Summary
Introduction
Alterations in homeostasis, and a subsequent increased risk for postoperative thromboembolic complications, are observed as a result of open surgery. Additionally, the stress response to surgical trauma precipitates a transient hypercoagulable state as well as inflammation. This study was conducted to evaluate the patterns in postoperative alterations of blood coagulation, and to detect their correlations with inflammatory markers.
Patients and methods
The study included 50 patients with comparable demographic data, who were randomly assigned to undergo abdominal surgery. No previous coagulation disorders were noted. Blood samples were collected preoperatively and 72 h postoperatively. The following parameters were measured: prothrombin time (PT) and activated partial thromboplastin time (APTT); fibrinogen (FIB), D-dimer (D-D), and C-reactive protein (CRP) levels; and platelet (PLT) count. Prophylactic doses of low molecular weight heparin were administered to all patients.
Results
The PT mean value significantly changed from 90.38% before surgery to 81.25% after surgery. No statistical difference was observed between APTT values before and after surgery. FIB levels significantly increased from 381.50 mg/dL preoperatively to 462.57 mg/dL postoperatively. Mean D-D levels also significantly increased from 235.54 μg/L preoperatively to 803.59 μg/L postoperatively. PLT count significantly declined after surgery. Mean CRP levels significantly increased from 12.33 mg/L preoperatively to 44.28 mg/L postoperatively. A strong correlation was observed between D-D and C-RP levels after surgery.
Conclusion
These results indicate that, despite administering an-tithromboembolic prophylaxis, a hypercoagulable state was observed following surgery. This state was enhanced by inflammation.
Keywords: Coagulation alteration, Surgical trauma, Inflammation
Introduction
Surgical trauma results in considerable alterations in the hemostatic system due to the activation of blood coagulation. Subsequently, it is associated with a significant risk of intraoperative or postoperative thromboembolic complications that can reach as high as 40–80% (1).
Inflammation and alterations in coagulation parameters constitute a serious challenge in the follow-up and success of surgical interventions (2–4). As a result of tissue injury (due to trauma) or open surgery, alterations in homeostasis are observed, and are associated with the risk of developing postoperative thromboembolic complications. The stress response to surgical trauma precipitates a transient hypercoagulable state and activates inflammation (5–7).
Therefore, we conducted a prospective randomized trial in order to study prevailing alterations in coagulation parameters and their correlation with inflammation following abdominal surgery.
Patients and methods
This prospective study included 50 patients, aged 45–55 years (mean age: 51 years), who were randomly assigned to undergo abdominal surgery at the Department of General Surgery, from October 2011 to May 2012. Patients on medication affecting coagulation (anticoagulants, antiaggregants, nonsteroidal anti-inflammatory drugs, and steroids), as well as patients with a preexisting disorder that could affect the coagulation system (sepsis, cancer, history of thrombosis, and recent surgery) were excluded from the study. Patients with preexisting coagulation derangements revealed through abnormalities in preoperative history, preoperative platelet (PLT) count, or prothrombin time (PT) and activated partial thromboplastin time (APTT) values were also excluded.
Procedure
Low molecular weight heparin was administered in prophylactic doses to all patients, before surgery and 3 days after surgery. Blood samples were collected before surgery and 72 h after the surgical operation. The following parameters were measured: PT and APTT, as well as C-reactive protein (CRP), fibrinogen (FIB), D-dimer (D-D) levels, and PLT count.
Anesthesia was administered to all patients by the same anesthesiology team; therefore, they all underwent the same anesthetic procedures. Additionally, the same surgical team performed all operations.
Determination of fibrinogen and prothrombin time
FIB levels were determined with an ACL-9000 Coagulation Analyzer, using a PT-FIB HS reagent, which is a high-sensitivity calcium thromboplastin that allows the simultaneous determination of PT and FIB levels. PT is expressed as activity percentage (%), ratio, seconds (s), and international normalized ratio (INR). FIB level is expressed in mg/dL, with a linearity of method range of 70–850 mg/dL.
Determination of activated partial thromboplastin time
APTT was measured using an ACL-9000 Coagulation Analyzer. Results are expressed as seconds (s) or ratio.
