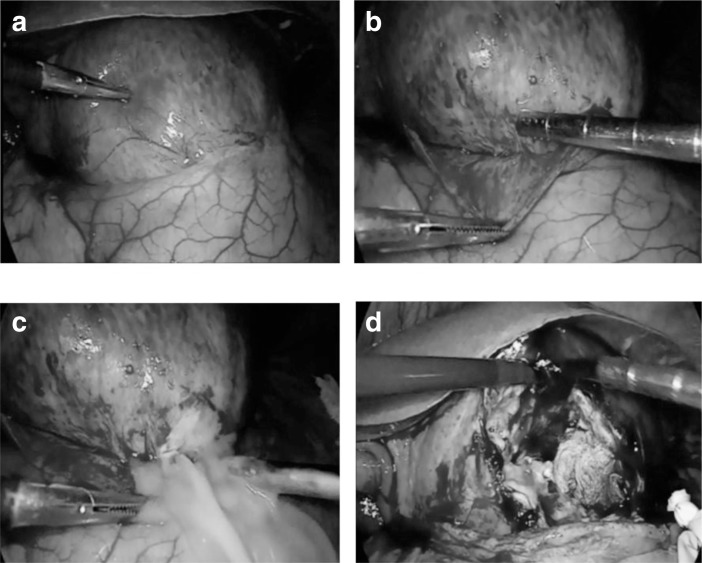
In accordance with the international scientific literature, the initial intention was to perform US- or CT-guided percutaneous drainage, but careful analysis of the features of the abscess, its size and above all, the presence of numerous septa and internal chambers –a prime obstacle to complete drainage of the contents – were indications for a minimally invasive laparoscopic approach. Pneumoperitoneum was induced using Hasson’s technique via trans-umbilical open laparoscopy (TUOL) (4) and two more trocars were placed in the left and right side (5). The procedure took about 45 minutes. Thorough exploration of the abdominal cavity revealed the anatomical connections between the abscess and organs and surrounding tissues, enabling its adequate isolation from the gastric wall and lesser omentum. At this point, a large fenestration was created in the anterior wall, of which part was taken for histological testing, the contents were drained and sent for microbiological tests, the area was cleaned with bactericidal solution and the internal septa were ruptured (Fig. 2). Finally, having controlled bleeding and checked for bile leakage, two gravity drains were placed in the residual cavity (6).
Fig. 2.
Intra-operative findings: a) identification of the abscess; b) cleavage by blunt dissection; c) drainage; d) rupture of the septum and lavage with bactericidal solution.
Results
The postoperative course was regular: the patient began passing gas on day 2 and began eating. The drains were removed on day 7 and the patient was discharged the following day, with his clinical picture and laboratory parameters having fully normalized. Follow up US exams confirmed the disappearance of the lesion.
Discussion
Liver abscesses were recognized as far back as Hippocrates, in 400 BC, who thought that prognosis was related to the type of fluid in the lesion. Osler, in 1890, was the first to describe the presence of amebae in a patient’s abscess and stools, but it was only in the early 20th century that amebae were correlated to the formation of a liver abscess. The etiology varies. In addition to pyogenic bacteria and amebae, other microorganisms, such as fungi and cytomegalovirus, can also cause liver abscesses, albeit rarely, especially in immunosuppressed patients. The most common causes of pyogenic abscesses are Escherichia coli, Klebsiella and Enterococcus. Among the anaerobic bacteria, Bacteroides, anaerobic streptococci and Fusobacterium predominate. Aerobic, anaerobic or microaerophilic streptococci are isolated in 25–30% of cultures from liver abscesses (7). In the USA, the incidence of pyogenic abscesses is 8–15 new cases/100,000 inhabitants/year, accounting for over 80% of cases. Abscesses due to amebae make up 10% of cases, and are more common in tropical areas, in tourists and in immigrants from developing countries, while fungi and other agents are responsible for less than 10%. Abscesses due to pyogenic bacteria and amebae seem to be more common in men, with the incidence peaking between the ages of 40 and 60 years.
