Summary
Adoptive cellular immunotherapy (ACT) is a potentially curative therapy for patients with advanced cancer. Eradication of tumor in mouse models and humans correlates with both a high dose of adoptively transferred cells and cells with a minimally differentiated phenotype that maintain replicative capacity and multipotency. We speculate that response to ACT not only requires transfer of cells with immediate cytolytic effector function to kill the bulk of fast-growing tumor, but also transfer of tumor-specific cells that maintain an ability for self-renewal and the capacity to produce a continual supply of cytolytic effector progeny until all malignant cells are eliminated. Current in vitro methods to expand cells to sufficient numbers and still maintain a minimally differentiated phenotype are hindered by the biological coupling of clonal expansion and effector differentiation. Therefore, a better understanding of the physiologic mechanism that couples cell expansion and differentiation in CD8+ T cells may improve the efficacy of ACT.
Keywords: adoptive cell transfer, T-cell based-therapy, effector differentiation, replicative senescence, CD8+ T cells, adoptive cellular immunotherapy
Introduction
‘Wolde ye bothe eate your cake, and haue your cake?’
-John Heywood, 1546
In some circumstances, you have to make a decision between two incompatible alternatives. The field of cell-based immunotherapy has long been struggling with its proverbial cake in trying to reconcile the capacity to expand CD8+ T cells in vitro without triggering effector differentiation and senescence. That is, the two most compelling correlates of response to adoptive cellular immunotherapy (ACT) in patients with metastatic cancer are number of cells transferred (the more, the better) and transfer of cells with a minimally differentiated phenotype (1). One explanation for this finding is that therapeutic response to ACT relies on an initial wave of cytotoxic T lymphocytes (CTLs) with immediate effector function to eradicate the bulk of tumor (transfer of large amount of cells), but also requires a continual renewal of CTLs mediated by cells with ongoing replicative capacity to ensure elimination of remaining malignant cells (transfer of minimally differentiated cells) (2).
Physiologic coupling of expansion and effector differentiation poses a major therapeutic obstacle to improving the efficacy of cell-based therapy for cancer because current methods to expand cells result in terminal differentiation and replicative senescence of the adoptively transferred cells (3). Therefore, efforts to uncouple this biologic process remain a major clinical priority. In this review, we evaluate the evidence that T-cell dose and differentiation status in ACT correlate with anti-tumor immunity, review the biologic mechanism underlying the coupling of expansion and effector differentiation, and highlight approaches to unhinge this process in ACT for the benefit of patients with metastatic cancer.
Adoptive cellular immunotherapy for cancer
Adoptive cellular immunotherapy with either tumor-infiltrating lymphocytes (TILs) or genetically modified T cells has resulted in complete and durable responses in patients with advanced hematologic and solid cancers (4). There are two general approaches of ACT to treat advanced cancer. Autologous CD8+ T cells can be genetically-modified to express a T-cell receptor or a chimeric antigen receptor (CAR) specific for an antigen expressed on tumor cells (5). Another approach involves isolating TILs from a surgically excised tumor, expanding TILs ex vivo, and subsequently infusing the cells back into the patient (6).
