Abstract
We previously reported that 18F-fluorodeoxyglucose positron emission tomography scan (FDG-PET) is almost universally positive in patients with T cell lymphoma. In the present analysis we examined the impact of FDG-PET on the initial staging of peripheral T cell lymphomas (PTCLs), and the prognostic value of interim FDG-PET. This retrospective analysis identified patients with mature T or natural killer (NK) lymphomas who had PET scans as part of initial staging or staging at relapse [(n = 95) (staging cohort)] in the PTCL database at Memorial Sloan-Kettering Cancer Center. A subset of these patients had repeat PET for interim restaging during initial therapy with curative intent [(n = 50) (interim restaging cohort)]. The frequency of specific T cell histologies included in this analysis were: PTCL not otherwise specified (NOS) (n = 35); angioimmunoblastic T cell lymphoma (AITL) (n = 17); anaplastic large cell lymphoma (ALCL), ALK-1+ (n = 11) and ALK-1− (n = 12); adult T cell lymphoma/leukemia (ATLL) (n = 7); NK/T cell lymphoma (NKTCL) (n = 10); and enteropathy-associated T cell lymphoma (EATL) (n = 3). In the staging cohort, 77 patients were newly diagnosed, and 18 had relapsed disease. Pretreatment FDG-PET was positive in 96% of patients. PET identified additional disease sites in 47/95 patients (50%) when added to conventional staging. Most frequently identified additional sites were: other nodal (n = 24); bone (n = 10); skin (n = 8); nasopharynx (n = 4); spleen (n = 3); and lung (n = 2). However, FDG-PET modified computed tomography (CT)-based staging in only 5/95 patients (5.2%): two patients were upstaged and three patients were downstaged. FDG-PET-based staging did not alter planned treatment for any patient. Interim restaging with PET was performed after a median of 4 cycles of chemotherapy. In this cohort, treatment regimens included cyclophosphamide, doxorubicin, vincristine and prednisone CHOP (n = 19); CHOP/ifosfamide, carboplatin and etoposide (ICE) (n = 26); and other (n = 7). Subsequently, 29 patients were consolidated with either autologous (n = 22) or allogeneic (n = 7) stem cell transplant. After a median follow-up of 3.4 years for surviving patients, those with negative interim PET had superior progression-free survival (PFS) compared to patients with positive interim PET (p = 0.03). There were no differences in overall survival (OS). In PTCL, FDG-PET commonly identifies additional sites of disease but infrequently impacts CT-based staging and does not influence therapy. Interim FDG-PET may predict for PFS. FDG-PET should be integrated into prospective trials to confirm these findings.
Keywords: Lymphoma and Hodgkin disease, FDG-PET, T cell lymphoma, prognosis, staging
Introduction
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has been found to be effective for initial staging and response evaluation in Hodgkin lymphoma and diffuse large B cell lymphoma [1–5]. The role of FDG-PET has been less well defined in other subtypes of lymphoma. The published experience in peripheral T cell lymphoma (PTCL) reports variable FDG avidity in this disease, depending on T cell subtype [6–13]. We previously reported that 90% of patients with PTCL had FDG-avid disease; FDG-avidity was detected throughout a range of T cell histologies [14].
Apart from its role in staging, FDG-PET may also be useful in the mid-treatment evaluation of patients with aggressive NHL [15 – 18]. In diffuse large B-cell lymphoma (DLBCL), a negative interim FDG-PET after two to four cycles of chemotherapy is predictive of a favorable outcome [16–19]. In contrast, the prognostic value of a positive interim FDG-PET is more ambiguous. Early studies suggested that an interim PET scan was strongly associated with failure to achieve or sustain a continuous complete remission (CR) [20]. More recent studies have suggested that a portion of patients with interim positive scans have favorable long-term outcomes. Our group has demonstrated a high false positive rate (when positive findings were biopsied for confirmation) for interim FDG-PET that did not correlate with outcome [21]. In PTCL, the ability of FDG-PET to influence prognosis remains unclear [2]. Most studies evaluating interim PET have included small numbers of patients with PTCL, although a recent analysis of 54 patients with PTCL suggested that a negative interim or post-therapy FDG-PET did not result in improved outcomes: overall survival (OS) was 76% for patients with a negative interim FDG-PET compared to 47% for patients with a positive interim FDG-PET (p = 0.16).