Determination of C-reactive protein level
We quantitatively determined CRP levels using an immune turbidimetric assay. Latex particles coated with antibody specific to human CRP aggregated in the presence of CRP from the sample, and formed immune complexes. These immune complexes resulted in an increase in light scattering proportional to the CRP concentration in the serum sample. Light scattering was measured using turbidity (absorbance) readings. CRP concentration was determined using a calibration curve developed from CRP standards of known concentrations (multistandard), with a linearity of the method range of 0.2–480 mg/dL.
Determination of D-dimer level
D-D levels were determined using a quantitative enzyme-linked immunosorbent assay based on the “sandwich” type immunoenzymatic method, with final measurements achieved through fluorescence detection. Assays were performed using a VIDAS Automated Immunoassay System (Biomerieux), with a measurement range of 45–10.000 μg/L. The reference value used was <500 μg/L.
Determination of platelet count
PLT count was automatically measured using a Pentra 80 cell-counter (Horiba), and confirmed by an indirect manual counting method involving a blood smear that was collected from the patient’s finger. After collection, the blood smear was fixed in methanol for 10 min, and then stained with Giemsa for 20 min before being used.
Statistical analysis
Continuous data are presented as average value and standard deviation. The student’s t-test for paired samples used to compare values between the 2 stages. Graphic lines were used for data presentation. The relationship between 2 variables was analyzed using Pearson’s and Kendall’s correlation coefficients. A difference with a p value less than 0.05 (p < 0.05) was considered statistically significant. Data analysis was carried out using the Statistical Package for the Social Sciences 11.5 software (SPSS, Chicago, IL).
Results
Prothrombin time
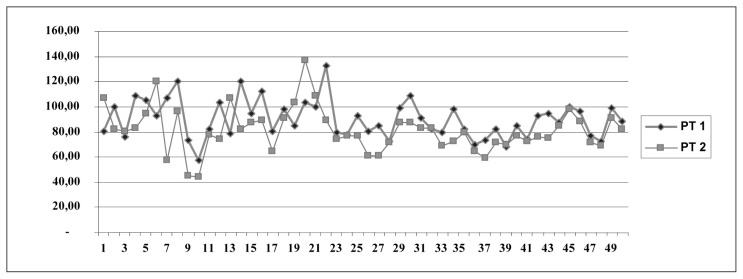
Data analysis using student’s t-test for paired samples showed a statistically significant difference between PT values before surgery (PT 1) and after surgery (PT 2; t = 3.446, df = 44, p = 0.001; Fig. 1).
Fig. 1.
PT values before surgery (PT 1) and after surgery (PT 2).
The mean PT value declined from 90.38% before surgery to 81.25% after surgery.
Activated partial thromboplastin time
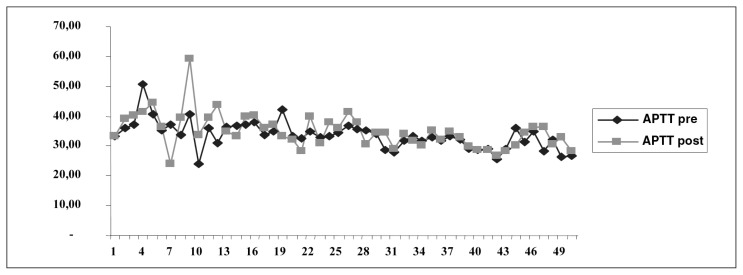
Data analysis using student’s t-test for paired samples showed no statistically significant difference between preoperative and postoperative APTT (t = 1,406, df = 44, p = 0.167; Fig. 2).
Fig. 2.
Pre- and postoperative APTT values.
In our study, we observed no statistically significant changes in APTT values before and after surgery (33.96 s versus 35.07 s, respectively). This observation could be explained by the fact that patients were receiving heparin medication in prophylactic but not therapeutic doses.
Fibrinogen
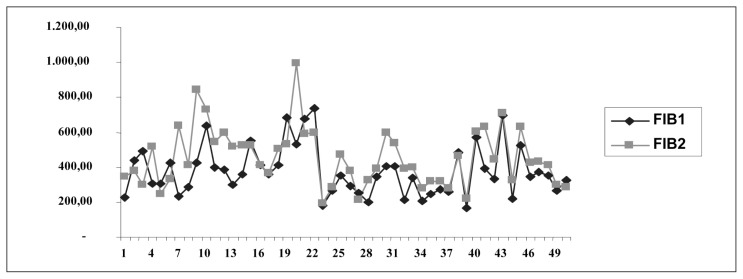
Data analysis using student’s t-test for paired samples revealed a statistically significant difference between FIB levels before surgery (FIB1) and after surgery (FIB2; t = 3,947, df = 44, p = 0,001; Fig. 3).