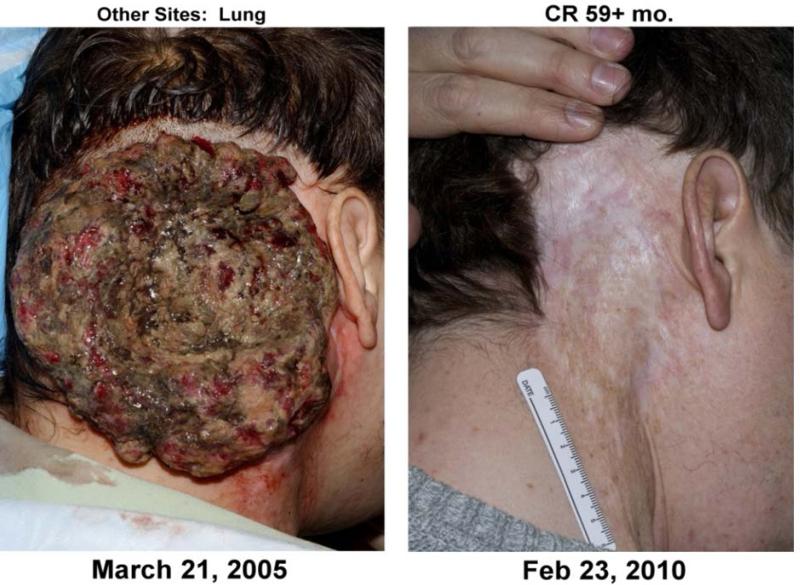
There have been dramatic responses to ACT. Shown in Fig. 1 is a 39-year-old man with metastatic melanoma that had previously failed anti-CTLA4 antibody therapy and three modalities of conventional therapy—radiation, surgery, and chemotherapy—but responded in a complete and durable manner to ACT using autologous tumor-reactive TILs. Of note, the primary lesion shown here was not surgically excised for TILs; rather, a metastasectomy of contralateral cervical lymph nodes was performed from which TILs were isolated. Complete regression of the pictured lesion was not at the hand of a surgical scalpel, but was observed with administration of a non-myeloablative preparative regimen and subsequent transfer of TILs and interleukin-2 (IL-2), establishing proof-of principle that cell-based therapy for advanced cancer is potentially curative even in bulky lesions that have failed all other treatment modalities.
Fig. 1. A 39-year-old man with metastatic melanoma (to lung) from right scalp primary (shown here) refractory to anti-CTLA4 antibody therapy, radiation, chemotherapy, and surgery who had a complete and durable response to cell-based immunotherapy using tumor-infiltrating lymphocytes.
CR= complete response by RECIST criteria 59 months after ACT transfer.
The promise of this potentially curative therapy for advanced cancer is especially timely given the sharp rise in the incidence of cancer worldwide. It is estimated that by 2030,13.2 million people will die from cancer each year (7). With the exception of chemotherapy for germ-cell tumors, however, there are currently few curative therapies for metastatic solid cancers (8). Although some patients have had a dramatic and complete response to ACT, the low frequency of such durable responses and limited cancer histologies for which ACT is effective has limited its widespread use as a standard therapy.
Considerable research effort has been devoted to determining the factors underlying the success of curative ACT, though to date, there are surprisingly few parameters that correlate with response. The size or location of tumor, for example, does not predict whether a patient will profit from an objective response to ACT (1). Much attention has been focused on the surface phenotype and functional features of adoptively transferred cells. Retrospective analyses of clinical trials administering TILs to patients with advanced melanoma have revealed that several parameters correlate with regression of tumor including: absolute number of transferred cells (9-11), telomere length of infused cells (4), shortened culture duration (9), CD27 expression on CD8+ T cells after withdrawal of IL-2 (4), and cells with a greater proliferative capacity and the ability to persist following cell transfer (12). In trying to distill these correlations to a unifying hypothesis to better understand how ACT is potentially curative in its current form, it seems that at least two conditions need to be met: (i) transfer of a large number of anti-tumor TILs and (ii) transfer of minimally differentiated TILs.
We speculate that a high dose of transferred CTLs with immediate effector function provides the bulk of killing shortly after transfer. This is especially relevant in patients with advanced disease whose metastatic deposits exhibit a rapid doubling time characteristic of early Gompertzian growth kinetics (13). There is some preclinical evidence to support this as it was observed that established OVA-expressing thymoma tumors showed greatest regression after adoptive transfer of OT1-specific CTL in the first 3 days after transfer (14). These CTLs are likely short-lived, however, and if they do not eradicate all malignant cells, the tumor will ultimately persist. If patients achieve a complete response to ACT [as measured by radiographic criteria called Response Evaluation Criteria In Solid Tumors (RECIST)], it is generally a long-lasting one. In 21 patients who achieved a complete response, 93% had no evidence of disease after a median follow-up of 8 years (4). In our experience, however, it can take up to several years for some patients to achieve a complete response (authors’ unpublished data). This observation suggests that cancer eradication continues long after the transfer of cells and must rely on the ability of the cells to self renew and produce a continual supply of cytolytic tumor-specific progeny.
