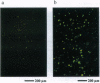
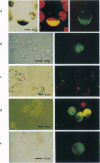
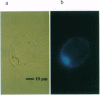
Abstract
The expression of the jellyfish green fluorescent protein (GFP) in plants was analyzed by transient expression in protoplasts from Nicotiana tabacum, Arabidopsis thaliana, Hordeum vulgare, and Zea mays. Expression of GFP was only observed with a mutated cDNA, from which a recently described cryptic splice site had been removed. However, detectable levels of green fluorescence were only emitted from a small number of protoplasts. Therefore, other mutations in the GFP cDNA leading to single-amino acid exchanges in the chromophore region, which had been previously studied in Escherichia coli, were tested in order to improve the sensitivity of this marker protein. Of the mutations tested so far, the exchange of GFP amino acid tyrosine 66 to histidine (Y66H) led to detection of blue fluorescence in plant protoplasts, while the exchange of amino acid serine 65 to cysteine (S65C) and threonine (S65T) increased the intensity of green fluorescence drastically, thereby significantly raising the detection level for GFP. For GFP S65C, the detectable number of green fluorescing tobacco (BY-2) protoplasts was raised up to 19-fold, while the fluorimetricly determined fluorescence was raised by at least 2 orders of magnitude.
Full text
PDF

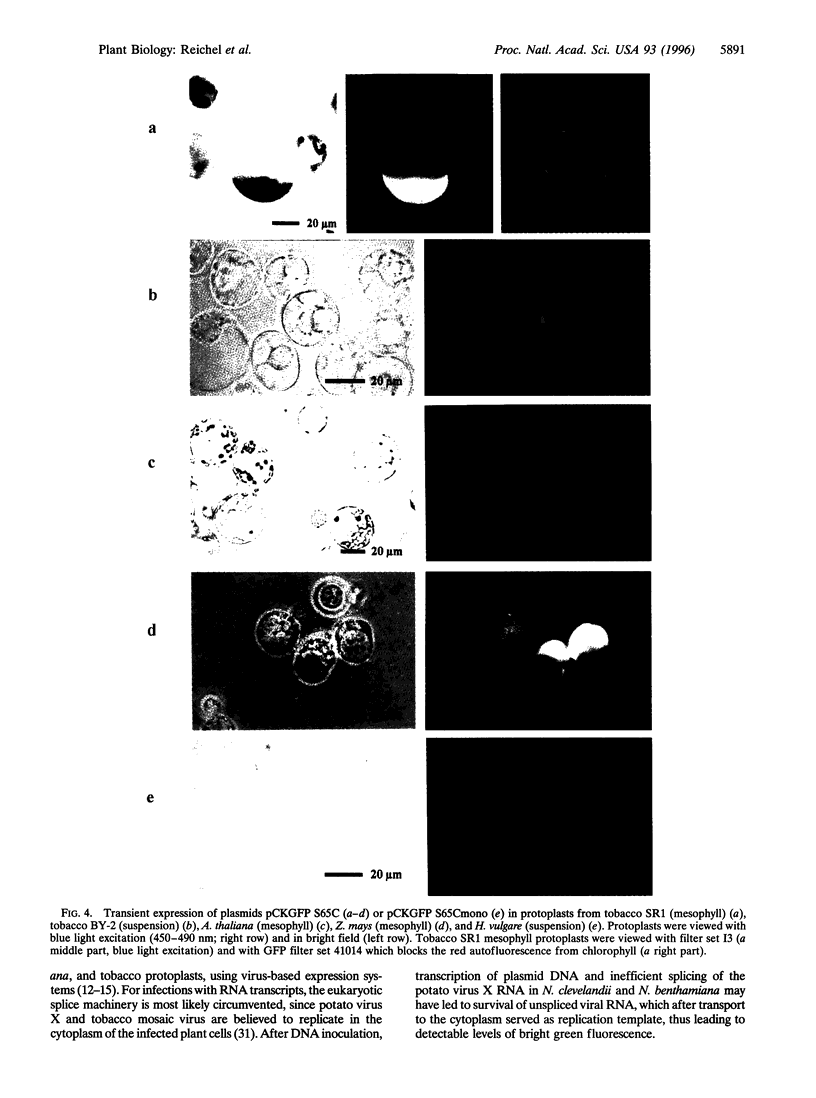


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D. C., Chapman S., Santa Cruz S. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J. 1995 Jun;7(6):1045–1053. doi: 10.1046/j.1365-313x.1995.07061045.x. [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Leinicke A. J. Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell. 1991 Sep;3(9):953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cody C. W., Prasher D. C., Westler W. M., Prendergast F. G., Ward W. W. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry. 1993 Feb 9;32(5):1212–1218. doi: 10.1021/bi00056a003. [DOI] [PubMed] [Google Scholar]
- Corbett A. H., Koepp D. M., Schlenstedt G., Lee M. S., Hopper A. K., Silver P. A. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995 Sep;130(5):1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt A. B., Heim R., Adams S. R., Boyd A. E., Gross L. A., Tsien R. Y. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995 Nov;20(11):448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- Delagrave S., Hawtin R. E., Silva C. M., Yang M. M., Youvan D. C. Red-shifted excitation mutants of the green fluorescent protein. Biotechnology (N Y) 1995 Feb;13(2):151–154. doi: 10.1038/nbt0295-151. [DOI] [PubMed] [Google Scholar]