Overall, there remains some ambiguity regarding the role of FDG-PET in staging and response assessment of PTCL. If FDG-PET is shown to be an early indicator of tumor chemo-sensitivity, it could be used to tailor therapeutic strategies. This retrospective analysis examined the utility of FDG-PET in the initial staging for patients with previously untreated or relapsed PTCL, and aimed to assess its value at interim evaluation for a subset of patients treated with curative intent.
Methods
We retrospectively reviewed the PTCL database at Memorial Sloan-Kettering Cancer Center and identified 95 patients with histologically proven mature T-cell or natural killer (NK) lymphomas who underwent FDG-PET as part of initial staging or staging at relapse [(staging cohort) (n = 95)]. For this study, 90.5% of FDG-PET scans (86/95) underwent independent repeat review by a nuclear medicine physician without knowledge of the patient’s clinical outcome. A subset of these patients underwent repeat FDG-PET for interim restaging while being treated with initial therapy for curative intent [(n = 50) (interim restaging cohort)]. All patients in this subset had newly diagnosed disease.
Staging
Patients were staged based on the Ann Arbor system, using helical computed tomography (CT) scan of the chest, abdomen and pelvis, physical examination and bone marrow biopsy. CT scans of the neck were not routinely performed. Staging FDG-PET scans were performed on state of the art PET/CT systems prior to the initiation of treatment at initial diagnosis or relapse. Interim FDG-PET scans were performed after 2–4 cycles of therapy. Patients were treated at the discretion of the treating attending.
A negative interim scan was defined as FDG uptake less than or equal to liver uptake at any site of FDG-positive disease identified in the baseline study. A positive scan was defined as any FDG uptake greater than liver background activity with a corresponding structural abnormality on CT scan [22].
Statistical analysis
Progression-free survival (PFS) was defined as the time interval from the end of therapy to progression of disease or date of death. Overall survival was defined as the time between diagnosis and the last day of follow-up or date of death. Survival curves were calculated using SPSS software by the Kaplan–Meier method and comparison between groups was performed by the log-rank test. Correlations were analyzed by χ2.
Results
Patient characteristics
We identified 95 patients with histologically proven mature T-cell or NK lymphoma treated at Memorial Sloan-Kettering Cancer Center between October 2001 and April 2010 who had undergone FDG-PET and diagnostic CT (covering at least chest, abdomen and pelvis) at disease baseline or at disease relapse (Table I). Sixty one percent were male, and 39% were female. Patients with primary cutaneous T-cell lymphomas were excluded. Fifty of these 95 patients with newly diagnosed disease also underwent interim (or repeat) restaging with FDG-PET as part of treatment administered with curative intent (Table I). Median age was 54 (range: 24–89).
Table I.
Patient characteristics.
| Characteristic | No. | % |
|---|---|---|
| Staging cohort | 95 | |
| Histology | ||
| PTCL, NOS | 35 | 36.8 |
| AITL | 17 | 18 |
| ALCL, ALK− or unknown | 12 | 12.6 |
| ALCL, ALK+ | 11 | 11.5 |
| NK/T cell lymphoma | 10 | 10.5 |
| ATLL | 7 | 7.3 |
| EATL | 3 | 3.1 |
| Disease state | ||
| Initial diagnosis | 77 | 81 |
| Relapsed disease | 18 | 19 |
| Gender | ||
| Male | 58 | 61 |
| Female | 37 | 39 |
| Interim restaging cohort | 50 | |
| Histology | ||
| PTCL-NOS | 19 | 38 |
| ALCL, ALK+ | 9 | 18 |
| NK/T cell lymphoma | 8 | 16 |
| AITL | 6 | 12 |
| ALCL, ALK− or unknown | 5 | 10 |
| EATL | 2 | 4 |
| ATLL | 1 | 1.42 |
| Treatment | ||
| Initial chemotherapy | ||
| CHOP | 19 | 38 |
| CHOP/ICE | 24 | 48 |
| Other* | 7 | 14 |
| Consolidative treatment | ||
| Autologous stem cell transplant | 22 | 44 |
| Allogeneic stem cell transplant | 7 | 14 |
PTCL, NOS, peripheral T cell lymphoma, not otherwise specified; AITL, angioimmunoblastic T cell lymphoma; ALCL, ALK−, anaplastic large cell lymphoma, anaplastic lymphoma kinase negative or unknown; ALCL, ALK+, anaplastic large cell lymphoma, anaplastic lymphoma kinase positive; NK/T cell lymphoma, natural killer/T cell lymphoma; ATLL, adult T cell leukemia/lymphoma, HTLV-1 associated; EATL, enteropathy associated T cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ICE, ifosfamide, carboplatin, etoposide.