Fig. 3.
FIB 1 (before surgery) and FIB 2 (after surgery) values.
Levels of FIB, a protein that is synthesized in the liver, are known to increase during inflammation. In our study, we observed a statistically significant increase in FIB levels from 381.56 mg/dL before surgery to 462.57 mg/dL after surgery. Surgical trauma and inflammation during necrosis result in an increase in FIB levels. However, we did not observe a critical reduction in FIB levels that would have indicated an increased risk of disseminated intravascular coagulation in any of the cases under study.
D-dimer
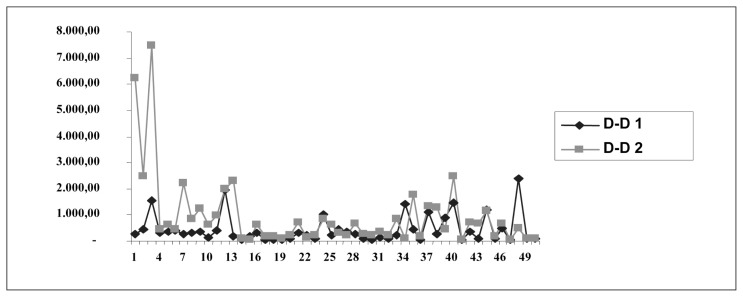
Data analysis using student’s t-test for paired samples demonstrated a statistically significant difference between D-D levels before surgery (D-D1) and after surgery (D-D2; t = 2.868, df = 44, p = 0.006; Fig. 4).
Fig. 4.
Presentation of D-D 1 (before surgery) and D-D 2 (after surgery) values.
An increase in D-D levels was observed after surgery compared to before surgery (803.59 g/mL versus 235.53 g/mL, respectively).
C-reactive protein
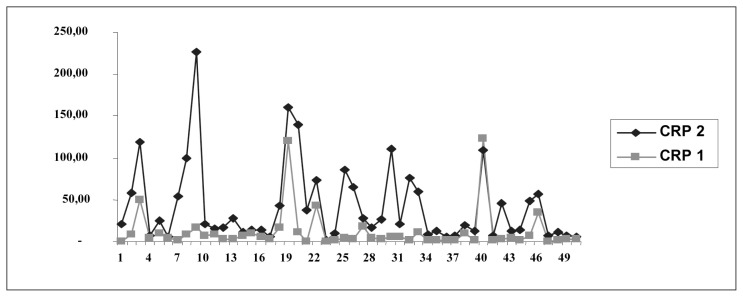
Data analysis using student’s t-test for paired of samples showed a statistically significant difference between CRP levels before surgery (CRP1) and after surgery (CRP2; t = 5.229, df = 44, p = 0.001; Fig. 5).
Fig. 5.
Presentation of CRP 1 (before surgery) and CRP 2 (after surgery) values.
Platelets
Data analysis using student’s t-test for paired samples revealed a statistically significant difference between preoperative and postoperative PLT count (t = 3.619, df = 44, p = 0.001; Table 1).
Table 1.
LEVELS OF PLATELETS BEFORE (PLT1) AND AFTER SURGERY (PLT2).
| M | N | SD | t | df | P | ||
|---|---|---|---|---|---|---|---|
| Pair 6 | PLT1 | 285.46 | 50 | 108.82 | −3.619 | 49 | 0.001 |
| PLT2 | 270.80 | 50 | 101.51 |
Correlation between CRP, PLT, and D-D levels before surgery
Using Pearson’s correlation coefficient, a statistically significant correlation was observed between CRP1 and PLT 1 levels (r = 0.417, p = 0.003); however, no statistically significant correlation was observed between CRP1 and D-D 1 levels (r = 0.061, p = 0.692; Table 2).
Table 2.
THE CORRELATION OF CRP1 WITH PLT1 AND D-DIMER1.
| PLT1 | D-dimer 1 | |
|---|---|---|
| CRP1 | r = 0.417 | r = 0.061 |
| P = 0.003 | P = 0.692 | |
| N = 50 | N = 45 |
This correlation was observed before surgery, and it could be explained by the fact that cytokines released in circulation, during inflammation, result in an increase in PLT count and thrombocytopoiesis.