- Ehrig T., O'Kane D. J., Prendergast F. G. Green-fluorescent protein mutants with altered fluorescence excitation spectra. FEBS Lett. 1995 Jun 26;367(2):163–166. doi: 10.1016/0014-5793(95)00557-p. [DOI] [PubMed] [Google Scholar]
- Galbraith D. W., Lambert G. M., Grebenok R. J., Sheen J. Flow cytometric analysis of transgene expression in higher plants: green-fluorescent protein. Methods Cell Biol. 1995;50:3–14. doi: 10.1016/s0091-679x(08)61018-3. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Amos B. GFP in plants. Trends Genet. 1995 Aug;11(8):328–329. doi: 10.1016/0168-9525(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995 Feb 23;373(6516):663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Heim R., Prasher D. C., Tsien R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein M., Epel B. L., Padgett H. S., Beachy R. N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995 Dec 22;270(5244):1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- Hu W., Cheng C. L. Expression of Aequorea green fluorescent protein in plant cells. FEBS Lett. 1995 Aug 7;369(2-3):331–334. doi: 10.1016/0014-5793(95)00776-6. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C., Gerdes H. H. Visualization of protein transport along the secretory pathway using green fluorescent protein. FEBS Lett. 1995 Aug 7;369(2-3):267–271. doi: 10.1016/0014-5793(95)00765-2. [DOI] [PubMed] [Google Scholar]
- Maas C., Laufs J., Grant S., Korfhage C., Werr W. The combination of a novel stimulatory element in the first exon of the maize Shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold. Plant Mol Biol. 1991 Feb;16(2):199–207. doi: 10.1007/BF00020552. [DOI] [PubMed] [Google Scholar]
- Maas C., Reichel C., Schell J., Steinbiss H. H. Preparation and transformation of monocot protoplasts. Methods Cell Biol. 1995;50:383–399. doi: 10.1016/s0091-679x(08)61045-6. [DOI] [PubMed] [Google Scholar]
- McLean B. G., Zupan J., Zambryski P. C. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995 Dec;7(12):2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D. C., Eckenrode V. K., Ward W. W., Prendergast F. G., Cormier M. J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992 Feb 15;111(2):229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- Sheen J., Hwang S., Niwa Y., Kobayashi H., Galbraith D. W. Green-fluorescent protein as a new vital marker in plant cells. Plant J. 1995 Nov;8(5):777–784. doi: 10.1046/j.1365-313x.1995.08050777.x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990 Oct;2(10):1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994 Jun 2;369(6479):400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]