Abstract
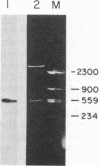
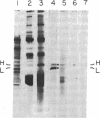
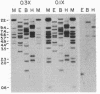
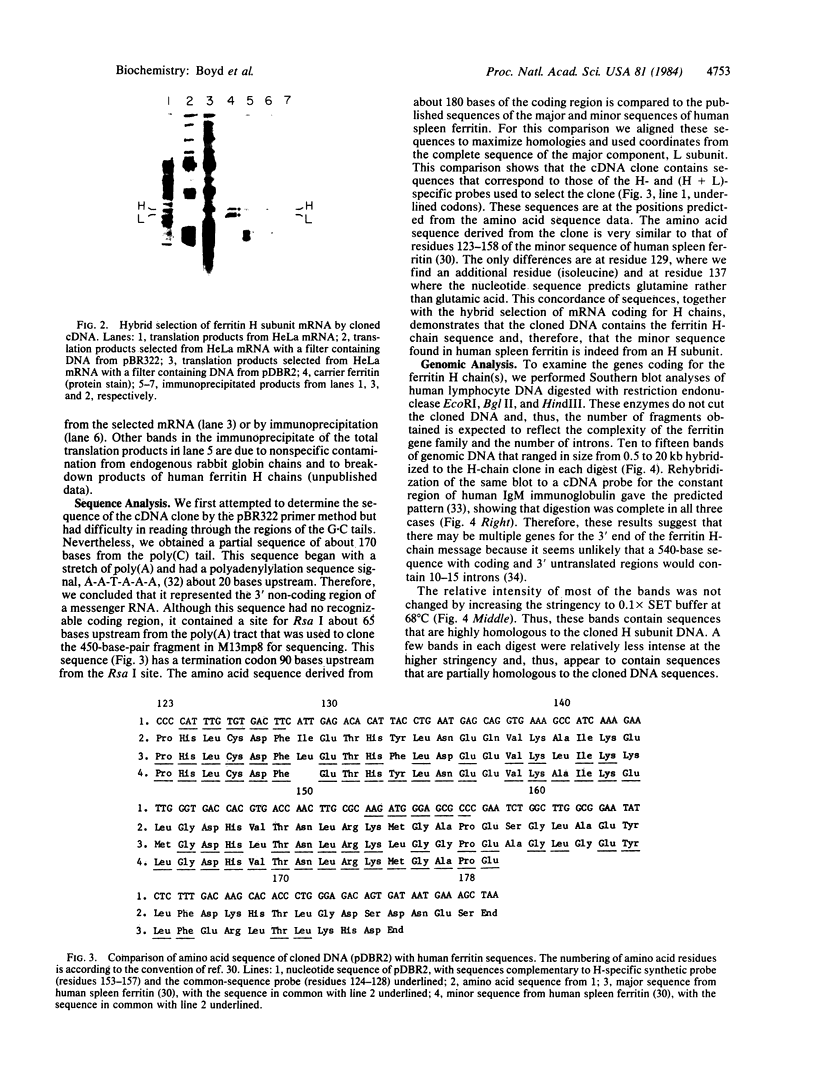
Ferritin, the main iron-storage protein, is composed of two partially homologous subunits, heavy (H) and light (L), with MrS of 21,000 and 19,000, respectively. We have isolated a cDNA clone for human ferritin H chains by screening a human lymphocyte cDNA library with synthetic oligodeoxyribonucleotides. The oligonucleotide sequences were derived from two pentapeptides found in human spleen ferritin. The selected clone hybridized to both probes and selected H-chain mRNA, but not L-chain mRNA, when hybridized to HeLa cell mRNA. These results indicate that the cloned DNA codes for a H chain of human ferritin. Since the amino acid sequence derived from the cloned DNA was almost identical to the partial amino acid sequence of a minor component found in human spleen ferritin, we conclude that the minor sequence found in human spleen ferritin must be a H subunit. Genomic analysis gives a complex pattern that suggests that ferritin H chains are encoded by a multigene family or have an unusually large number of exons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert E., Coston R. L., Drysdale J. W. Carcino-foetal human liver ferritins. Nature. 1973 Mar 16;242(5394):194–196. doi: 10.1038/242194a0. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyard S. H., Stammers D. K., Harrison P. M. Electron density map of apoferritin at 2.8-A resolution. Nature. 1978 Jan 19;271(5642):282–284. doi: 10.1038/271282a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. J., Leibold E. A., Munro H. N. Isolation of cDNA clones for the light subunit of rat liver ferritin: evidence that the light subunit is encoded by a multigene family. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1265–1269. doi: 10.1073/pnas.80.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Ralph P., Dörner M. H., Lu L., Castro-Malaspina H. Monocyte-macrophage-derived acidic isoferritins: normal feedback regulators of granulocyte-macrophage progenitor cells in vitro. Blood. 1982 Sep;60(3):595–607. [PubMed] [Google Scholar]
- Costanzo F., Santoro C., Colantuoni V., Bensi G., Raugei G., Romano V., Cortese R. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984 Jan;3(1):23–27. doi: 10.1002/j.1460-2075.1984.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S. J., Wagstaff M., Worwood M. Detection of a glycosylated subunit in human serum ferritin. Biochem J. 1981 Dec 1;199(3):565–571. doi: 10.1042/bj1990565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Adelman T. G., Arosio P., Casareale D., Fitzpatrick P., Harzard J. T., Yokota M. Human isoferritins in normal and disease states. Semin Hematol. 1977 Jan;14(1):71–88. [PubMed] [Google Scholar]
- Dörner M. H., Silverstone A., Nishiya K., de Sostoa A., Munn G., de Sousa M. Ferritin synthesis by human T lymphocytes. Science. 1980 Aug 29;209(4460):1019–1021. doi: 10.1126/science.6967622. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hazard J. T., Drysdale J. W. Ferritinaemia in cancer. Nature. 1977 Feb 24;265(5596):755–756. doi: 10.1038/265755a0. [DOI] [PubMed] [Google Scholar]
- Jones T., Spencer R., Walsh C. Mechanism and kinetics of iron release from ferritin by dihydroflavins and dihydroflavin analogues. Biochemistry. 1978 Sep 19;17(19):4011–4017. doi: 10.1021/bi00612a021. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Lavoie D. J., Ishikawa K., Listowsky I. Correlations between subunit distribution, microheterogeneity, and iron content of human liver ferritin. Biochemistry. 1978 Dec 12;17(25):5448–5454. doi: 10.1021/bi00618a019. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Leung D. W., Gillam S., Astell C. R., Montgomery D. L., Hall B. D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979 Apr;16(4):753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Nakai S., Honjo T. Cloning of human immunoglobulin mu gene and comparison with mouse mu gene. Nucleic Acids Res. 1980 Dec 20;8(24):5983–5991. doi: 10.1093/nar/8.24.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Drysdale J. Evidence for distinct mRNAs for ferritin subunits. Biochem Biophys Res Commun. 1981 Jan 30;98(2):507–511. doi: 10.1016/0006-291x(81)90869-x. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Drysdale J. Studies on heterogeneity in ferritin subunits. Biochim Biophys Acta. 1983 Feb 28;743(1):98–105. doi: 10.1016/0167-4838(83)90422-3. [DOI] [PubMed] [Google Scholar]
- Woods D., Crampton J., Clarke B., Williamson R. The construction of a recombinant cDNA library representative of the poly(A)+ mRNA population from normal human lymphocytes. Nucleic Acids Res. 1980 Nov 25;8(22):5157–5168. doi: 10.1093/nar/8.22.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]