When framed in this context, one of the main limitations to improving the curative potential of ACT is perhaps an inability of the transferred cells to infiltrate the tumor in sufficient quantity and persist for an adequate amount of time to kill all malignant cells. Therefore, the efficacy of ACT may be improved with transfer of a large number of cytolytic effectors that constitute the first wave of anti-tumor immunity to destroy the bulk of the tumor burden. The second wave ensures that all malignant tumor cells are eliminated and relies on the ability of a subpopulation of transferred CD8+ T cells to persist for a long period of time and continually produce cytolytic effectors. With this conceptualization of ACT as a two-prong therapy, it is vital that the adoptively transferred ACT product has adequate proportions of both short-lived effector cells and long-lived T cells with the capacity to produce an ongoing source of tumor-specific progeny.
CD8+ T-cell nomenclature in context of chronic tumor challenge: a vague memory
The biologic phenomenon of immunological memory at an organismal level was recognized well before the establishment of the field of immunology. The Greek historian Thucydides, for example, noted in reference to a plague that struck Athens in 490 BC, ‘the same man was never attacked twice—never at least fatally’ (15). It was later recognized, of course, that CD8+ T lymphocytes were largely responsible for mediating immunological memory in the context of episodic microbial infections. Immunologists largely focused subsequent research efforts on elucidating the underlying molecular mechanisms of T-cell memory in an effort to improve vaccines for infectious disease (3).
The other principle biologic challenge of CD8+ T cells, however, is to maintain a continuous production of cytolytic effector T cells (CTLs) in the face of persistent infections or cancer. This is arguably a more important task for the immune system given that a failure to eradicate the initial foreign threat could be lethal to the organism and render subsequent challenges from the same offender impossible, thereby obviating the need for a memory response (3). Although the molecular machinery that enables continual production of CTLs during persistent antigen challenge may have similar components to the signaling architecture that results in a memory response, particularly with regard to molecular programs governing cellular senescence, the two can be viewed as distinct biological phenomenon (2).
Understanding the molecular architecture that mediates continual replication of CD8+ T cells without clonal senescence during a chronic microbial infection or cancer may lead to strategies to improve the efficacy of ACT. In particular, uncoupling the physiologic process of T-cell expansion and concomitant effector differentiation could enable both a continuous population of terminally differentiated CTLs and a population of minimally differentiated T cells that maintain replicative capacity.
This is a notably different task than trying to elucidate the mechanisms of a memory response to episodic challenge with foreign antigen. Unfortunately, the nomenclature describing CD8+ T-cell ontogeny in the context of persistent antigen challenge is dominated by memory terminology (16). Discrete subsets of antigen-experienced CD8+ T cells are referred to as memory cells—central memory (Tcm) or effector memory (Tem) T cells—even in the context of chronic antigen challenge. The usage of memory to describe CD8+ T-cell subsets responding to chronic challenge is far too commonly used to change now (we are guilty of it, too). Ultimately, efforts to improve the efficacy of ACT are not focused on expanding the pool of memory T cells per se but rather to provide a continuous, uninterrupted production of cancer-specific CTLs until the tumor is completely eradicated.
T-cell dose is a determinant of response to cell-based therapy for cancer
Before a CD8+ T cell can kill a malignant cell, it first must find it. This extraordinary challenge comes into high relief with a simple consideration of the spatial dimensions involved in this process. The human body consists of 1013 somatic cells in a volume of 70 liters (17). It is within this vast landscape that T cells, themselves occupying a mere 290 femtoliters (representing one-one-hundred-trillionth of the organismal volume), must navigate to find their tumor targets (18).
Understanding the mechanisms by which CD8+ T cells are able to cover so much ground in search of their targets is in nascent stages, but one recent observation by Hunter and colleagues (19) is particularly intriguing. Using two-photon microscopy it was shown that T cells do not engage in Brownian walk to survey peripheral tissue for cognate antigen. Rather, they employ Levy walks, which are thought to cover far more territory. Here, the metaphor of a lymphocyte as a shark trying to find its prey in a vast ocean is not too far off the mark because both sharks and lymphocytes use Levy walking as a way to find what they are looking for in a space that dwarfs their own stature. It may be that a large dose of cells is correlated with response to ACT simply because it favors the probability that tumor-specific T cells co-localize with their target.
