Abstract
Cellular life depends on protein transport and membrane traffic. In multicellular organisms, membrane traffic is required for extracellular matrix deposition, cell adhesion, growth factor release, and receptor signaling, which are collectively required to integrate the development and physiology of tissues and organs. Understanding the regulatory mechanisms that govern cargo and membrane flow presents a prime challenge in cell biology. Extracellular matrix (ECM) secretion remains poorly understood, although given its essential roles in the regulation of cell migration, differentiation, and survival, ECM secretion mechanisms are likely to be tightly controlled.
Recent studies in vertebrate model systems, from fishes to mammals and in human patients, have revealed complex and diverse loss-of-function phenotypes associated with mutations in components of the secretory machinery. A broad spectrum of diseases from skeletal and cardiovascular to neurological deficits have been linked to ECM trafficking. These discoveries have directly challenged the prevailing view of secretion as an essential but monolithic process. Here, we will discuss the latest findings on mechanisms of ECM trafficking in vertebrates.
Keywords: ECM, collagen secretion, membrane traffic, vertebrate animal models, cartilage and bone
1. Introduction
Extracellular matrix (ECM) is a complex non-cellular structure synthesized by all tissues and is composed of water, proteins, and polysaccharides, as well as mineral deposits in skeletal tissues (Bosman and Stamenkovic, 2003). ECM composition is unique to each tissue and is deposited by fibroblasts or other specialized cells. For example, epithelial cells secrete basement membrane proteins, such as collagens, fibronectin and laminin, whereas chondrocytes and osteocytes secrete type II and type I collagens that are characteristic of mature cartilage and bone, respectively (Gay et al., 1976; Li et al., 1995; Reddi et al., 1977). Large structural ECM proteins are typically fibrillar, including collagens, fibronectin, and laminins. Collagens, for example, constitute over 30% of a total protein mass in multicellular organisms (Ishikawa and Bachinger, 2013) and are rapidly secreted during development or in response to pathological conditions such as wound healing after tissue injury (e.g. skin damage, myocardial infarction, liver cirrhosis) (Cleutjens et al., 1995; Clore et al., 1979; Gay et al., 1975; Pinzani et al., 2011).
The rapid secretion of large cargos such as collagens requires unique regulatory mechanisms to assure availability of specialized transport machinery (Melville et al., 2011; Saito et al., 2009a). Procollagen has been extensively used as a model-cargo in secretory pathway studies (Arnold and Fertala, 2013; Bonfanti et al., 1998; Ishikawa and Bachinger, 2013; Stephens and Pepperkok, 2002). As with all ECM proteins, procollagen is synthesized and initially post-translationally modified in the Endoplasmic Reticulum (ER), from which it is transported in a COPII (coat protein II complex)-dependent manner to the ER-to-Golgi intermediate compartment (ERGIC) en route to the Golgi complex, where further post-translational processing occurs (Canty and Kadler, 2005). Procollagen is then transported in tubular carriers to be secreted to the extracellular space, where it is cleaved and assembled into higher-order structures (Arnold and Fertala, 2013; Ishikawa and Bachinger, 2013; Polishchuk et al., 2003; Polishchuk et al., 2009).
The first leg of this journey is export from the ER, which is mediated by the COPII complex (Figure 1). Pioneering work using yeast genetics first identified 23 genes whose products are required for secretory activity (Kaiser and Schekman, 1990; Novick et al., 1980). Among them were components of the COPII complex (Barlowe et al., 1994). COPII formation is initiated when the cytoplasmic GTPase Sar1 undergoes a conformational change upon GTP binding and associates with the ER membrane (Barlowe et al., 1993; Kuge et al., 1994; Nakano and Muramatsu, 1989). Sar1 then recruits Sec23/Sec24 heterodimers to form the “inner coat” complex (Bi et al., 2002; Matsuoka et al., 1998). Two additional ER associated proteins, Sec12 that acts as a GEF (guanine nucleotide exchange factor) for Sar1 (Barlowe and Schekman, 1993) and Sec16 that is a large scaffold protein shown to associate with ER Exit Sites (Connerly et al., 2005; Espenshade et al., 1995; Watson et al., 2006) and contribute to initiation of vesicle formation. While Sec23 serves as GAP (GTPase activating protein) for Sar1, resulting in coat dissociation from the vesicle membrane (Yoshihisa et al., 1993), Sec24 acts as a cargo adaptor by selecting distinct proteins for ER exit (Miller et al., 2002). Assembly of the inner coat is followed by recruitment of Sec13-Sec31 heterotetramer of the “outer coat” complex, which is thought to stabilize the coat (Bhattacharya et al., 2012; Bi et al., 2007; Copic et al., 2012; Stagg et al., 2006; Tang et al., 2000). The molecular nature of these processes has been reviewed elsewhere (Brandizzi and Barlowe, 2013; Szul and Sztul, 2011).
Figure 1. The secretory module, Transcription Factor–COPII Adaptor–ECM Cargo, operates in a spatio-temporal manner.
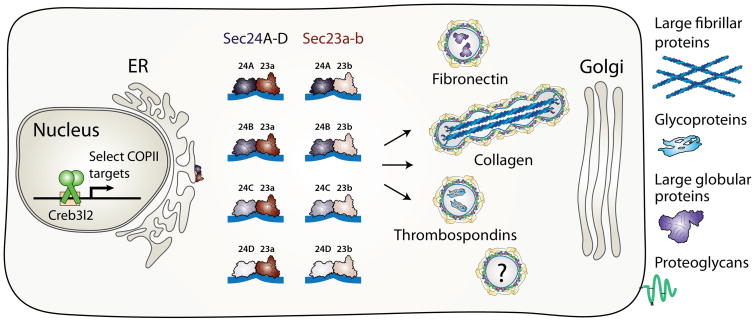
Recent discovery of a “secretory module” consisting of Creb3L2, a transcription factor that regulates the expression of Sec23A-Sec24D, which then facilitate procollagen cargo traffic during embryonic skeletal development, provided the first evidence for the “secretory code”. The existence of such a secretory code is supported by studies with mutant animal models and human patient samples discussed in this review. To date only the secretory modules for type I and type II collagens were tested, which were conducted primarily in the zebrafish system using feelgood-crusher-bulldog mutations (creb3L2-sec23A-sec24D, respectively). It is hypothesized that unknown transcription factors regulate the expression of distinct COPII cargo adaptors (Sec24A-D and Sec23A-B), leading to preferential availability of the various coat components that are required for transport of distinct ECM cargos, such as fibronectin and thrombospondins, at any given time point. Evidence suggests that a diverse array of COPII coats containing specific combinations of core components and associated proteins is required for transport of structurally divergent cargos, such as globular, fibrillar, or transmembrane proteins. Further studies will be required to unravel the complexity of the secretory code to understand how the system integrates cellular operations at regulatory and structural levels to a spatio-temporal manner in a living organism.
Unlike the baker’s yeast genome (Saccharomyces cerevisiae) that harbors single copies of these essential genes, vertebrate genomes have an expanded repertoire of COPII genes, including Sar1a and Sar1b (Jones et al., 2003; Loftus et al., 2012), Sec23a and Sec23b (Paccaud et al., 1996; Wadhwa et al., 1993), Sec24A, Sec24B, Sec24C and Sec24D (Tang et al., 1999), Sec13 (Swaroop et al., 1994), Sec31a and Sec31b (Stankewich et al., 2006; Tang et al., 2000), (Figure 1). Gene multiplication of the coat components might have been evolutionarily driven by expansion of the genomes to accommodate novel extracellular matrix proteins and more complex body plans.
Many of the additional COPII paralogs seem to be specific to vertebrates and might be associated with unique functions that are essential for vertebrate development, including organ structures that are supported by diverse types of basement membranes and an internal skeleton primarily composed of mineralized ECM of cartilage and bone (Braasch and Postlethwait, 2012, Forster et al., 2010, Norum et al., 2010). Thus it is not surprising that many loss-of-function mutations in the trafficking machinery components result in skeletal dysmorphology.
Here we will discuss cargo- and tissue-specific functions of the COPII machinery, post-translational modifications, phosphorylation and ubiquitylation of COPII proteins, and the effects they have on vesicle biogenesis. In addition we will discuss auxiliary proteins such as cargo receptors and guide proteins that were shown to assist in loading of ECM macromolecules into vesicular carriers. Finally, one of the most intriguing unanswered questions in regulation of secretion is transcriptional control of the secretory machinery. Most coat genes are ubiquitously expressed but are enriched in specific tissues at defined developmental time points or in pathological conditions. However, little is known about transcriptional control of secretion with only a single factor of the OASIS family, Creb3L2, implicated in the process so far.
In this review we will focus on recently discovered trafficking mechanisms in the initial leg of the secretory pathway, from the ER to Golgi, by highlighting studies of vertebrate model organisms and human genetic mutations.