Most pathogens reach the liver via the portal system. In normal conditions, the immune system of healthy subjects prevents the colonization of the sinusoids and parenchyma through intrahepatic elimination. Predisposing factors are thus necessary for a liver abscess to arise. These include trauma, necrotic or hemorrhagic areas, tumors, obstruction and/or primary or secondary malignant stenosis of the bile ducts and/or portal branches, arterial microemboli in systemic sepsis, perfusion defects, iatrogenic cell necrosis after chemoembolization and post-transplant mini-microabscess (MMA) syndrome. However, distal abdominal infections are most commonly implicated. Dieulafoy coined the term la foie appendiculaire to describe a picture of multiple liver abscesses following perforated appendicitis, which is in fact one of the most common infectious foci. Other foci include cholecystitis, pylephlebitis, perihepatic and subphrenic abscesses, diverticulitis, IBD and pelvic sepsis (7). However, despite considerable technological progress, the etiology of a large number of liver abscesses is never discovered. The incidence is three times higher in the right lobe than the left. This is probably due to the predominant flow from the superior mesenteric vein to the right lobe, as well as its larger size. Only 1% of patients present bilobar abscesses. Most caseloads report a similar incidence of single and multiple abscesses. The size can vary from less than a millimeter to several centimeters. From an anatomical perspective, the pyogenic membrane consists of three layers: inner (necrosis and fibrin), intermediate (granulation tissue) and outer (fibrous tissue), which delimits the surrounding parenchymal process (8).
Conclusions
US- or CT-guided percutaneous drainage of liver abscesses has proved simple and effective, with a success rate of 80–87% (1), and is currently considered the gold standard for the treatment of this condition. However, it is not suitable for all liver abscesses (9, 10). Large abscesses with strong walls and multiple chambers cannot be effectively evacuated or fully drained, making recurrence more likely. Laparoscopic drainage is an effective alternative in such cases, assuring better results (2, 11).
References
- 1.Dominguez-Guzman DJ, Moreno-Portillo M, Garcia-Flores C, et al. Laparoscopic drainage of liver abscess. Initial experience Cir Cir 2006May–Jun743189–94. [PubMed] [Google Scholar]
- 2.Tay KH, Ravintharan T, Hoe MN, et al. Laparoscopic drainage of liver abscesses. Br J Surg. 1998 Mar;85(3):330–2. doi: 10.1046/j.1365-2168.1998.00617.x. [DOI] [PubMed] [Google Scholar]
- 3.Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A. Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol. 2012 Apr 7;18(13):1425–37. doi: 10.3748/wjg.v18.i13.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuveri M, Calò PG, Medas F, Tuveri A, Nicolosi A. Limits and advantages of fundus-first laparoscopic cholecystectomy: lessons learned. J Laparoendosc Adv Surg Tech A. 2008 Feb;18(1):69–75. doi: 10.1089/lap.2006.0194. [DOI] [PubMed] [Google Scholar]
- 5.Aydin Cemalettin, Piskin Turgut, Sumer Faith, et al. Laparoscopic drainage of pyogenic liver abscesses. JSLS, Journal of the Society of Laparoendoscopic Surgeons. 2010;14:418–20. doi: 10.4293/108680810X12924466006567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Weu, Lee Wei-Jei, Wei Po-li, et al. Laparoscopic drainage of pyogenic liver abscesses. Surg Today. 2004;34:323–325. doi: 10.1007/s00595-003-2709-x. [DOI] [PubMed] [Google Scholar]
- 7.Tarcoeanu E, Vlad N, Moldovanu R, et al. Pyogenic liver abscesses Chirurgia (Bucur) 2008July–Aug1034417–27. [PubMed] [Google Scholar]
- 8.Heneghan Helen M, Healy Nuala A, Martin Sean T, et al. Modern Management of Pyogenic Hepatic Abscess: a case series and review of literature. Heneghan et al. BMC Research Notes. 2011;4:80. doi: 10.1186/1756-0500-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung YFA, Tan YM, Lui HF, Tay KH, et al. Management of pyogenic liver abscesss-percutaneous or open drainage? Singapore Med J. 2007;48(12):1158. [PubMed] [Google Scholar]
- 10.Kayaalp C, Yoll S, Nessar G. Drainage of liver abscess via laparoscopic trocar with local anesthesia. Surg Laparosc Endosc Percutan Tech. 2003;13:121–124. doi: 10.1097/00129689-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Tu Jin-Fu, Huang Xiu-Fang, Hu Ru-Ying, et al. Comparison of laparoscopic and open surgery for pyogenic liver abscess with biliary pathology. World Journal of Gastroenterology. 2011 Oct 14;17(38):4339–4343. doi: 10.3748/wjg.v17.i38.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]