Includes VACOP (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin, n = 1), cyclophosphamide, pentostatin (n = 1), GND (gemcitabine, vinorelbine, doxorubicin, n = 1), IVAC (ifosfamide, etoposide, cytarabine, n = 1), SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide, n = 2), ICE/CBV (ifosfamide, carboplatin, etoposide/cyclophosphamide, carmustine, etoposide, n = 1).
Initial staging and new sites of disease
FDG-PET was positive at initial staging in 96% (91/95) of patients. New nodal and extranodal disease sites were identified in 50% (47/95) of patients. Since FDG-PET scan routinely images from the base of the skull, sites in the neck and supraclavicular regions are included in the imaging. Since CT of the neck is not routinely used in staging (clinical evaluation is done), the most frequently found additional nodal sites were in the neck (11/95) and supraclavicular area (4/95) (Table II). The most frequently identified extranodal site of disease was bone (10/95) (Table II). Bony sites of disease were identified radiographically but were not confirmed by biopsy. All sites of disease identified in the head and neck (neck, n = 11; supraclavicular, n = 4; nasopharyngeal, n = 4; tonsil, n = 1; parotid, n = 1; base of tongue, n = 1) were noted because the FDG-PET examination routinely covers the body from the skull base to the mid-thigh. FDG-PET also documented epiglottic disease in one patient that was later found to be a primary squamous cell carcinoma.
Table II.
New sites of disease identified by FDG-PET.
| New disease site | No. | % |
|---|---|---|
| Nodal | ||
| Neck | 11 | 11.5 |
| Supraclavicular | 4 | 4.2 |
| Axillae | 3 | 3.2 |
| Paratracheal | 3 | 3.2 |
| Epitrochlear/iliac/inguinal | 3 | 3.2 |
| Abdominal | 2 | 2.1 |
| Extranodal | ||
| Bone | 10 | 10.5 |
| Skin | 8 | 8.5 |
| Lung/liver | 5 | 5.2 |
| Spleen | 3 | 3.2 |
| Breast | 2 | 2.1 |
| Soft tissue | 2 | 2.1 |
| Nasopharynx | 4 | 4.2 |
| Parotid | 1 | 1 |
| Adrenal | 1 | 1 |
| Tonsil | 1 | 1 |
| Base of tongue | 1 | 1 |
FDG-PET, 18F-fluorodeoxyglucose positron emission tomography.
Alteration of stage
FDG-PET would have altered the clinical stage in 5.2% of patients (5/95) as compared to CT. Two patients were up-staged (stage I increased to stage III; stage II increased to stage IV), while three patients were down-staged. The change in stage did not result in any treatment alterations, largely due to the use of combination chemotherapy regardless of stage, and short course chemotherapy was not performed (Table III).
Table III.
Change in stage based on FDG-PET.
| Stage change | Reason |
|---|---|
| Upstage | |
| 1. Stage I → III | Axillary, inguinal lymph nodes detected |
| 2. Stage II → IV | Nasopharynx, tonsil lesions detected |
| Downstage | |
| 1. Stage IV → III | Lung lesions not detected by FDG-PET |
| 2. Stage IV → III | Lung lesions not detected by FDG-PET |
| 3. Stage III → 0 | No FDG uptake in neoplastic lesions |
FDG-PET, 18F-fluorodeoxyglucose positron emission tomography.