2. ER-to-Golgi transport is facilitated by Coat Protein II (COPII) vesicular carriers
2.1. Cargo selection by Sec24 components of the inner coat
Tandem genome duplication expanded the ancestral Sec24 gene to two syntenic groups, one of Sec24A and Sec24B and the second of Sec24C and Sec24D (Tang, et al., 1999). The four genes are highly divergent in sequence between the two groups (20% similarity) and approximately 50% similar between each pair, but each paralog is highly conserved within vertebrates (up to 90% sequence similarity between fish and human) (Sarmah et al., 2010). Pioneering work on transport signal recognition by the Sec24 paralogs using model cargo in Sec24-depleted HeLa cells revealed for the first time selectivity and redundancy in the cargo selection process (Wendeler et al., 2007).
Recent in vivo evidence obtained from phenotype-driven genetic screens in zebrafish, medaka and mouse have begun to uncover the complexity of Sec24-based cargo selection. To date, only Sec24D has been directly implicated in ECM secretion. The zebrafish mutant bulldog/sec24d fails to secrete type II collagen and matrilin from chondrocytes, fibroblasts and notochord sheath cells, leading to severe craniofacial dysmorphology, short body length and kinked pectoral fins (Sarmah et al., 2010). This phenotype is largely recapitulated by the vbi/sec24d medaka mutant carrying a nonsense mutation predicted to truncate a C-terminal portion of Sec24D protein (Ohisa et al., 2010). In both zebrafish and medaka, Sec24D-deficient chondrocytes accumulate type II collagen in distended rough endoplasmic reticulum (rER). However, other ECM and transmembrane proteins appear to be trafficked normally to the extracellular space and plasma membrane, including fibronectin, cadherin, and β1-integrin (Table 1). In mouse, however, gene-trap mediated knockout of Sec24D gene leads to pre-implantation lethality with no discernible phenotype in a haploinsufficient condition (Baines et al., 2013). The mouse data confirm that cargos that are required as early as the 8-cell stage are transported in a Sec24D-dependent manner. The inability to study Sec24D-dependent ECM transport during organogenesis in global mouse knockouts is complemented by studies in teleost fish such as zebrafish and medaka, which receive maternal Sec24D protein and mRNA that allow for normal gastrulation. Thus, fish models are ideally suited to study cargo adaptor functions at later developmental stages (Melville and Knapik, 2011, Ohisa et al., 2010, Sarmah et al., 2010).
Table 1.
The effects of trafficking machinery depletion on cargo transport
| Trafficking machinery component | Cargo | Effect of depletion on cargo transport | Tissue/Cell Type | Organism | Reference |
|---|---|---|---|---|---|
| Sec23a | Collagen I | Accumulation in the ER | Fibroblasts | Human | Boyadjiev et al., 2006 |
| Collagen II | Accumulation in the ER | Chondrocytes | Zebrafish | Lang et al., 2006 | |
| Sec24B | Vangl2 | Failure of localization to plasma membrane | Neural Tube | Mouse |
Merte at al., 2010 Wansleeben et al., 2010 |
| Sec24D | Collagen II | Accumulation in the ER | Chondrocytes | Zebrafish | Sarmah et al., 2010 |
| Matrilin | Accumulation in the ER | Chondrocytes | |||
| Fibronectin | Unaffected | Chondrocytes | |||
| Integrin β1 | Unaffected | Chondrocytes | |||
| Pan-cadherin | Unaffected | Chondrocytes | |||
| Collagen II | Intracellular accumulation | Chondrocytes | Medaka | Ohisa et al., 2010 | |
| Collagen II | Intracellular accumulation | Notochord | |||
| Collagen II | Intracellular accumulation | Myoseptum | |||
| Sar1b | Chylomicron | Retention in membrane-bound compartments | Enterocytes | Human | Dannoura et al., 1999 |
| Sec13 | Collagen I | Defective secretion and deposition | Fibroblasts | Human | Townley et al, 2008 |
| Opsin | Intracellular accumulation | Photoreceptor cells | Zebrafish | Schmidt et al, 2013 | |
| Syntaxin-3A | Unaffected | Photoreceptor cells | |||
| TANGO1 | Collagen VII | Accumulation in the ER | Fibroblasts | Human | Saito et al., 2009b |
| Collagen I | Intracellular accumulation | Chondrocytes | Mouse | Wilson et al., 2011 | |
| Collagen II | Intracellular accumulation | Chondrocytes | |||
| Collagen III | Intracellular accumulation | Chondrocytes | |||
| Collagen IV | Intracellular accumulation | Endothelial cells | |||
| Collagen VII | Intracellular accumulation | Embryonic fibroblasts | |||
| Collagen IX | Intracellular accumulation | Embryonic fibroblasts | |||
| COMP | Intracellular accumulation | Chondrocytes | |||
| Fibronectin | Unaffected | Chondrocytes | |||
| Aggrecan | Unaffected | Chondrocytes | |||
| cTAGE5 | Collagen VII | Accumulation in the ER | A431 cells | Human | Saito et al., 2011 |
| Sedlin | Collagen I | Accumulation in the ER | Fibroblasts | Human | Venditti et al., 2012 |
| Collagen II | Accumulation in the ER | Chondrocytes |
ER: Endoplasmic Reticulum, Vangl2: Van Gogh-like protein 2, TANGO1: Transport and Golgi organization 1, COMP: Cartilage oligomeric matrix protein, cTAGE5: cutaneous T-cell lymphoma-associated antigen 5.
Although Sec24D and Sec24C cargo adaptors recognize similar cargo binding motifs in in vitro assays (Wendeler et al., 2007), they appear to transport unique cargos in cell culture and in vivo conditions. For example, Sec24C has been shown to be essential for secretion of neurotransmitter transporters (Sucic et al., 2011) and the fusion of prechylomicron transport vesicles with Golgi membranes (Siddiqi et al., 2010). Furthermore, although Sec24D depletion in zebrafish results in both craniofacial skeleton deficits as well as impaired notochord extension (axial skeleton), Sec24C depletion only affects notochord extension and not the head skeleton. Notably, combined depletion of Sec24C and Sec24D results in a significantly more severe phenotype. These findings suggest that Sec24C and Sec24D are exclusively required for secretion of select notochord basement membrane matrix proteins, whereas other matrix proteins are secreted in a redundant fashion by the two paralogs (Melville and Knapik, 2011, Sarmah et al., 2010). So far, only a few ECM cargos have been matched with specific adaptors. Future in depth studies will be needed to establish a combinatorial network of cargos and their respective adaptors and to understand how they are regulated to meet the secretory demand of different tissues during embryonic development as well as pathological and homeostasis conditions.
Although no human syndromes affecting Sec24 paralogs were known, SEC24B mutations were recently identified in patients carrying severe neural tube defects (Yang et al., 2013). Prior work in mouse mutants helped to explain how Sec24B is required for neural tube closure. Analyses in mice obtained from a phenotype-driven chemical screen have shown that Sec24B is essential for the secretion of Vangl2 (Van Gogh-like 2), a protein known to act in planar cell polarity (PCP) and the gastrulation movements of convergence and extension during early embryonic development. Defects in these processes result in craniorachischisis and neural tube closure defects in Sec24B mouse mutants (Merte et al., 2010, Wansleeben et al., 2010). Although Vangl2 is not a matrix protein per se, its function has been shown to regulate MMP-14 (membrane type-1 matrix metalloproteinase), which is involved in fibronectin remodeling (Williams et al., 2012a, Williams et al., 2012b) during gastrulation (Latimer and Jessen, 2010). It will be interesting to determine whether a single or multiple cargo adaptors execute ER egress of multiple components of a single pathway such as PCP.
2.2. Sec23A and Sec23B paralogs perform tissues specific functions
The discovery of tissue-specific phenotypes in carriers of Sec23A mutations challenged the prevailing view that Sec24 adaptors are solely responsible for cargo specificity of COPII carriers. Vertebrate genomes harbor two paralogs of the ancestral Sec23 gene, Sec23A and Sec23B (Paccaud et al., 1996). Unbiased, phenotype-driven genetic screens in vertebrate animal models and disease genotyping in human patients provided the first evidence that Sec23A and Sec23B are not only acting in tissue specific manner, but also are differentially used in ECM macromolecule traffic.
crusher, a zebrafish sec23a mutant, was isolated in a genetic screen for phenotypes affecting embryonic patterning and organogenesis (Driever et al., 1996, Knapik, 2000, Neuhauss et al., 1996). crusher was shown to carry a nonsense mutation at amino acid 402 in the Sec23a gene, resulting in a predicted stop codon and truncation of almost half of the protein (Lang et al., 2006). Craniofacial dysmorphology and shorter body length are the predominant phenotypes of sec23a mutants, indicating deficits in skeletal development. At the subcellular level, crusher chondrocytes exhibit distended rough endoplasmic reticulum (rER) in electron micrographs (TEM) and accumulate type II collagen deposits as shown by immunofluorescence (IF). This intracellular backlog consequently leads to reduced Collagen and Matrilin content in cartilage ECM.
