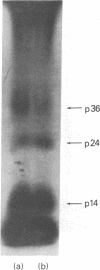
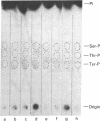
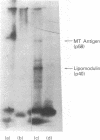
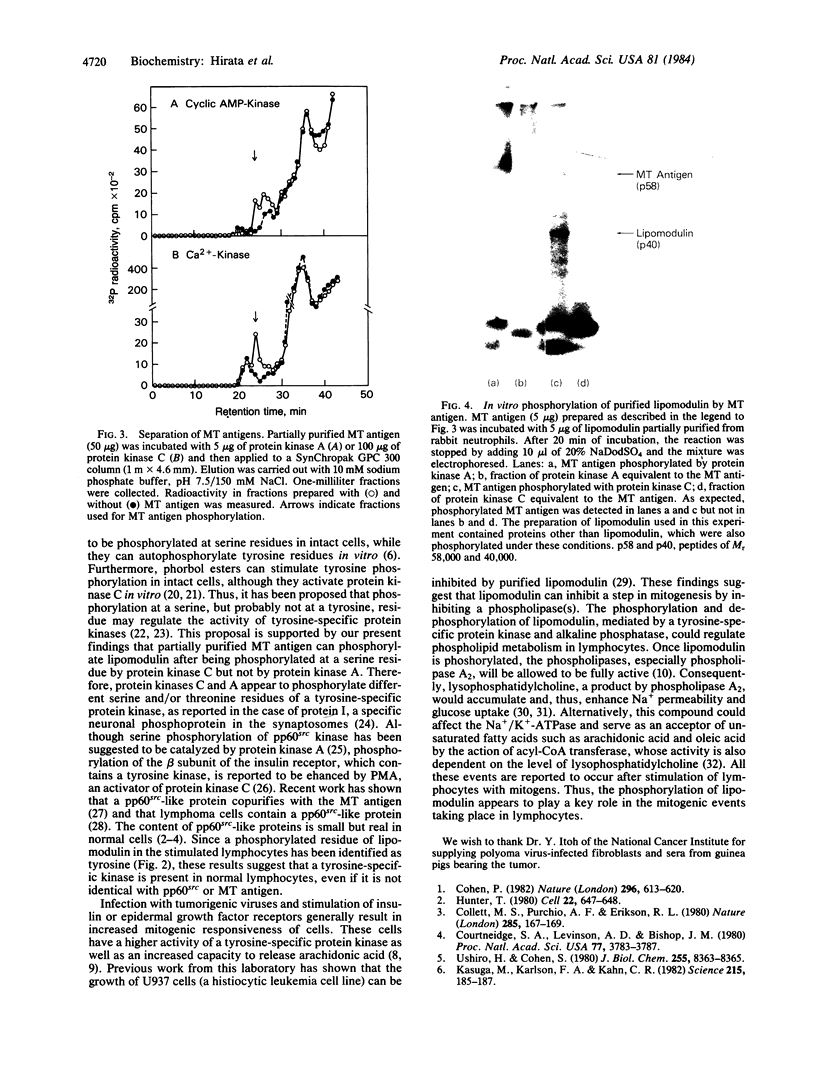
Abstract
When murine thymocytes were stimulated by mitogens such as concanavalin A, the Ca2+ ionophore A23187, or 4 beta-phorbol 12-myristate 13-acetate, there was a marked increase of 32P incorporation into immunoprecipitable lipomodulin, a phospholipase inhibitory protein. These compounds enhanced 45Ca2+ influx into thymocytes, which, in turn, increased protein phosphorylation, probably by Ca2+- and phospholipid-dependent protein kinase (protein kinase C). Cyclic 8-bromo-AMP, an inhibitor of lymphocyte mitogenesis, blocked the mitogen-stimulated phosphorylation of lipomodulin, although it stimulated the protein phosphorylation via cyclic AMP-dependent kinase (protein kinase A). On electrophoresis, the hydrolysates of 32P-labeled lipomodulin showed a single radioactive spot, which comigrated with authentic phosphotyrosine. The partially purified middle-sized tumor antigen was able to phosphorylate lipomodulin after being phosphorylated by protein kinase C but not by the catalytic subunit of protein kinase A. Our findings suggest that the activity of a tyrosine-specific kinase, which phosphorylates lipomodulin in vivo as well as in vitro, is stimulated by protein kinase C and inhibited by protein kinase A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R., Martinez R., Nakamura K. D., Weber M. J. A tumor promoter stimulates phosphorylation on tyrosine. Biochem Biophys Res Commun. 1983 Sep 15;115(2):536–543. doi: 10.1016/s0006-291x(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma protein with an in vitro site of tyrosine phosphorylation homologous to that in pp60src. J Biol Chem. 1982 Dec 10;257(23):13877–13879. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Biosynthesis and biological actions of prostaglandins and thromboxanes. Arch Biochem Biophys. 1982 Apr 1;214(2):431–445. doi: 10.1016/0003-9861(82)90047-9. [DOI] [PubMed] [Google Scholar]
- Hattori T., Hoffman T., Hirata F. Differentiation of a histiocytic lymphoma cell line by lipomodulin, a phospholipase inhibitory protein. Biochem Biophys Res Commun. 1983 Mar 16;111(2):551–559. doi: 10.1016/0006-291x(83)90342-x. [DOI] [PubMed] [Google Scholar]
- Hirata F., Iwata M. Role of lipomodulin, a phospholipase inhibitory protein, in immunoregulation by thymocytes. J Immunol. 1983 Apr;130(4):1930–1936. [PubMed] [Google Scholar]