Interim restaging
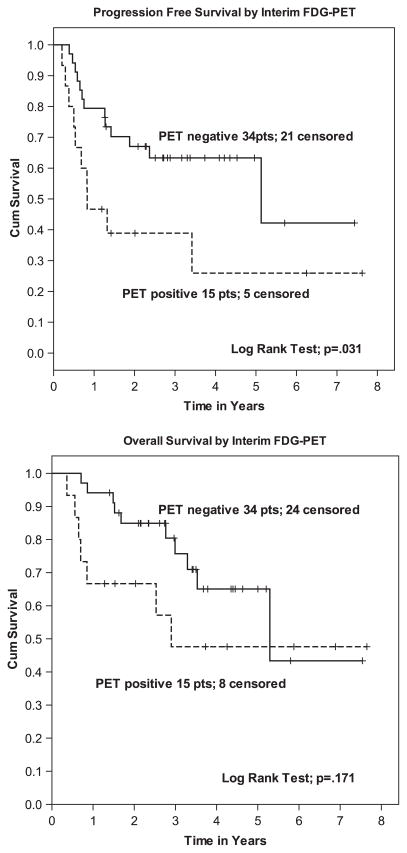
After a median of four cycles of chemotherapy (range 2–10), 50 of the 95 patients underwent interim PET. Most frequent histologies for patients undergoing interim FDG-PET were PTCL, not otherwise specified (NOS) (n = 19), angioimmunoblastic T cell lymphoma (AITL) (n = 6), anaplastic large cell lymphoma (ALCL) ALK+ (n = 9) and ALCL ALK− or unknown (n = 5). Fifteen patients had positive interim FDG-PET scans, 34 had negative FDG-PET scans and one patient had an equivocal scan. At median follow-up for surviving patients of 3.4 years, patients with negative interim PET had superior PFS of 5.1 years as compared to 10 months of patients with positive interim PET (p = 0.03) (Figure 1). Sixty-three percent of patients with negative interim PET were alive at median follow-up vs. 26% of those with positive interim PET; however, this difference was not statistically significant (p = 0.171) (Figure 1). When comparing results of interim PET positivity when PET was performed after ≥ 4 cycles of chemotherapy vs. < 4 cycles, there was no difference in PFS (p = 0.47 by Fisher’s exact test). For patients undergoing autologous (ASCT) or allogeneic stem cell transplant (Allo), median OS and PFS were not reached.
Figure 1.
Progression-free and overall survival based on interim FDG-PET.
Eight out of 15 patients with positive interim PET (53%) remained alive at last follow-up. These patients went on to receive further therapy: three patients received an allogeneic transplant, one patient underwent ASCT, two patients received salvage treatment (one with cyclophosphamide, etoposide, procarbazine, prednisone [CEPP] followed by romidepsin, the other with gemcitabine, cyclophosphamide and etoposide). Two patients remain alive and well without disease.
Univariate analysis
In order to explore the correlation between other factors and interim FDG-PET results, we evaluated whether the prognostic index for T-cell lymphoma score (PIT), International Prognostic Index (IPI) or transplant status influenced the ability to achieve a negative interim FDG-PET. By a Pearson χ2 test, it was found that interim FDG-PET status was not associated with PIT score, IPI score or further consolidative treatment with ASCT or Allo (p = 0.415, 0.356, 0.260, respectively.) We found no associations between any histologic subtype and interim PET positivity (p = 0.75 by Fisher’s exact test). Specifically, when evaluating patients with ALK+ ALCL compared with all other histologies, there was no difference in survival (p = 0.81).
Discussion
In this retrospective analysis, FDG-PET demonstrated disease avidity in 96% of patients at the time of initial staging. FDG-PET consistently identified more extranodal disease compared to contrast-enhanced CT scan. Though clinical stage would have been altered in 5.2% of patients, this did not lead to changes in planned management. More importantly, we found that interim FDG-PET provides prognostic information in PTCL, which provides a rationale for future studies investigating whether changes in management might improve the outcome of these patients.
FGD-PET in staging of PTCL
FDG-PET has been shown to alter stage in approximately 20% of patients with lymphoma (largely Hodgkin lymphoma [HL] or DLBCL), accompanied by alteration in management in up to 15% of patients [2,23]. In our series of PTCL, FDG-PET identified new disease sites in 50% of patients (47/95). This is consistent with our previously reported data [14]. Although clinical stage would have been altered in only 5.2% of patients, with no change in treatment, the more accurate description of the extent of disease may prove useful in baseline evaluation, follow-up and response assessment. The most sites of disease shown only on FDG-PET involved lymph nodes in the neck, and extranodal disease in bone. The increased identification of cervical nodes is largely secondary to the clinical rather than radiographic evaluation of the neck in patients not participating in clinical trials at our institution. This is similar to Bishu et al., who reported that PET had excellent sensitivity at nodal and non-cutaneous extranodal sites, and detected more osseous sites than did CT [11].
Additional cutaneous sites of disease were noted in 8.4% of patients. Previous studies noted variable performance of FDG-PET in detecting cutaneous lesions [8,13,24]. The patients included in the present series did not include those with cutaneous T-cell lymphoma; hence, these represent additional cutaneous sites of systemic disease.