In parallel with discoveries of sec23a function in zebrafish, mutations in the human SEC23A gene were reported to cause cranio-lenticulo-sutural-dysplasia (CLSD), an autosomal recessive disorder characterized by facial dysmorphism and axial skeleton defects (Boyadjiev et al., 2006). Electron microscopy and IF studies in fibroblasts isolated from CLSD patients revealed dilated ER structures and accumulation of procollagen in rER cisternae. To date two missense mutations located near the Sec31 binding site of the SEC23A folded protein were identified in patients. The F382L mutation was shown to hinder SEC23A ability to recruit the SEC13-SEC31 outer coat and to ultimately prevent vesicle budding (Fromme et al., 2007), whereas the M702V appears to activate SAR1B more efficiently than the wild type allele, resulting in premature dissociation of the COPII coat from ER membranes (Boyadjiev et al., 2011, Kim et al., 2012). Although other cargo molecules are packaged into COPII vesicles normally, procollagen accumulates in the ER of M702V mutant fibroblasts. These results suggest that COPII vesicles with a longer occupancy on ER membrane may be required to form sufficiently large carriers to transport procollagen (Kim, et al., 2012). The zebrafish crusher and two CLSD mutations affect approximately the same region of the Sec23A protein and result in remarkably similar phenotypes. This highlights the high degree of conservation of COPII-mediated collagen transport and establishes zebrafish as a strong in vivo system to model human mutations (Vacaru et al., 2013).
SEC23B mutations in humans, in contrast to those of SEC23A, are linked to Congenital Dyserythropoietic Anemia Type II (CDAII), an autosomal recessive disease characterized by ineffective erythropoiesis, hemolysis, and presence of multinucleated erythroblasts in bone marrow. The precise molecular mechanisms leading to these phenotypes are not understood, but transmission electron micrographs (TEM) of erythrocytes in peripheral blood showed double plasma membranes, and SDS-PAGE experiments revealed hypoglycosylation of membrane proteins (Bianchi et al., 2009), suggesting a requirement of SEC23B in the trafficking of components of glycosylation pathways. Over 50 variants in SEC23B have been identified throughout the length of the coding region, and most of the patients are homozygous for missense mutations or compound heterozygotes presenting primarily with an anemia phenotype (Iolascon et al., 2010, Khoriaty et al., 2012, Russo et al., 2010, Schwarz et al., 2009). No homozygotes for nonsense mutations have been identified in approximately 370 reported cases (Iolascon et al., 2012). Interestingly, three independent gene-trap insertion lines in mouse, each of which results in predicted null alleles, do not present an anemia phenotype and die at birth with profound developmental and exocrine organ (pancreas, salivary and intestinal glands) secretion deficits (Tao et al., 2012). However, zebrafish larvae depleted in sec23b present an anemia phenotype and hemolysis similar to CDAII patients (Figure 2A,B; Schwarz et al., 2009), and in addition they lack the entire neural crest-derived craniofacial skeleton (Lang et al., 2006, Schwarz et al., 2009). At present it is not clear whether the range of phenotypes represents species-specific functional differences (human-mouse) or variations between hypomorph and null alleles. One potential explanation for the tissue-specific phenotypes observed with Sec23a and Sec23b mutations is that the two paralogs are required to transport distinct cargos. This notion, however, contrasts with the prevailing view that Sec24, but not Sec23, participates in COPII cargo selection. Whether Sec23b is required (directly or indirectly) to transport unique cargos from those of Sec23a remains unknown, and future studies will be needed to address these questions to better explain tissue- and species-specific phenotypes that have been observed.
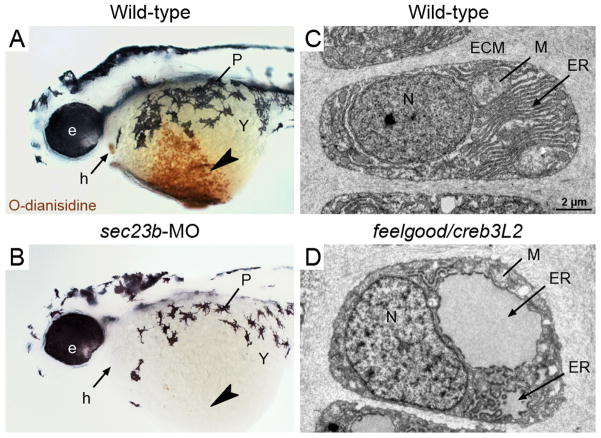
Figure 2. Deficits in secretory machinery lead to tissue-specific phenotypes.
A,B. Wild-type 2 day zebrafish embryos stained with o-dianisidine, which is oxidized by heme in the presence of peroxide and colored brown, display abundant hemoglobin within the ducts of Cuvier (arrowhead) and the heart (arrow). B. Sec23b morphants, which have defects in erythrocyte development, are deficient of hemoglobin at 2 days. C,D. Transmission Electron Micrographs (TEM) images of wild-type zebrafish chondrocytes (3-day old) show characteristics of highly secretory cells, including abundant rough ER (arrow), mitochondria (line), and Golgi. D. feelgood/creb3L2 mutant chondrocytes contain highly distended rER (arrow) due to collagen backlog. Symbols: e, eye; h, heart; P, pigment; Y, yolk; ECM, extracellular matrix; ER, endoplasmic reticulum; M, mitochondria; N, nucleus.
Although Sec23 is not known to directly carry cargo-binding functions, Sec23a and Sec23b could indirectly convey cargo specificity of the COPII coat by selective interaction with Sec24 proteins, which are known to directly interact with cargos (Barlowe, 2003, Mancias and Goldberg, 2008, Miller et al., 2002, Miller et al., 2003). In this scenario, Sec23a may be viewed as a critical partner for a collagen-specific Sec24 paralog. Alternatively Sec23 could participate in direct cargo/receptor/adapter binding through undiscovered mechanisms. The complexity of ECM cargo selection is just being uncovered in an in vivo setting of a vertebrate body, and it has become clear that this is a complex problem that will require extensive investigation.
2.3. Sar1A and Sar1B may differentially contribute to carrier size
Sar1 is a small GTP-binding protein that initiates COPII coat assembly on ER membranes (Aridor et al., 2001, Bielli et al., 2005, Kuge et al., 1994). Similar to other COPII components, vertebrate genomes harbor two Sar1 genes, Sar1A and Sar1B (He et al., 2002, Jones et al., 2003; Levic et al., 2013). The two paralogs are highly conserved and the human genes vary by only 20 residues, whereas the fish and mammalian homologs share over 90% identity. Despite this remarkable similarity in sequence the two paralogs function in a distinct manner.
The distinct functions may potentially be explained by differential interactions of Sar1A and Sar1B with the outer COPII coat. Under Sec31-stimulated conditions, Sar1B hydrolyzes GTP more slowly than Sar1A possibly due to higher binding affinity of Sec13-Sec31 to Sar1A than to Sar1B containing coats (Fromme et al., 2007). Conceivably, this high-affinity binding of Sar1A to the outer coat could lead to tightly packaged small vesicles, whereas loosely packed Sar1B -outer coat complex may allow for larger COPII carriers (Fromme et al., 2008). Although this could potentially help to explain how enlarged COPII carriers are formed, it remains unknown whether the different binding affinities of Sar1A and Sar1B for the COPII outer coat observed in vitro translate into functional differences in a physiological context. Alternatively, for example, Sar1b may accommodate the formation of larger sized carriers primarily due to its slower rate of GTP hydrolysis, which slow the kinetics of vesicle budding to allow larger vesicles to form.
Consistent with the carrier size hypothesis, chylomicrons (250 nm in size) are significantly larger than typical 90 nm COPII vesicles, and have been observed to be secreted in a Sar1B-dependent rather than Sar1A-dependent manner (Shoulders et al., 2004). Mutations in human SAR1B were shown to cause chylomicron retention disease (CMRD), a lipid absorption disorder characterized by deficits in intestinal lipid uptake and hypocholesterolemia (Jones, et al., 2003). Besides lipid malabsorption, some CMRD patients are diagnosed with other symptoms, including exocrine pancreatic insufficiency, decreased bone mineral density, and cerebellar ataxia. The pathophysiology of the disease is not understood; however, sar1b loss-of-function experiments in zebrafish embryos showed not only intestinal lipid absorption deficits but also craniofacial dysmorphology due to failure of type II collagen secretion to extracellular cartilage matrix (Levic et al., 2013). These findings suggest that Sar1b is needed not only for secretion of lipids in large chylomicrons, but also large extracellular matrix proteins such as collagen.