Correlations between CRP, PLT, and D-D levels after surgery
Using Kendal’s correlation coefficient, we observed no statistically significant correlation between CRP2 and PLT2 levels (r = 0.417, p = 0.332); however, a statistically significant correlation was observed between CRP2 and D-D2 levels (r = 0.230, p = 0.028; Table 3).
Table 3.
THE CORRELATION OF CRP2 WITH PLT2 AND D-DIMER2.
| PLT2 | D-dimer 2 | |
|---|---|---|
| CRP2 | r = 0.417 | r = 0.230 |
| P = 0.332 | P = 0.028 | |
| N = 50 | N = 44 |
Discussion
After surgery two potentially important alterations in homeostasis increasing the risk of thromboembolic complications occur in patients. The first alteration is represented by a tendency toward hypercoagulability (8), and the second alteration is represented by an initial enhancement of fibrinolysis, followed by a decline in fibrinolytic activity (9).
Most studies that have evaluated perioperative alterations in homeostasis, have examined them in association with open surgery (10–12). The risk of developing deep venous thrombosis after open surgery can be as high as 40–80%, while the incidence of fatal pulmonary embolism is estimated at 1–5% (1,13).
In order to further understand this issue, we compared perioperative changes in the coagulation pathway before and after surgery, in the context of a prospective randomized trial. Anesthesia was administered to all patients by the same anesthesiology team; therefore, they all underwent the same anesthetic procedures. Additionally, the same surgical team performed all operations. This is an essential element of randomization, since the training and experience of an individual surgeon play an important role in the outcome of surgery (14). Studies have shown that postoperative coagulation can be affected by many factors, including the type of operation performed (15) and the type of anesthesia administered (16).
Prothrombin is a protein that is synthesized in the liver in a vitamin K-dependent manner. No previous studies have reported any inflammation-related increase in prothrombin synthesis. However, the decline in PT that we observed after surgery may be explained by the body’s distress following surgical intervention, and the subsequent reduction in prothrombin synthesis from heparin after surgery (4,17–20).
FIB is an acute-phase protein that is synthesized by the liver and that plays a key role in blood clotting. During clot formation, FIB is converted to fibrin via the enzymatic activity of thrombin (21). Low levels may indicate increased degradation (fibrinolysis), while increased levels, which are often observed after inflammation, reflect the close association between stress and coagulation activation (22). In our study, the significant increase in plasma FIB levels after surgery indicates an early and prolonged increase in the activation of coagulation. Defining and tracking FIB values are important for surveying such patients, but not for assessing the degree of inflammation and postoperative necrosis (5,23). Previous studies have reported that such increases in FIB levels are caused by the surgical event itself (24). Many patients had very high levels after surgery, and in many cases, even higher levels were detected prior to surgery. High FIB levels before surgery are probably related to the patient’s primary pathology.
During fibrinolysis, fibrin and FIB are broken down into various fibrin/FIB degradation products, including the terminal product D-D, in a process mediated by plasmin (25). Therefore, elevated D-D plasma levels are also indicative of recent or ongoing fibrinolysis (26). However, a constant elevation of D-D plasma levels raises suspicion of deep vein thrombosis (27). The postoperative increase in D-D levels observed in our study is indicative of fibrinolysis activation following surgery. This group of patients with the highest D-D levels strongly indicate the need for anticoagulant therapy as well as for careful evaluation of all clinical and biochemical indicators during postsurgical follow-up. D-Ds are soluble fibrin degradation products, and the determination of D-D level has been used for its high sensitivity and specificity in detecting thromboembolic events that occur during surgery (28,20). D-D is a cross-linked fibrin degradation product that forms as a result of fibrin breakdown. D-D levels frequently increase after surgery or trauma, and they indicate the presence of an intravascular clot that has undergone lysis (29).
PT and APTT are also used as part of a broad test for coagulation (30). The INR = PTISI formula, where ISI is the international sensitivity index of the specific thromboplastin reagent used in a laboratory, allows for better standardization through adjusting for reagent variability (31,32). In our study, we did not observe significant differences between the measured values of these markers before and after open surgery. We also note that most patients received low molecular weight heparin, which has little impact on APTT and does not require strict laboratory monitoring (3,7, 33).