Interim FDG-PET in PTCL
In this dataset, interim FDG-PET had significant prognostic value, and the ability to achieve a negative interim FDG-PET was an important predictor of PFS in patients with PTCL. However, this did not translate into a benefit in OS, likely because definitive curative therapy does not exist for this cohort of patients. Therefore, OS may not have been improved because of late relapse. Several studies have confirmed the utility of interim FDG-PET as a prognostic biomarker in DLBCL and HL (reviewed in references [1] and [2]); however, response assessment with FDG-PET for PTCL remains largely unexplored. A recent study by Cahu et al. [25] evaluated interim FDG-PET in 54 patients with PTCL. Using a three-point scale (1 = low, 2 = moderate and 3 = high), they found no significant differences in 4-year OS between patients with negative vs. positive interim PET. OS was 76% for patients with a negative interim FDG-PET compared to 47% for patients with a positive interim FDG-PET (p = 0.16). Using the same method for PFS assessment, the 4-year PFS estimate was 69% for patients with a negative interim FDG-PET and 49% for patients with a positive interim FDG-PET, respectively (p = 0.10) [25]. This differs from our findings, which demonstrate that assessment of metabolic activity by FDG-PET at interim restaging in PTCL can predict outcome. Of note, to our knowledge, ours is the first study to use a five-point scale to grade FDG uptake in interim scans, and dichotomized findings as either positive (uptake higher than reference liver background) or negative (uptake less than liver background). This may explain some of the differences between the two studies. Our data justify further exploration of interim FDG-PET in PTCL [16,17,22,26].
As a retrospective study, ours has several limitations. One limitation of our study is the small number of patients with positive interim FDG-PET (n = 15). This partially reflects physician choice in omitting PET scans in patients with clinical evidence of disease progression or lack of improvement while on chemotherapy administered with curative intent. Consequently our analysis may be underpowered to detect a broader difference between positive and negative interim scans, which may have contributed to our inability to detect a difference in OS between patients whose interim FDG-PET was positive or negative (p = 0.171). This is further confounded by the inability to cure many patients with T-cell lymphomas even with current intensive chemotherapy approaches. However, as treatment paradigms change with the integration of new therapy, this may differ. Perhaps for the same reason, PIT or IPI scores did not segregate patients in any way and did not affect the achievement of negative interim FDG-PET. Differences based on IPI or PIT may emerge in larger datasets. Additionally, patients with negative interim FDG-PET were more likely to receive consolidative therapy, which may have contributed to their superior PFS and OS and confounds the interpretation of outcome as solely reflective of FDG-PET status. Finally, the majority of our initial therapy programs for PTCL include planned high dose therapy in first remission. As a result, patients with negative interim PET were more likely to receive consolidation with high dose therapy as compared to those with persistent positive interim PET and less responsive disease, 77% (17/22) vs. 22% (2/9). Another limitation of our study is that a significant portion of the new lesions found on FDG-PET were detected in the head and neck area, where CT of the neck was not performed. However in the National Comprehensive Cancer Center (NCCN) guidelines, CT of the neck in staging of TCL is recommended only under certain circumstances. This underscores the point that if neck disease is clinically underappreciated, FDG-PET may be a useful tool in identifying these additional areas of disease involvement.
Our results show value in including FDG-PET at initial staging in PTCL as it detected additional sites of disease in 50% of patients, despite a small alteration in stage. More importantly, we believe that FDG-PET is useful in interim restaging in patients with PTCL to predict outcome. Our findings support early stratification with FDG-PET to propose a more risk-adapted approach in treating PTCL. Identifying high-risk patients with this imaging modality could lead to the earlier implementation of alternative and novel therapies to improve patient outcomes.