Interestingly, no mutations in the SAR1A coding region have been identified in patients (Charcosset et al., 2008, Kumkhaek et al., 2008) and depletion of Sar1a in zebrafish did not result in a gross dysmorphology (Levic et al., 2013). Recent experiments in mammalian cell culture setting examined essential roles of Sar1a and Sar1b and revealed that cells depleted in both paralogs were still capable of secreting small globular proteins (VSV-G) in a COPII-independent manner using a previously uncharacterized, atypical COPI-dependent secretory mechanism. Procollagen type I, however, was retained in the ER and did not sort to ER exit sites (Cutrona et al., 2013). These findings open a new, intriguing possibility for ECM transport mechanisms, particularly because COPI genetic mutations in zebrafish have deficits in secretion of notochord basement membrane proteins (Coutinho et al., 2004). For example, copa depletion results in a highly similar phenotype in the craniofacial skeleton compared to Sec23a mutants alone; however, combined copa/sec23a mutants had a significantly shorter axial skeleton than either condition alone (Lang et al., 2006). These results suggest that distinct trafficking pathways are required for morphogenesis of the two tissues, which are composed of distinct ECM components. Further investigations are needed to better understand the function of the COPII inner coat in ECM secretion, as well as the regulatory mechanisms that provide the proper combinations and stoichiometry of different inner coat paralogs to meet the secretory demand of various tissues and organs – the so-called secretory code, which remains an elusive riddle in cell biology (Figure 1).
2.4. Sec13-Sec31of the outer coat contribute to cargo specificity
Sec13-Sec31 heterotetramers form an outer shell of the COPII coat (Stagg et al, 2006) and are thought to provide stability to its rigid structure (Copic et al., 2012). Most vertebrate genomes contain at least two Sec31 genes, but only one Sec13 (Stankewich et al., 2006, Swaroop et al., 1994, Tang et al, 2000). To date no human patients have been reported to carry mutations in genes of the outer coat. The unknown nature of the Sec13 and Sec31 syndromes might be a result of in utero lethality, or alternatively might reflect redundant and nonessential functions of the genes in human development. Further characterization of loss-of-function phenotypes in vertebrate animal models will help narrow down the spectrum of human syndromes that could be tested for potential mutations in Sec13 and Sec31.
Sec13 has been suggested to play critical roles in development of craniofacial structures. In zebrafish, knockdown of Sec13 leads to morphological abnormalities in craniofacial cartilage elements, short pectoral fins, small eyes and cardiac edema, likely resulting from defects in proteoglycan and collagen secretion (Townley et al., 2008). At the molecular level, Sec13 depletion in human cells in culture led to loss of Sec31 on the outer coat of COPII vesicles, although budding and curvature of the vesicles were unaffected in TEM analyses (Niu et al., 2012, Townley et al., 2008). Furthermore, Sec13 depleted cells failed to export collagen from distended rER, whereas tsO45-VSV-G-YFP, a secretory cargo marker, and other small soluble cargos were normally secreted. Although the bases for the secretion of small soluble cargos were not investigated, a proposed COPI-dependent mechanism could potentially explain the results (Cutrona et al., 2013).
Sec13 was also implicated in the development of digestive system organs. The zebrafish sec13 genetic mutation leads to hypoplastic intestine, exocrine pancreas, and liver. The gene appears to be dispensable for initial specification and patterning stages of development, but is essential for tissue growth and cell proliferation (Niu et al., 2012). Similar morphological deficits in intestinal epithelium morphology were observed in a morphant model, further emphasizing the requirement of Sec13-Sec31-driven secretion in the development of digestive system (Townley et al., 2012). A small eye phenotype in Sec13-depleted zebrafish embryos was linked to degeneration of photoreceptor cells, in addition to impaired trafficking of collagen in retinal pigment epithelium, supporting the model that efficient assembly of the COPII outer coat is required for trafficking of large ECM cargos in skeletal tissues, tubular organs and the retina (Schmidt et al., 2013).
Collectively, these studies underscore the importance of a fully functional Sec13-Sec31 outer coat for the development of various, highly secretory organs such as cartilage, exocrine pancreas, liver, and retina. The outer coat appears to be required for the transport of bulky collagen cargos as opposed to small soluble proteins. Further studies in vertebrate model systems and patient samples will be required to identify other ECM cargos secreted in a Sec13-31-dependent manner and to identify determinants of the secretory code.
3. Transcriptional regulation of ECM secretion
An unsolved problem in cell biology is how regulatory mechanisms directing development and adult homeostasis communicate with secretory pathway programs to assure sufficient and timely availability of cargo-specific coats. In a living multicellular organism, the secretory pathway responds to constant demands for cargo delivery during development, physiological changes, and tissue repair. The transcriptional and other signaling mechanisms that govern these functions are just being illuminated. The first known bona fide transcriptional regulator of the secretory machinery has recently been identified in mice, zebrafish and flies (Abrams and Andrew, 2005, Fox et al., 2010, Melville et al., 2011, Saito et al., 2009a, Tanegashima et al., 2009).
Creb3L2 (cAMP responsive element binding protein-3 like 2), also known as BBF2H7, has been linked to collagen secretion in vertebrates. Creb3L2 is an ER-resident, transmembrane transcription factor that is highly expressed in chondrocytes. After COPII-dependent ER export, Creb3L2 is cleaved in the Golgi to an active, soluble peptide that dimerizes and then is translocated to the nucleus to activate the expression of target genes (Lui et al., 2008, Panagopoulos et al., 2007). Creb3L2 knockout mice display chondrodysplasia and die shortly after birth (Saito et al., 2009a). Proliferating chondrocytes in E18.5 stage mice have distended ER and intracellular collagen II accumulation. In cultured ATDC5 cells, luciferase assays revealed that Sec23a promoter activity is regulated by CREB3L2, while chromatin immunoprecipitation showed binding of CREB3L2 to Sec23a promoter region. Taken together, this study linked Creb3L2-mediated transcriptional regulation of Sec23A expression to collagen trafficking.
Concurrent study of the zebrafish feelgood mutation identified a missense variant in the creb3L2 coding region resulting in a hypomorphic allele (Melville et al., 2011). Luciferase assays of the feelgood variant revealed reduced transcriptional activity to approximately 50% and consequently, significant decrease in Sec23a expression by quantitative PCR experiments. Further analyses showed that, in addition to Sec23a, Creb3L2 is required for the transcription of specific subsets of the COPII machinery, such as Sec24D but not Sec24C. In addition, trafficking of collagen II in chondrocytes and collagen IV in notochord sheath cells were disrupted, whereas other cargos including laminins were not affected. These data suggests that spatio-temporal expression of COPII components is differentially regulated by Creb3L2 and possibly by other, yet unknown transcription factors. TEM analyses in feelgood chondrocytes revealed protein backlog and distended ER structure as well as progressive loss of cartilage matrix. One potential interpretation of the finding is that in the initial phase of collagen secretion the cargo load is modest and available coats are sufficient to initiate the process. As matrix secretion increases, however, the available coats are not able to meet the demands of the cell and ECM deposition decreases, resulting in progressive reduction of the collagen fibrils in growing skeletal tissues. Creb3L2 is, so far, the only transcription factor identified as a regulator of COPII-dependent collagen secretion. Future studies are needed to discover transcriptional regulatory mechanisms for the specific COPII components and mediators of post-translational modifications important for ECM secretion.
4. Post-translational modifications of the trafficking machinery
Recent findings have highlighted the importance of an additional level of regulation in ECM secretion through post-translational modifications of COPII components, which have been shown to regulate COPII vesicle architecture and assembly. For example, phosphorylation and ubiquitination of COPII proteins can change the affinity of individual coat components to each other and can impact vesicle size.
4.1. Monoubiquitination
Monoubiquitination (addition of a single ubiquitin molecule) has emerged recently as a novel mechanism to modulate protein function (Lauwers et al., 2009), unlike polyubiquitination, which has been known to earmark proteins for proteasomal degradation (Angers et al., 2006). The Cullin3 (Cul3, cullin-based E3 ligase) and KLHL12 (kelch-like family member 12) complex has been shown to function as a ubiquitin ligase that monoubiquitinates Sec31 (Jin et al., 2012, Stephens, 2012). This modification is required to drive procollagen secretion. The concerted action of the cytoplasmic Cul3-KLHL12 complex, under overexpression conditions in a mammalian cell culture system, leads to production of enlarged COPII coated carriers that are up to 500 nm in diameter – large enough to accommodate procollagen molecules. However, this modification is not required for trafficking of smaller or more flexible cargoes such as fibronectin, EGF receptors, or integrin β1 (Jin et al., 2012). Despite this significant discovery, the precise mechanism of how Sec31 monoubiquitination regulates the size of COPII coated carriers in collagen traffic is unknown. Particularly, it remains to be established how the cytoplasmic Cul3-KLHL12 complex recognizes secretory cargo within the ER lumen, which is separated from the complex by ER membranes. Conceivably, the extra time required to package bulky cargo like collagen could result in Sec31 occupying a budding vesicle longer, which may result in its targeting by the Cul3-KLHL12 complex. In any case, Sec31 appears to be a key target for post-translational regulation of COPII carriers’ size as it is both monoubiquitinated and phosphorylated.