CRP reduces thrombin generation through inactivating factors V and VIII. Measurement of CRP level is most frequently used to evaluate injury to body tissues or to detect an inflammatory event occurring in the body (5,20,23). CRP levels typically increase during the acute phase of inflammation (19), since cytokines produced during inflammation and necrosis can stimulate CRP production in the liver (34,35). CRP plays a role in the non-specific immune system, and its production is increased in response to damage, possibly because it promotes the neutrophil-mediated destruction of pathogenic microorganisms. High levels of CRP indicate the need for more intensive treatment with heparin, since higher levels of inflammation significantly favor the formation of thrombi (5).
Thrombocytosis is reportedly associated with inflammation and necrosis (18). In our study, we observed a reduction in PLT count, possibly in the context of changes in hematological parameters after surgical intervention. We note that all of the patients in our study were administered prophylactic treatment with fraxiparine immediately after surgery. Several previous studies have reported a reduction in PLT count caused by heparin and heparin fractions, indicating the need for the extended follow-up of patients receiving heparin treatment (20,36,37). Heparin itself, as well as anti-heparin antibodies have been associated with a decline in PLT count.
In this study, no correlation was observed between PLT count and inflammation after surgery, possibly due to the postoperative impact of heparin or other factors on PLT count (2,6,20,36). On the other hand, a statistically significant correlation was observed between CRP and D-D levels after surgery. This correlation supports the observation that inflammation stimulates coagulation, resulting in a hypercoagulable state that should be considered during postoperative patient follow-up (5,20,23).
Previous studies have demonstrated that open surgeries lead to higher activation of the clotting system than laparoscopic procedures (7,18–20), which would explain the differences between our values and those reported by laparoscopy studies. Comparative studies of hemostasis after open and laparoscopic surgery have used several surgical models other than laparoscopic and open cholecystectomy (38,39). In a randomized study, Milic et al.(10) only compared perioperative plasma levels of prothrombin fragments (F1+2), D-D, and antithrombin, as well as APTT and the incidence of postoperative deep vein thrombosis, between open and laparoscopic cholecystectomy patients. They were unable to demonstrate a significant difference in the plasma levels of the aforementioned markers between the 2 groups of patients. On the other hand, the incidence of postoperative deep vein thrombosis was significantly higher in the open cholecystectomy group than in the laparoscopic cholecystectomy group.
Additionally, in a randomized trial, Prisco et al.(40) compared laparoscopic and open cholecystectomy by studying perioperative changes in FIB, PT, and D-D values. They observed significantly higher postoperative FIB levels in the open surgery group than in the laparoscopic group, as well as a significant increase in postoperative PT in both groups. They concluded that laparoscopic cholecystectomy induces the activation of the clotting system, despite the activation being mild and brief.
Nguyen et al.(39) compared PT values as well as plasminogen, FIB, D-D, and CRP plasma levels between patients randomly assigned to open or laparoscopic gastric bypass (GBP) surgery. They found an insignificant increase in PT values and an insignificant decrease in plasminogen levels in both groups after surgery. On the other hand, D-D plasma levels were significantly higher after surgery in the open GBP group compared to the laparoscopic GBP group.
In another nonrandomized comparative study involving laparoscopic cholecystectomy (n = 20) and low-risk open surgery (Bassini herniorrhaphy, n = 12), Martinez-Ramos et al.(18) reported significantly higher postoperative FIB levels than preoperative levels in both groups, 24 h after surgery.
Conclusions
Our results showed that open surgery leads to the activation of the clotting system, which represents an increase in thromboembolic risk for patients. We conclude that, in addition to considering the patient’s pathology, the selection of patients for surgery should be based on biochemical indicators, such as CRP and FIB levels, as well as PLT count and PT value. Additionally, preoperative high CRP and D-D levels should indicate the need for prophylactic preliminary anticoagulant therapy using maximal allowable dose levels, as well as for a cautious postoperative follow-up period.
These recommendations are based on findings of studies that only involved open surgery. Several randomized comparative studies that include both open and laparoscopic surgery should be conducted for further recommendation.