Supplementary Material
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Zelenetz AD, Abramson JS, Advani RH, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin’s lymphomas. J Natl Compr Canc Netw. 2010;8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 2.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29:1844–1854. doi: 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 3.Terasawa T, Lau J, Bardet S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27:1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2. 2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:589–597. doi: 10.6004/jnccn.2012.0061. [DOI] [PubMed] [Google Scholar]
- 6.Wu HB, Wang QS, Wang MF, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun. 2010;31:195–200. doi: 10.1097/MNM.0b013e32833310fa. [DOI] [PubMed] [Google Scholar]
- 7.Khong PL, Pang CB, Liang R, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87:613–621. doi: 10.1007/s00277-008-0494-8. [DOI] [PubMed] [Google Scholar]
- 8.Kako S, Izutsu K, Ota Y, et al. FDG-PET in T-cell and NK-cell neoplasms. Ann Oncol. 2007;18:1685–1690. doi: 10.1093/annonc/mdm265. [DOI] [PubMed] [Google Scholar]
- 9.Karantanis D, Subramaniam RM, Peller PJ, et al. The value of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography in extranodal natural killer/T-cell lymphoma. Clin Lymphoma Myeloma. 2008;8:94–99. doi: 10.3816/CLM.2008.n.010. [DOI] [PubMed] [Google Scholar]
- 10.Suh C, Kang YK, Roh JL, et al. Prognostic value of tumor 18F-FDG uptake in patients with untreated extranodal natural killer/T-cell lymphomas of the head and neck. J Nucl Med. 2008;49:1783–1789. doi: 10.2967/jnumed.108.053355. [DOI] [PubMed] [Google Scholar]
- 11.Bishu S, Quigley JM, Schmitz J, et al. F-18-fluoro-deoxy-glucose positron emission tomography in the assessment of peripheral T-cell lymphomas. Leuk Lymphoma. 2007;48:1531–1538. doi: 10.1080/10428190701344915. [DOI] [PubMed] [Google Scholar]
- 12.Storto G, Di Giorgio E, De Renzo A, et al. Assessment of metabolic activity by PET-CT with F-18-FDG in patients with T-cell lymphoma. Br J Haematol. 2010;151:195–197. doi: 10.1111/j.1365-2141.2010.08335.x. [DOI] [PubMed] [Google Scholar]
- 13.Otero HJ, Jagannathan JP, Prevedello LM, et al. CT and PET/CT findings of T-cell lymphoma. AJR Am J Roentgenol. 2009;193:349–358. doi: 10.2214/AJR.08.1398. [DOI] [PubMed] [Google Scholar]
- 14.Feeney J, Horwitz S, Gonen M, et al. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195:333–340. doi: 10.2214/AJR.09.3665. [DOI] [PubMed] [Google Scholar]
- 15.Jerusalem G, Beguin Y, Fassotte MF, et al. Persistent tumor 18F-FDG uptake after a few cycles of polychemotherapy is predictive of treatment failure in non-Hodgkin’s lymphoma. Haematologica. 2000;85:613–618. [PubMed] [Google Scholar]
- 16.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–1363. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 17.Haioun C, Itti E, Rahmouni A, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 18.Mikhaeel NG, Hutchings M, Fields PA, et al. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 19.Safar V, Dupuis J, Itti E, et al. Interim [18F]fluorodeoxyglucose positron emission tomography scan in diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy plus rituximab. J Clin Oncol. 2012;30:184–190. doi: 10.1200/JCO.2011.38.2648. [DOI] [PubMed] [Google Scholar]
- 20.Zinzani PL, Gandolfi L, Broccoli A, et al. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer. 2011;117:1010–1018. doi: 10.1002/cncr.25579. [DOI] [PubMed] [Google Scholar]
- 21.Moskowitz CH, Schoder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28:1896–1903. doi: 10.1200/JCO.2009.26.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meignan M, Gallamini A, Itti E, et al. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leuk Lymphoma. 2012;53:1876–1881. doi: 10.3109/10428194.2012.677535. [DOI] [PubMed] [Google Scholar]
- 23.Partridge S, Timothy A, O’Doherty MJ, et al. 2-Fluorine-18-fluoro-2-deoxy-D glucose positron emission tomography in the pretreatment staging of Hodgkin’s disease: influence on patient management in a single institution. Ann Oncol. 2000;11:1273–1279. doi: 10.1023/a:1008368330519. [DOI] [PubMed] [Google Scholar]
- 24.Valencak J, Becherer A, Der-Petrossian M, et al. Positron emission tomography with [18F] 2-fluoro-D-2-deoxyglucose in primary cutaneous T-cell lymphomas. Haematologica. 2004;89:115–116. [PubMed] [Google Scholar]
- 25.Cahu X, Bodet-Milin C, Brissot E, et al. 18F-fluorodeoxyglucose-positron emission tomography before, during and after treatment in mature T/NK lymphomas: a study from the GOELAMS group. Ann Oncol. 2011;22:705–711. doi: 10.1093/annonc/mdq415. [DOI] [PubMed] [Google Scholar]
- 26.Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose compared to standard procedures for staging patients with Hodgkin’s disease. Haematologica. 2001;86:266–273. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.