4.2. Phosphorylation
Although Sec31 was initially detected as a phosphoprotein many years ago (Salama et al., 1997, Shugrue et al., 1999), Casein kinase II (CK2) has only recently been identified as a kinase responsible for Sec31 phosphorylation. This modification was suggested to regulate Sec31 association with ER membranes through interaction with the COPII inner coat (Koreishi et al., 2013). Ultracentrifugation assays concluded that phosphorylated Sec31 has reduced membrane association, whereas a non-phosphorylatable Sec31 mutant remains at ER exit sites longer and bound more strongly to Sec23. A model where Sec31 phosphorylation interferes with its binding to the COPII inner coat, which ultimately delays vesicle budding, has been proposed (Koreishi et al., 2013). Although preliminary, this model could explain regulatory mechanisms that control COPII vesicle size. Notably, the function of other kinases in phosphorylation of COPII outer coat components has not been investigated in vivo (Dephoure et al., 2008, Franz-Wachtel et al., 2012, Olsen et al., 2006). Identification and characterization of the phosphatase(s) responsible for dephosphorylation of COPII coat subunits will help in further understanding the molecular mechanisms underlying the regulation of collagen secretion
Akt (Protein kinase B) has been shown to phosphorylate recombinant human SEC24C and SEC24D. SEC24 proteins phosphorylated by Akt show greater affinity toward Sec23 as detected by co-IP using CHO-7 cells (Sharpe et al., 2011). Although the specific role of Sec24D phosphorylation in collagen trafficking has not been investigated, differential phosphorylation of individual Sec24 paralogs could explain COPII coat diversity in accommodating ECM cargo.
Collagen secretion is critical to homeostasis of the arterial wall, and malfunction can result in blood vessel rupture with detrimental consequences to the organism. PKCδ (Protein kinase C-, member of the family of serine/threonine kinases) has been implicated in collagen I secretion in smooth muscle cells. PKCδ knockout mice display reduced collagen I deposition to the arterial wall and intracellular accumulation in surrounding smooth muscle cells. Backlogged collagen I was primarily found in the TGN (trans-Golgi network) of the Golgi complex. PKCδ-null smooth muscle cells exhibited reduced levels of the Rho GTPase Cdc42, and restoration of Cdc42 rescued collagen I secretion defects (Lengfeld et al., 2012). This study supports a model where PKCδ phosphorylates yet unidentified targets in a Cdc42-dependent manner that is required for collagen I secretion. This study has identified a novel phenotype in the collagen secretory pathway and future studies might uncover its specific functions in ECM cargo transit through the Golgi complex (Lengfeld et al., 2012).
5. Auxiliary proteins supporting collagen secretion
Recent phenotype-driven genetic screens have identified two novel proteins that are associated with COPII coat machinery and are essential for efficient packaging of large ECM cargos. Tango1/Mia3 and cTage5 are transmembrane proteins associated with ER (Saito et al., 2009b, Saito et al., 2011). They bind to Sec23-Sec24 subunits of COPII coat on the cytoplasmic side and to procollagen on the ER luminal side. Tango1 and cTage5 are postulated to aid cargo selection and concentration into carriers, as well as to delay vesicle scission to allow extra time for cargo loading.
5.1. Tango1
Tango1 (transport and Golgi organization 1) was identified in a genome-wide screen for genes required for constitutive protein secretion using Drosophila S2 cells (Bard et al., 2006). Tango1 was shown to reside at ER exit sites and potentially act as a guide protein for large cargo loading (Figure 3) (Saito et al., 2009b). The protein contains two well-characterized domains: a C-terminal proline rich domain (PRD) required for localization to ER exit sites, and a luminal SH3 domain interacting with procollagen, as shown by immunoprecipitation assays using transfected COS7 cells. Tango1 is essential for collagen VII transport, and its depletion in cultured skin fibroblasts results in a backlog of collagen VII in the ER (Saito et al., 2009b). The same study showed that Tango1 does not get packaged into COPII vesicles, but remains at ER exit sites after collagen loading is completed. Tango1 is suggested to work as a guide by binding to collagen via its SH3 (SRC homology 3) domain to help bulky collagen molecules to get pushed into vesicles. Aside from its interaction with collagen, Tango1 can also bind to the Sec23-Sec24 inner coat through its proline-rich domain (Bi et al., 2007, Shaywitz et al., 1997, Shugrue et al., 1999). Tango1 is proposed to prevent binding of Sec31 during budding to slow down vesicle biogenesis while procollagen molecules are being loaded; once the loading process is completed, Sec31 can be recruited to a procollagen-filled vesicle upon dissociation of Tango1 from both collagen and Sec23-Sec24 complex via a conformational change (Figure 3).
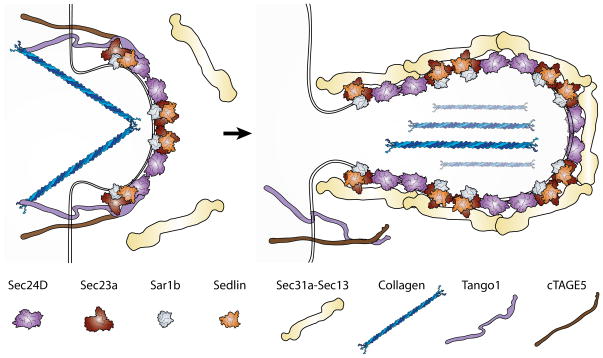
Figure 3. Packaging of procollagen fibrils into large COPII carriers.
Procollagen is a rigid, fibrillar protein aggregate that is significantly larger than the typical size COPII-coated vesicles. Recent work has uncovered auxiliary proteins that aid in the packaging and transport of procollagen into mega-sized COPII carriers. Procollagen is initially loaded into budding vesicles through the concerted action of transmembrane proteins TANGO1 and cTAGE5, which both bind to the Sec23-Sec24 inner coat complex on the cytoplasmic side and collagen on the luminal side. TANGO1/cTAGE5 interaction with the inner coat is thought to inhibit the association of the COPII outer coat complex with the inner coat, which delays the fission of vesicles from the ER exit sites and results in the formation of large-size carriers. TANGO1 is also essential for recruiting Sedlin, which interacts with Sar1 and provides efficient cycling of Sar1-GTP hydrolysis, further delaying coat dissociation from the membranes. After procollagen loading is completed, TANGO1 undergoes a conformational change and dissociates from both procollagen and the inner coat complex but is left behind in the ER membrane after COPII carrier fission. Recruitment of Sec13-Sec31 outer coat is the final step of coat formation before fission.
Developmental roles of Tango1 were investigated in mouse models. Tango1 knockout mice exhibit global secretion defects in collagen I, II, III, IV, VII and IX from various cell types such as chondrocytes, fibroblasts and endothelial cells. Overall development of Tango1 knockout mice is severely compromised, resulting in dwarfism and edema (Wilson et al., 2011). In Tango1-null mice ECM deposition is affected, leading to deficits in cartilage and bone development. These analyses in knockout mouse have revealed critical and widespread roles for Tango1 in vertebrate development, especially for global collagen secretion and skeletogenesis.
5.2. cTAGE5
cTAGE5 (cutaneous T-cell lymphoma-associated antigen 5) was originally found to be overexpressed in various tumors and considered a tumor-specific antigen (Heckel et al., 1997), and has recently been associated with collagen trafficking in mammalian cells. cTAGE5 is an integral membrane protein that localizes to ER exit sites. It contains a transmembrane domain, a proline rich-domain and coiled-coil domains. Immunoprecipitation experiments showed that cTAGE5, via its coiled coil motifs, interacts with Tango1 in mammalian cells. Yeast-two-hybrid assays revealed the interaction between the proline-rich domain of cTAGE5 and Sec23-Sec24 inner COPII coat complex. Moreover, knockdown of cTAGE5 in mammalian cells resulted in accumulation of collagen VII within the ER (Saito et al., 2011). These results suggest that cTAGE5 may serve as an essential co-receptor for Tango1 to facilitate packaging of procollagen into COPII carriers. This notion is supported by the following observations: (1) cTAGE5 can directly interact with Tango1 and (2) knockdown of cTAGE5 leads to collagen export defects regardless of the presence of proper localization and expression of Tango1.
5.3. Sedlin
Although Tango1 and cTAGE5 have been known to facilitate loading of collagen into COPII vesicles, their action can only partially explain the mechanisms that govern the growth of large COPII carriers. Sedlin, a TRAPP (TRAfficking Protein Particle) component that was shown to be defective in spondyloepiphyseal dysplasia tarda (SEDT) patients (Davis et al., 2013, Gedeon et al., 1999, Gedeon et al., 2001, Matsui et al., 2001, Mumm et al., 2000, Mumm et al., 2001) has recently been shown to be required for procollagen export from the ER. The SEDLIN gene is mutated in SEDT patients with chondrogenesis defects. In Sedlin-depleted chondrocytes, procollagen accumulates in ER while small cargo is trafficked properly. Further analysis showed that Sedlin is recruited to ER exit sites in a Tango1-dependent manner. In the absence of Sedlin, the Sar1-GTPase cycle is hyperactive, which results in premature membrane constrictions as detected by electron tomography and 3D reconstruction analysis in fibroblasts from SEDT patients (Venditti et al., 2012). A current model postulates that Tango1-mediated Sedlin recruitment to ER exit sites facilitates efficient Sar1-GTPase cycles to stabilize inner COPII coat and prevent premature membrane constrictions.