References
- 1.Clagett GP, Anderson FA, Jr, Geerts W, Heit JA, Knudson M, Lieberman JR, Wheeler HB. Prevention of venous thromboembolism. Chest. 1998;114:531S–560S. doi: 10.1378/chest.114.5_supplement.531s. [DOI] [PubMed] [Google Scholar]
- 2.Clagett GP, Reisch IS. Prevention of venous thromboembolism in general surgical patients. Result of meta-analysis. Ann Surg. 1988;208:227–240. doi: 10.1097/00000658-198808000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leizorovicz A, Haugh MC, Chapuis FR. Low molecular weight heparin in prevention of perioperative thrombosis. BMJ. 1992;305:913–920. doi: 10.1136/bmj.305.6859.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntz J. Duration of deep vein thrombosis prophylaxis in the surgical patients and its relation to quality issues. Am J Surg. 2010;200:413–421. doi: 10.1016/j.amjsurg.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 7.Tsiminikakis N, Chouillard E, Tsigris C, Diamantis T, Bongiorni C, Ekonomou C, Antoniou C, Bramis I. Fibrinolytic and coagulation pathways after laparoscopic and open surgery: a prospective randomized trial. Surg Endosc. 2009;23:2762–9. doi: 10.1007/s00464-009-0486-3. [DOI] [PubMed] [Google Scholar]
- 8.Tuman KJ, Spiess BD, McCarthy R, Ivancovich AD. Effects of progressive blood loss on coagulation as measured by thromboelastography. Anesth Analg. 1987;66:856–863. [PubMed] [Google Scholar]
- 9.Murphy W, Davies M, Eduardo A. The hemostatic response to surgery and trauma. Br J Anaesth. 1993;70:205–213. doi: 10.1093/bja/70.2.205. [DOI] [PubMed] [Google Scholar]
- 10.Milic DJ, Pejcic VD, Zivic SS, Jovanovic SZ, Stanojkovic ZA, Jankovic RJ, Pecic VM, Nestorovic MD, Jankovic ID. Coagulation status and the presence of postoperative deep vein thrombosis in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2007;21:1588–1592. doi: 10.1007/s00464-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 11.Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Haemoost. 1998;24:33–34. doi: 10.1055/s-2007-995821. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld BA, faraday N, Campbell D, Dorman T, Clarkson K, Siedler A, Breslow MJ, Bell W. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology. 1993;79:255–261. doi: 10.1097/00000542-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaides A, Berkqvist D, Hull R. Prevention of venous thromboembolism. International Consensus Statement. Int Angiol. 1997;16:3. [PubMed] [Google Scholar]
- 14.Mc Ardle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. Br Med J. 1991;302:1501–1505. doi: 10.1136/bmj.302.6791.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caprini JA, Arcelus JI, Laubach M, Size G, Hoffman KN, Coats RW, II, Blattner S. Postoperative hypercoagulability and deep-vein thrombosis after laparoscopic cholecystectomy. Surg Endosc. 1995;9:304–309. doi: 10.1007/BF00187774. [DOI] [PubMed] [Google Scholar]
- 16.Davis FM, McDermott E, Hickton C, Wells E, Heaton DC, Laurenson VG, Gillespie WJ, Foate J. Influence of spinal and general anesthesia an hemostasis during total hip arthroplasty. Br J Anaesth. 1987;59:561–571. doi: 10.1093/bja/59.5.561. [DOI] [PubMed] [Google Scholar]
- 17.Divita G, Frazzeta M, Sciume C, Lauria G, Patti R, Leo P. Changes in the hemostatic system after laparoscopic cholecystectomy. Chir. 2000;21:213–8. [PubMed] [Google Scholar]
- 18.Martinez-Ramos C, Lopez-Pastor A, Nunez-Pena JR, Gopegui M, Sanz-Lopez R, Jorgensen T, Pastor L, Fernandez-Chacon JL, Tahames-Escobar S. Changes in hemostases after laparoscopy. Surg Endosc. 1999;13:476–9. doi: 10.1007/s004649901016. [DOI] [PubMed] [Google Scholar]
- 19.Schietroma M, Giuliani A, Agnifili A, Lely L, Carlei F, Pescosolido A, Amicucci G. Changes in blood coagulation, fibrinolysis and cytokine profile during laparoscopic and open cholecystectomy. Chir Ital. 2008;60:179–88. [PubMed] [Google Scholar]
- 20.Diamantis T, Tsiminikakis N, Skordylaki A. Alterations of hemostasis after laparoscopic and open surgery. Hematology. 2007;12:561–70. doi: 10.1080/10245330701554623. [DOI] [PubMed] [Google Scholar]