In advanced stages of melanoma the mass of tumor in a patient can be measured in kilograms and consist of 3×108 melanoma cells per gram of tumor (20-21). In this case, the burden of locating a tumor is probably lessened, because the tumor burden is so large. The formidable task of eradicating every malignant cell within the tumor is the next challenge that confronts CD8+ T cells, especially considering that much of the tumor killing is thought to be mediated by CTLs through cell-contact-dependent lysis of tumor with perforin and granzyme (14). Recent attempts to quantify the concentration of intratumoral antigen-specific CD8+ T cells needed to kill 100% of clonogenic tumor cells showed that greater than 107 activated OT-1 cells/ml was required to kill 100% of SIINFEKL-B16 cells/ml by 7 days in a collagen-fibrin gel model (21).
It is therefore not surprising that the absolute number of transferred T cells in ACT is a determinant of response to therapy in patients with advanced melanoma. In both preclinical murine models and several human ACT TIL trials, the absolute number of infused CD8+ T cells has correlated with the likelihood of objective response. Klebanoff et al. (1) demonstrated in mice with established B16 melanoma a dose-response relationship between the number of transferred CD8+ T cells and the strength of tumor regression. This corroborated the results of several human TIL trials in patients with metastatic melanoma in which significant correlations were demonstrated between a high number of infused cells and objective response to therapy (9, 11).
T-cell differentiation status correlates with anti-tumor immunity
That cell dose correlates with response to ACT can be considered as intuitive, as transfer of minimally differentiated cells that lack cytolytic function is not. In other words, it was initially thought that transfer of highly cytolytic terminally differentiated effector T cells would result in greater eradication of tumor in patients with advanced cancer (22). Clinical protocols for ACT were initially designed to select TILs for adoptive transfer that demonstrated the ability to produce large quantities of INFγ and cytolytic function in vitro when cocultured with autologous tumor, though this selection method has never shown to improve efficacy of ACT (23-25). Rather, there is increasing evidence that adoptive transfer of minimally differentiated T cells into patients with advanced melanoma results in improved anti-tumor immunity (26).
The rationale behind transfer of less-differentiated cells is that they maintain proliferative capacity and can produce effector progeny continuously, thereby providing a continual source of cytolytic effector cells (3). Proof-of-principle that transfer of less-differentiated T cells improved anti-tumor immunity was first demonstrated in preclinical mouse models. The Pmel-1 T-cell receptor (Pmel-TCR) transgenic mouse, whose T cells are specific for the Db-restricted melanoma-associated antigen hgp100, has played a key experimental role in elucidating causal relationships between T-cell differentiation and anti-tumor efficacy (27). Pmel T cells that were repeatedly stimulated in vitro and designated as early Teff (1 stimulation), intermediate Teff (2 stimulations), or late Teff (3 stimulations) cells showed progressively diminished capacity to produce IL-2 with each stimulation (28). Late Teff acquired potent capacity for cytolysis and IFNγ release, but showed increased expression of the replicative senescence marker killer cell lectin-like receptor G1 (KLRG-1) and demonstrated poor persistence when adoptively transferred (29). In contrast, early Teff cells had increased expression of CD62L, CCR7, CD27, IL-7Rα, and had greater persistence and increased capacity for tumor regression when transferred into mice with established B16 melanoma (28, 30).
Because conventional memory T cells were observed in viral challenge models to have greater multipotency and replicative capacity than their terminally differentiated CTL counterparts, it was hypothesized that adoptive transfer of minimally differentiated memory T cells may mediate improved cancer regression (31-33). Preliminary experiments compared Tcm to Tem cells and showed that Tcm cells had greater engraftment and proliferative capacity compared to Tem cells that translated into improved tumor regression by Tcm cells (34). Subsequent experiments evaluating the anti-tumor immunity of stem-cell memory T cells (Tscm) showed superior in vivo expansion, persistence, and anti-tumor efficacy compared to their Tcm and Tem counterparts (35-36).