- Hirata F., Notsu Y., Iwata M., Parente L., DiRosa M., Flower R. J. Identification of several species of phospholipase inhibitory protein(s) by radioimmunoassay for lipomodulin. Biochem Biophys Res Commun. 1982 Nov 16;109(1):223–230. doi: 10.1016/0006-291x(82)91588-1. [DOI] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hirata F., Toyoshima S., Axelrod J., Waxdal M. J. Phospholipid methylation: a biochemical signal modulating lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1980 Feb;77(2):862–865. doi: 10.1073/pnas.77.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Ku Y., Kishimoto A., Takai Y., Ogawa Y., Kimura S., Nishizuka Y. A new possible regulatory system for protein phosphorylation in human peripheral lymphocytes. II. Possible relation to phosphatidylinositol turnover induced by mitogens. J Immunol. 1981 Oct;127(4):1375–1379. [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J., Chinkers M., Brugge J. S. A major cytoplasmic glucose-regulated protein is associated with the Rous sarcoma virus pp60src protein. J Biol Chem. 1982 Aug 10;257(15):8604–8607. [PubMed] [Google Scholar]
- Ogawa Y., Takai Y., Kawahara Y., Kimura S., Nishizuka Y. A new possible regulatory system for protein phosphorylation in human peripheral lymphocytes. I. Characterization of a calcium-activated, phospholipid-dependent protein kinase. J Immunol. 1981 Oct;127(4):1369–1374. [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Gelehrter T. D., Legg A., Pettican P. Melittin stimulates Na entry, Na-K pump activity and DNA synthesis in quiescent cultures of mouse cells. Cell. 1981 Mar;23(3):781–788. doi: 10.1016/0092-8674(81)90442-6. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T. Serum stimulation of phospholipase A2 and prostaglandin release in 3T3 cells is associated with platelet-derived growth-promoting activity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):137–141. doi: 10.1073/pnas.77.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Selective dephosphorylation of proteins containing phosphotyrosine by alkaline phosphatases. J Biol Chem. 1981 Aug 10;256(15):8197–8201. [PubMed] [Google Scholar]
- Szamel M., Resch K. Modulation of enzyme activities in isolated lymphocyte plasma membranes by enzymatic modification of phospholipid fatty acids. J Biol Chem. 1981 Nov 25;256(22):11618–11623. [PubMed] [Google Scholar]
- Toyoshima S., Hirata F., Axelrod J., Beppu M., Osawa T., Waxdal M. J. The relationship between phospholipid methylation and calcium influx in murine lymphocytes stimulated with native and modified Con A. Mol Immunol. 1982 Feb;19(2):229–234. doi: 10.1016/0161-5890(82)90335-2. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]