- 21.Podolnikova NP, Yakubenko VP, Volkov GL, Plow EF, Ugarova TP. Identification of a novel biding site for platelet integrins alpha IIb beta 3 (GpIIbIIIa) and alpha 5 beta 1 in the gamma C-domain of fibrinogen. J Biol Chem. 2003;278:32251–32258. doi: 10.1074/jbc.M300410200. [DOI] [PubMed] [Google Scholar]
- 22.Freyburger G, Janvier G, Dief S, Boisseau MR. Fibrinolytic and hemorheologic alterations during and after elective aortic graft surgery: implications for postoperative management. Anesth Analg. 1993;76:504–512. doi: 10.1213/00000539-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–8. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 24.Dindo D, Breitenstein S, Hahnloser D, Seifert B, Yakarisik S, Asmis LM, Muller MK, Clavien PA. Kinetics of D-dimer after general surgery. Blood Coagul Fibrinolysis. 2009;20:347–52. doi: 10.1097/MBC.0b013e32832a5fe6. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuizen W. New strategies in the determination of fibrin and fibrinogen derivatives by monoclonal antibodies. Blut. 1988;57:285–291. doi: 10.1007/BF00320354. [DOI] [PubMed] [Google Scholar]
- 26.Hirsh J, Lee A. How we diagnose and treat deep vein thrombosis. Blood. 2002;99:3102–3110. doi: 10.1182/blood.v99.9.3102. [DOI] [PubMed] [Google Scholar]
- 27.Boneu B, Bes G, Pelzer H, Sie P, Boccalon H. Thrombin antithrombin III complexes and prothrombine fragments 1+2: diagnostic value in clinically suspected deep vein thrombosis. Thromb Haemost. 1991;65:28–31. [PubMed] [Google Scholar]
- 28.Sandrick K. Using new D-dimer tests to rule out venous thromboembolism. CAP Today. 2000;14:40–2. [PubMed] [Google Scholar]
- 29.Owings JT, Gosselin RC, Battistella FD, Anderson JT, Petrich M, Larkin EC. Whole blood D-dimer assay: an effective noninvasive method to rule out pulmonary embolism. J Trauma. 2000;48:795–799. doi: 10.1097/00005373-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kovacs MJ, Wong A, MacKinnon K, Weir K, Keeny M, Boyle E, Cruickshank M. Assessment of the validity of the INR system for patients with liver impairment. Thromb Haemost. 1994;71:727–730. [PubMed] [Google Scholar]
- 31.Gallus AS, Baker RI, Chong BH, Ockelford PA, Street AM. Consensus guidelines for warfarin therapy. Recommendations from the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2000;172:600–605. [PubMed] [Google Scholar]
- 32.Baglin TP, Keeling DM, Watson HG. British Committee for standards in Haematology. Guidelines on oral anticoagulation (warfarin): 3rd Edition-2005 update. Br J Haematol. 2006;132:277–285. doi: 10.1111/j.1365-2141.2005.05856.x. [DOI] [PubMed] [Google Scholar]
- 33.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 34.Bllou SP, Lozaznksi G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 35.Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 36.Amiral J, Bridey F, Dreyfus M, Vissoc AM, Fressinaud E, Wolf M, Meyer D. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68:95–6. [PubMed] [Google Scholar]
- 37.Greinacher A, Eicher P, Lubenow N, Kwasny H, Luz M. Heparin induced thrombocytopenia with thromboembolic complication: meta analysis of 2 prospective trials to assess the value of parenteral treatment with lepirudin and its therapeutic aPTT range. Blood. 2000;96:846–851. [PubMed] [Google Scholar]
- 38.Neudecker J, Junghans T, Ziemer S, Raue W, Schwenk W. Prospective randomized trial to determine the influence of laparoscopic and conventional colorectal resection on intravasal fibrinolytic capacity. Surg Endosc. 2003;17:73–77. doi: 10.1007/s00464-002-9028-y. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen NT, Owings JT, Gosselin R, Pevec WC, Lee SJ, Goldman C, Wolfe BM. Systemic coagulation and fibrinolysis after laparoscopic and open gastric bypass. Arch Surg. 2001;136:909–916. doi: 10.1001/archsurg.136.8.909. [DOI] [PubMed] [Google Scholar]
- 40.Prisco D, De Gaudio AR, Carla R, Gori AM, Fedi S, Cella AP, Gensini GF, Abbate R. Videolaparoscopic cholecystectomy induces a haemostasis activation of a lower degree than does open surgery. Surg Endosc. 2000;14:170–174. doi: 10.1007/s004649900093. [DOI] [PubMed] [Google Scholar]