5.4. ECM trafficking mechanisms
ECM macromolecules may require specialized trafficking carriers and mechanisms because of their large size and complex assembly. Recent reports have revealed that large cargo, like procollagen, use a specialized combination of COPII components to build large transport carriers for ER exit. Others have shown that COMP (cartilage oligomeric matrix protein) /thrombospondin 5 traffic is integrated with secretion of other matrix proteins, such as collagen IX, which reveals potentially novel ways of packaging and selecting ECM cargo (Chen et al., 2004; Hecht et al., 2005; Posey et al., 2004)). Similar regulatory mechanisms may apply to cartilage ECM proteoglycan secretion as recently shown by genetic mutations disrupting proteoglycan synthesis pathways (Eames et al., 2010; Eames et al., 2011)
Thrombosponsins (TSP) are calcium-binding, glycosylated oligomeric ECM proteins that function in diverse cellular and physiological processes such as cell adhesion, tumor growth, platelet aggregation, and angiogenesis, as well as assembly of skeletal matrix (Adams & Lawler, 2004). TSPs exert many of these functions through interactions with other extracellular proteins (Adams & Lawler, 2011). Several mutations in TSP-5/COMP result in skeletal dysplasia, namely pseudoachondroplasia (PSACH) and multiple epiphysial dysplasia (EDM1) (Briggs et al, 1995; Briggs et al, 1998; Hecht et al, 2005; Hecht et al, 1995; Posey et al, 2004). Interestingly, chondrocytes expressing mutant COMP alleles retain not only COMP in the ER but also other ECM proteins, such as type IX collagen and matrilin-3 (Hecht et al, 2005; Hecht et al, 2004; Merritt et al, 2007). A model to explain this finding has been proposed in which mutant COMP may promote an interaction of type-IX collagen and matrilin-3 around a type-II collagen based core, which would otherwise form in ECM, resulting in an accumulation of several matrix components in the ER cisternae (Merritt et al, 2007). This study proposes a novel concept by suggesting that the intracellular trafficking of select ECM proteins may be facilitated by other secretory ECM molecules.
Concluding Remarks
Despite sustained interest, little is known about fundamental cargo selection mechanisms of the COPII inner coat, and numerous questions remain. For example, are Sec24 paralogs the only inner coat subunits that function in cargo selection, or are the Sec23 paralogs also capable of influencing cargo selection/traffic? Are all Sec23 and Sec24 paralogs present at stoichiometric levels within individual cells during embryonic development, tissue repair, and at homeostatic conditions? Alternatively, does transcriptional and post-translational regulation dynamically balance the relative levels of inner coat paralogs to meet secretory demand? What types of cargos use specific adaptors and in which tissues, and are the processes of cargo and adapter expression regulated coordinately or separately during cellular differentiation? Is there an overarching “secretory code” that could predict cargo-adaptor relationships, and could such understanding be used therapeutically as “druggable targets” to promote or suppress the secretion of factors involved in processes such as cancer metastasis and cellular differentiation? What are the functions of conserved and divergent domains in cargo adaptors, and do these domains confer cargo specificity directly or indirectly?
These unanswered questions have critical human health implications because the coordinated function of secretory pathway is essential for organ and cell physiology, particularly for the establishment of all basement membranes and extracellular matrices, as well as for influencing cell polarity, migration, adhesion, and the deployment of specific receptors and growth factors. All of these events are tightly regulated, and the precise availability of COPII components appears to be required to deliver these molecules in a coordinated spatiotemporal fashion. In summary, recent discoveries of secretory machinery functions in ECM transport using in vivo vertebrate model systems, as well as elegant in vitro and culture models, have created new paradigms for understanding secretory biology. Continued research will help to translate these principles into a therapeutic framework.
Acknowledgments
FUNDING
The authors were supported in part by the Zebrafish Initiative of the Vanderbilt University Academic Venture Capital Fund, the NIH NIDCR grant R01 DE018477 (E.W.K.), T32GM008554, the Cellular, Biochemical and Molecular Sciences Training Program (D.B.M.), NRSA F31DE022226 and T32HD007502 Training Program in Developmental Biology (D.S.L.), and the Vanderbilt International Scholar Program (GU).
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development. 2005;132:2743–58. doi: 10.1242/dev.01863. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–8. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–3. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, et al. The Sar1 Gtpase Coordinates Biosynthetic Cargo Selection with Endoplasmic Reticulum Export Site Assembly. J Cell Biol. 2001;152:213–30. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold WV, Fertala A. Skeletal diseases caused by mutations that affect collagen structure and function. Int J Biochem Cell Biol. 2013;45:1556–67. doi: 10.1016/j.biocel.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Baines AC, Adams EJ, Zhang B, Ginsburg D. Disruption of the sec24d gene results in early embryonic lethality in the mouse. PLoS One. 2013;8:e61114. doi: 10.1371/journal.pone.0061114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439:604–7. doi: 10.1038/nature04377. [DOI] [PubMed] [Google Scholar]
- Barlowe C. Molecular recognition of cargo by the COPII complex: a most accommodating coat. Cell. 2003;114:395–7. doi: 10.1016/s0092-8674(03)00650-0. [DOI] [PubMed] [Google Scholar]
- Barlowe C, d’Enfert C, Schekman R. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem. 1993;268:873–9. [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R. SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–9. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharya N, JOD, Stagg SM. The structure of the Sec13/31 COPII cage bound to Sec23. J Mol Biol. 2012;420:324–34. doi: 10.1016/j.jmb.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419:271–7. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13:635–45. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Fermo E, Vercellati C, Boschetti C, Barcellini W, Iurlo A, et al. Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat. 2009;30:1292–8. doi: 10.1002/humu.21077. [DOI] [PubMed] [Google Scholar]
- Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–24. doi: 10.1083/jcb.200509095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Mironov AA, Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, et al. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Fromme JC, Ben J, Chong SS, Nauta C, Hur DJ, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38:1192–7. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, Kim SD, Hata A, Haldeman-Englert C, Zackai EH, Naydenov C, et al. Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion. Clin Genet. 2011;80:169–76. doi: 10.1111/j.1399-0004.2010.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Postlethwait JH. Polyploidy in Fish and the Teleost Genome Duplication. In: Soltis PS, Soltis DE, editors. Polyploidy and Genome Evolution. Berlin Heidelberg: Springer-Verlag; 2012. pp. 341–83. [Google Scholar]
- Brandizzi F, Barlowe C. Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol. 2013;14:382–92. doi: 10.1038/nrm3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- Briggs MD, Mortier GR, Cole WG, King LM, Golik SS, Bonaventure J, et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am J Hum Genet. 1998;62:311–9. doi: 10.1086/301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Charcosset M, Sassolas A, Peretti N, Roy CC, Deslandres C, Sinnett D, et al. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol Genet Metab. 2008;93:74–84. doi: 10.1016/j.ymgme.2007.08.120. [DOI] [PubMed] [Google Scholar]
- Chen TL, Stevens JW, Cole WG, Hecht JT, Vertel BM. Cell-type specific trafficking of expressed mutant COMP in a cell culture model for PSACH. Matrix Biol. 2004;23:433–44. doi: 10.1016/j.matbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–38. [PMC free article] [PubMed] [Google Scholar]
- Clore JN, Cohen IK, Diegelmann RF. Quantitation of collagen types I and III during wound healing in rat skin. Proc Soc Exp Biol Med. 1979;161:337–40. doi: 10.3181/00379727-161-40548. [DOI] [PubMed] [Google Scholar]
- Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, et al. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–47. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Copic A, Latham CF, Horlbeck MA, D’Arcangelo JG, Miller EA. ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p. Science. 2012;335:1359–62. doi: 10.1126/science.1215909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho P, Parsons MJ, Thomas KA, Hirst EM, Saude L, Campos I, et al. Differential requirements for COPI transport during vertebrate early development. Dev Cell. 2004;7:547–58. doi: 10.1016/j.devcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Cutrona MB, Beznoussenko GV, Fusella A, Martella O, Moral P, Mironov AA. Silencing of mammalian Sar1 isoforms reveals COPII-independent protein sorting and transport. Traffic. 2013;14:691–708. doi: 10.1111/tra.12060. [DOI] [PubMed] [Google Scholar]
- Dannoura AH, Berriot-Varoqueaux N, Amati P, Abadie V, Verthier N, Schmitz J, et al. Anderson’s disease: exclusion of apolipoprotein and intracellular lipid transport genes. Arterioscler Thromb Vasc Biol. 1999;19:2494–508. doi: 10.1161/01.atv.19.10.2494. [DOI] [PubMed] [Google Scholar]
- Davis EE, Savage JH, Willer JR, Jiang YH, Angrist M, Androutsopoulos A, et al. Whole exome sequencing and functional studies identify an intronic mutation in TRAPPC2 that causes spondyloepiphyseal dysplasia tarda (SEDT) Clin Genet. 2013 doi: 10.1111/cge.12189. [DOI] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A. 2008;105:10762–7. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Eames BF, Singer A, Smith GA, Wood ZA, Yan YL, He X, et al. UDP xylose synthase 1 is required for morphogenesis and histogenesis of the craniofacial skeleton. Dev Biol. 2010;341:400–15. doi: 10.1016/j.ydbio.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eames BF, Yan YL, Swartz ME, Levic DS, Knapik EW, Postlethwait JH, et al. Mutations in fam20b and xylt1 reveal that cartilage matrix controls timing of endochondral ossification by inhibiting chondrocyte maturation. PLoS Genet. 2011;7:e1002246. doi: 10.1371/journal.pgen.1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol. 1995;131:311–24. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster D, Armbruster K, Luschnig S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol. 2010;20:62–8. doi: 10.1016/j.cub.2009.11.062. [DOI] [PubMed] [Google Scholar]
- Fox RM, Hanlon CD, Andrew DJ. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J Cell Biol. 2010;191:479–92. doi: 10.1083/jcb.201004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz-Wachtel M, Eisler SA, Krug K, Wahl S, Carpy A, Nordheim A, et al. Global detection of protein kinase D-dependent phosphorylation events in nocodazole-treated human cells. Mol Cell Proteomics. 2012;11:160–70. doi: 10.1074/mcp.M111.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme JC, Orci L, Schekman R. Coordination of COPII vesicle trafficking by Sec23. Trends Cell Biol. 2008;18:330–6. doi: 10.1016/j.tcb.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Fromme JC, Ravazzola M, Hamamoto S, Al-Balwi M, Eyaid W, Boyadjiev SA, et al. The genetic basis of a craniofacial disease provides insight into COPII coat assembly. Dev Cell. 2007;13:623–34. doi: 10.1016/j.devcel.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay S, Fietzek PP, Remberger K, Eder M, Kuhn K. Liver cirrhosis: immunofluorescence and biochemical studies demonstrate two types of collagen. Klin Wochenschr. 1975;53:205–8. doi: 10.1007/BF01468808. [DOI] [PubMed] [Google Scholar]
- Gay S, Muller PK, Lemmen C, Remberger K, Matzen K, Kuhn K. Immunohistological study on collagen in cartilage-bone metamorphosis and degenerative osteoarthrosis. Klin Wochenschr. 1976;54:969–76. doi: 10.1007/BF01468947. [DOI] [PubMed] [Google Scholar]
- Gedeon AK, Colley A, Jamieson R, Thompson EM, Rogers J, Sillence D, et al. Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat Genet. 1999;22:400–4. doi: 10.1038/11976. [DOI] [PubMed] [Google Scholar]
- Gedeon AK, Tiller GE, Le Merrer M, Heuertz S, Tranebjaerg L, Chitayat D, et al. The Molecular Basis of X-Linked Spondyloepiphyseal Dysplasia Tarda. Am J Hum Genet. 2001;68:1386–97. doi: 10.1086/320592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Dai F, Yu L, She X, Zhao Y, Jiang J, et al. Identification and characterization of nine novel human small GTPases showing variable expressions in liver cancer tissues. Gene Expr. 2002;10:231–42. doi: 10.3727/000000002783992406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JT, Hayes E, Haynes R, Cole WG. COMP mutations, chondrocyte function and cartilage matrix. Matrix Biol. 2005;23:525–33. doi: 10.1016/j.matbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Makitie O, Hayes E, Haynes R, Susic M, Montufar-Solis D, et al. Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. J Orthop Res. 2004;22:759–67. doi: 10.1016/j.orthres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hecht JT, Nelson LD, Crowder E, Wang Y, Elder FF, Harrison WR, et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat Genet. 1995;10:325–9. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- Heckel D, Brass N, Fischer U, Blin N, Steudel I, Tureci O, et al. cDNA cloning and chromosomal mapping of a predicted coiled-coil proline-rich protein immunogenic in meningioma patients. Hum Mol Genet. 1997;6:2031–41. doi: 10.1093/hmg/6.12.2031. [DOI] [PubMed] [Google Scholar]
- Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- Iolascon A, Esposito MR, Russo R. Clinical aspects and pathogenesis of congenital dyserythropoietic anemias: from morphology to molecular approach. Haematologica. 2012;97:1786–94. doi: 10.3324/haematol.2012.072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iolascon A, Russo R, Esposito MR, Asci R, Piscopo C, Perrotta S, et al. Molecular analysis of 42 patients with congenital dyserythropoietic anemia type II: new mutations in the SEC23B gene and a search for a genotype-phenotype relationship. Haematologica. 2010;95:708–15. doi: 10.3324/haematol.2009.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Bachinger HP. A molecular ensemble in the rER for procollagen maturation. Biochim Biophys Acta. 2013;1833:2479–91. doi: 10.1016/j.bbamcr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, et al. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–33. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Khoriaty R, Vasievich MP, Ginsburg D. The COPII pathway and hematologic disease. Blood. 2012;120:31–8. doi: 10.1182/blood-2012-01-292086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SD, Pahuja KB, Ravazzola M, Yoon J, Boyadjiev SA, Hammamoto S, et al. The [corrected] SEC23-SEC31 [corrected] interface plays critical role for export of procollagen from the endoplasmic reticulum. J Biol Chem. 2012;287:10134–44. doi: 10.1074/jbc.M111.283382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapik EW. ENU mutagenesis in zebrafish--from genes to complex diseases. Mamm Genome. 2000;11:511–9. doi: 10.1007/s003350010098. [DOI] [PubMed] [Google Scholar]
- Koreishi M, Yu S, Oda M, Honjo Y, Satoh A. CK2 phosphorylates Sec31 and regulates ER-To-Golgi trafficking. PLoS One. 2013;8:e54382. doi: 10.1371/journal.pone.0054382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, et al. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumkhaek C, Taylor JG, Zhu J, Hoppe C, Kato GJ, Rodgers GP. Fetal haemoglobin response to hydroxycarbamide treatment and sar1a promoter polymorphisms in sickle cell anaemia. Br J Haematol. 2008;141:254–9. doi: 10.1111/j.1365-2141.2008.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet. 2006;38:1198–203. doi: 10.1038/ng1880. [DOI] [PubMed] [Google Scholar]
- Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biol. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Jacob C, Andre B. K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol. 2009;185:493–502. doi: 10.1083/jcb.200810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld J, Wang Q, Zohlman A, Salvarezza S, Morgan S, Ren J, et al. Protein kinase C delta regulates the release of collagen type I from vascular smooth muscle cells via regulation of Cdc42. Mol Biol Cell. 2012;23:1955–63. doi: 10.1091/mbc.E11-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–30. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Loftus AF, Hsieh VL, Parthasarathy R. Modulation of membrane rigidity by the human vesicle trafficking proteins Sar1A and Sar1B. Biochem Biophys Res Commun. 2012;426:585–9. doi: 10.1016/j.bbrc.2012.08.131. [DOI] [PubMed] [Google Scholar]
- Lui WO, Zeng L, Rehrmann V, Deshpande S, Tretiakova M, Kaplan EL, et al. CREB3L2-PPARgamma fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res. 2008;68:7156–64. doi: 10.1158/0008-5472.CAN-08-1085. [DOI] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. Embo j. 2008;27:2918–28. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Yasui N, Ozono K, Yamagata M, Kawabata H, Yoshikawa H. Loss of the SEDL gene product (Sedlin) causes X-linked spondyloepiphyseal dysplasia tarda: Identification of a molecular defect in a Japanese family. Am J Med Genet. 2001;99:328–30. doi: 10.1002/ajmg.1179. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–75. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- Melville DB, Knapik EW. Traffic jams in fish bones: ER-to-Golgi protein transport during zebrafish development. Cell Adh Migr. 2011;5:114–8. doi: 10.4161/cam.5.2.14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville DB, Montero-Balaguer M, Levic DS, Bradley K, Smith JR, Hatzopoulos AK, et al. The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Dis Model Mech. 2011;4:763–76. doi: 10.1242/dmm.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt TM, Bick R, Poindexter BJ, Alcorn JL, Hecht JT. Unique Matrix Structure in the Rough Endoplasmic Reticulum Cisternae of Pseudoachondroplasia Chondrocytes. Am J Pathol. 2007;170:293–300. doi: 10.2353/ajpath.2007.060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, et al. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol. 2010;12:41–6. doi: 10.1038/ncb2002. sup pp 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Antonny B, Hamamoto S, Schekman R. Cargo selection into COPII vesicles is driven by the Sec24p subunit. Embo j. 2002;21:6105–13. doi: 10.1093/emboj/cdf605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, et al. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- Mumm S, Christie PT, Finnegan P, Jones J, Dixon PH, Pannett AA, et al. A five-base pair deletion in the sedlin gene causes spondyloepiphyseal dysplasia tarda in a six-generation Arkansas kindred. J Clin Endocrinol Metab. 2000;85:3343–7. doi: 10.1210/jcem.85.9.6840. [DOI] [PubMed] [Google Scholar]
- Mumm S, Zhang X, Vacca M, D’Esposito M, Whyte MP. The sedlin gene for spondyloepiphyseal dysplasia tarda escapes X-inactivation and contains a non-canonical splice site. Gene. 2001;273:285–93. doi: 10.1016/s0378-1119(01)00571-6. [DOI] [PubMed] [Google Scholar]
- Nakano A, Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989;109:2677–91. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, et al. Mutations affecting craniofacial development in zebrafish. Development. 1996;123:357–67. doi: 10.1242/dev.123.1.357. [DOI] [PubMed] [Google Scholar]
- Niu X, Gao C, Jan Lo L, Luo Y, Meng C, Hong J, et al. Sec13 safeguards the integrity of the endoplasmic reticulum and organogenesis of the digestive system in zebrafish. Dev Biol. 2012;367:197–207. doi: 10.1016/j.ydbio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Norum M, Tang E, Chavoshi T, Schwarz H, Linke D, Uv A, et al. Trafficking through COPII Stabilises Cell Polarity and Drives Secretion during Drosophila Epidermal Differentiation. PLoS One. 2010:5. doi: 10.1371/journal.pone.0010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–15. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ohisa S, Inohaya K, Takano Y, Kudo A. sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev Biol. 2010;342:85–95. doi: 10.1016/j.ydbio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Paccaud JP, Reith W, Carpentier JL, Ravazzola M, Amherdt M, Schekman R, et al. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol Biol Cell. 1996;7:1535–46. doi: 10.1091/mbc.7.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos I, Moller E, Dahlen A, Isaksson M, Mandahl N, Vlamis-Gardikas A, et al. Characterization of the native CREB3L2 transcription factor and the FUS/CREB3L2 chimera. Genes Chromosomes Cancer. 2007;46:181–91. doi: 10.1002/gcc.20395. [DOI] [PubMed] [Google Scholar]
- Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–90. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS. Mechanism of Constitutive Export from the Golgi: Bulk Flow via the Formation, Protrusion, and En Bloc Cleavage of large trans-Golgi Network Tubular Domains. Mol Biol Cell. 2003;14:4470–85. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk RS, Capestrano M, Polishchuk EV. Shaping tubular carriers for intracellular membrane transport. FEBS Lett. 2009;583:3847–56. doi: 10.1016/j.febslet.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Posey KL, Hayes E, Haynes R, Hecht JT. Role of TSP-5/COMP in pseudoachondroplasia. Int J Biochem Cell Biol. 2004;36:1005–12. doi: 10.1016/j.biocel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Reddi AH, Gay R, Gay S, Miller EJ. Transitions in collagen types during matrix-induced cartilage, bone, and bone marrow formation. Proc Natl Acad Sci U S A. 1977;74:5589–92. doi: 10.1073/pnas.74.12.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Esposito MR, Asci R, Gambale A, Perrotta S, Ramenghi U, et al. Mutational spectrum in congenital dyserythropoietic anemia type II: identification of 19 novel variants in SEC23B gene. Am J Hematol. 2010;85:915–20. doi: 10.1002/ajh.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009a;11:1197–204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009b;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, et al. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22:2301–8. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman RW. Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell. 1997;8:205–17. doi: 10.1091/mbc.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS One. 2010;5:e10367. doi: 10.1371/journal.pone.0010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Cavodeassi F, Feng Y, Stephens DJ. Early stages of retinal development depend on Sec13 function. Biol Open. 2013;2:256–66. doi: 10.1242/bio.20133251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Iolascon A, Verissimo F, Trede NS, Horsley W, Chen W, et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet. 2009;41:936–40. doi: 10.1038/ng.405. [DOI] [PubMed] [Google Scholar]
- Sharpe LJ, Luu W, Brown AJ. Akt phosphorylates Sec24: new clues into the regulation of ER-to-Golgi trafficking. Traffic. 2011;12:19–27. doi: 10.1111/j.1600-0854.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem. 1997;272:25413–6. doi: 10.1074/jbc.272.41.25413. [DOI] [PubMed] [Google Scholar]
- Shoulders CC, Stephens DJ, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opin Lipidol. 2004;15:191–7. doi: 10.1097/00041433-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Shugrue CA, Kolen ER, Peters H, Czernik A, Kaiser C, Matovcik L, et al. Identification of the putative mammalian orthologue of Sec31P, a component of the COPII coat. J Cell Sci. 1999;112 (Pt 24):4547–56. doi: 10.1242/jcs.112.24.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi S, Siddiqi SA, Mansbach CM., 2nd Sec24C is required for docking the prechylomicron transport vesicle with the Golgi. J Lipid Res. 2010;51:1093–100. doi: 10.1194/jlr.M002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–8. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- Stankewich MC, Stabach PR, Morrow JS. Human Sec31B: a family of new mammalian orthologues of yeast Sec31p that associate with the COPII coat. J Cell Sci. 2006;119:958–69. doi: 10.1242/jcs.02751. [DOI] [PubMed] [Google Scholar]
- Stephens DJ. CELL BIOLOGY: Collagen secretion explained. Nature. 2012;482:474–5. doi: 10.1038/482474a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DJ, Pepperkok R. Imaging of procollagen transport reveals COPI-dependent cargo sorting during ER-to-Golgi transport in mammalian cells. J Cell Sci. 2002;115:1149–60. doi: 10.1242/jcs.115.6.1149. [DOI] [PubMed] [Google Scholar]
- Sucic S, El-Kasaby A, Kudlacek O, Sarker S, Sitte HH, Marin P, et al. The serotonin transporter is an exclusive client of the coat protein complex II (COPII) component SEC24C. J Biol Chem. 2011;286:16482–90. doi: 10.1074/jbc.M111.230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Yang-Feng TL, Liu W, Gieser L, Barrow LL, Chen KC, et al. Molecular characterization of a novel human gene, SEC13R, related to the yeast secretory pathway gene SEC13, and mapping to a conserved linkage group on human chromosome 3p24-p25 and mouse chromosome 6. Hum Mol Genet. 1994;3:1281–6. doi: 10.1093/hmg/3.8.1281. [DOI] [PubMed] [Google Scholar]
- Szul T, Sztul E. COPII and COPI traffic at the ER-Golgi interface. Physiology (Bethesda) 2011;26:348–64. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Zhao H, Rebbert ML, Dawid IB. Coordinated activation of the secretory pathway during notochord formation in the Xenopus embryo. Development. 2009;136:3543–8. doi: 10.1242/dev.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Kausalya J, Low DY, Lock ML, Hong W. A family of mammalian proteins homologous to yeast Sec24p. Biochem Biophys Res Commun. 1999;258:679–84. doi: 10.1006/bbrc.1999.0574. [DOI] [PubMed] [Google Scholar]
- Tang BL, Zhang T, Low DY, Wong ET, Horstmann H, Hong W. Mammalian homologues of yeast sec31p. An ubiquitously expressed form is localized to endoplasmic reticulum (ER) exit sites and is essential for ER-Golgi transport. J Biol Chem. 2000;275:13597–604. doi: 10.1074/jbc.275.18.13597. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhu M, Wang H, Afelik S, Vasievich MP, Chen XW, et al. SEC23B is required for the maintenance of murine professional secretory tissues. Proc Natl Acad Sci U S A. 2012;109:E2001–9. doi: 10.1073/pnas.1209207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- Townley AK, Feng Y, Schmidt K, Carter DA, Porter R, Verkade P, et al. Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci. 2008;121:3025–34. doi: 10.1242/jcs.031070. [DOI] [PubMed] [Google Scholar]
- Townley AK, Schmidt K, Hodgson L, Stephens DJ. Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion. J Cell Sci. 2012;125:673–84. doi: 10.1242/jcs.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacaru AM, Unlu G, Spitzner M, Mione M, Knapik EW, Sadler KC. In vivo cell biology using zebrafish provides insight into development and disease. J Cell Sci. 2013 doi: 10.1242/jcs.140194. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, et al. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337:1668–72. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa R, Kaul SC, Komatsu Y, Ikawa Y, Sarai A, Sugimoto Y. Identification and differential expression of yeast SEC23-related gene (Msec23) in mouse tissues. FEBS Lett. 1993;315:193–6. doi: 10.1016/0014-5793(93)81161-r. [DOI] [PubMed] [Google Scholar]
- Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–73. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–87. doi: 10.1111/j.1600-0854.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep. 2007;8:258–64. doi: 10.1038/sj.embor.7400893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Cantrell VA, Mundell NA, Bennett AC, Quick RE, Jessen JR. VANGL2 regulates membrane trafficking of MMP14 to control cell polarity and migration. J Cell Sci. 2012a;125:2141–7. doi: 10.1242/jcs.097964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Mundell N, Dunlap J, Jessen J. The planar cell polarity protein VANGL2 coordinates remodeling of the extracellular matrix. Commun Integr Biol. 2012b:325–8. doi: 10.4161/cib.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DG, Phamluong K, Li L, Sun M, Cao TC, Liu PS, et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol. 2011;193:935–51. doi: 10.1083/jcb.201007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Zhou XY, Wang QQ, Li H, Chen Y, Lei YP, et al. Mutations in the COPII Vesicle Component Gene SEC24B are Associated with Human Neural Tube Defects. Hum Mutat. 2013 doi: 10.1002/humu.22338. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–8. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]