The initial starting population, whether naive T cells (Tn) or Tcm, from which adoptively transferred Teff are derived has also shown to impact in vivo persistence and anti-tumor efficacy (37). Teff cells derived from Tn cells had diminished KLRG-1 expression and increased production of IL-2 compared to Teff cells derived from Tcm cells (38). Tn-derived Teff cells also demonstrated superior anti-tumor eradication compared to those Teff cells derived from Tcm cells in both mouse and human (38-39). In summary, experimental evidence in both mouse and human has supported the notion that adoptive-transfer of minimally differentiated CD8+ T cells improves engraftment, persistence, and improved anti-tumor immunity tumor compared to terminally differentiated T cells.
Physiologic coupling of IL-2-mediated T-cell expansion and effector differentiation
The coupling of cellular expansion and differentiation has been observed in diverse biologic systems including the adaptive immune response to foreign antigen. Biologic coupling of T cell expansion with effector differentiation is mediated by IL-2 and is a critical feature of the adaptive response because it enables efficient and coordinated clearance of antigen (Figure 2A) (2-3). In the non-physiologic setting of ACT, however, the coupling of cell expansion and effector differentiation poses a major therapeutic obstacle to improving the efficacy of ACT which relies on both a large number of transferred cells and cells that have undergone minimal differentiation prior to adoptive transfer.
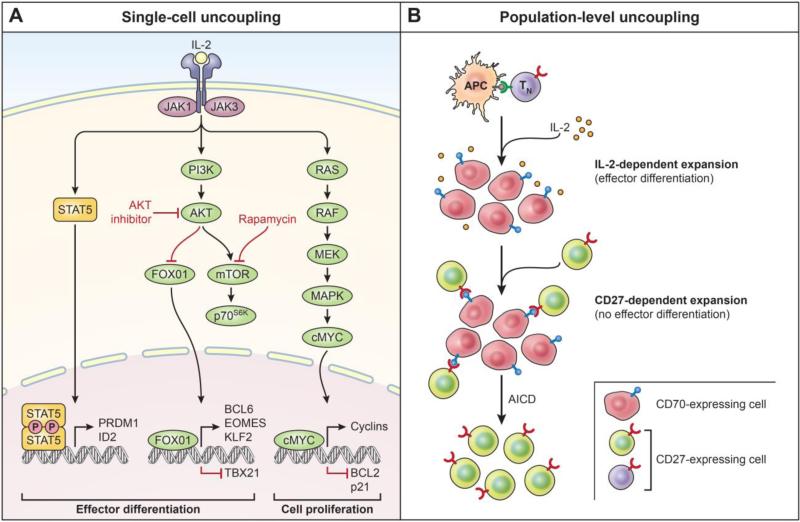
Fig. 2. Single-cell and population-level uncoupling of expansion and effector differentiation in CD8+ T cells.
(A) Interleukin-2 (IL-2) serves to couple CD8+ T-cell expansion and differentiation by activation of several signaling pathways including the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway, the phosphoinositide-3-kinase (PI3K)–AKT pathway and the mitogen-activated protein kinase (MAPK) pathway. Effector differentiation is driven by activation of AKT and nuclear exclusion of Forkhead box 01 (FOXO1), thereby inhibiting Eomesodermin (EOMES), B-cell CLL/lymphoma 6 (BCL6), and Kruppel-like factor 2 (KLF2) and resulting in expression of T-BET (encoded by T-box 21 (TBX21). STAT5 activation drives expression of pro-differentiation molecules B-lymphocyte-induced maturation protein 1 (BLIMP1, encoded by PR domain containing 1, with ZNF domain (PRDM1) and inhibitor of DNA-binding 2 (ID2). IL-2 signaling drives cell expansion through the RAS/RAF/mitogen-activated protein kinase (MAPK) pathway and expression of cMYC which drives proliferation by upregulating cyclins and downregulation of p21 and pro-apoptotic molecule B-cell lymphoma 2 (Bcl-2). Pharmacologic inhibition of select pathways by agents such as Akt inhibitor or rapamycin may uncouple cell proliferation and effector differentiation in a cell-intrinsic manner. p70S6K, p70 ribosomal protein S6 kinase; mTOR, mammalian target of rapamycin. (B) The establishment of a self-renewing subset of CD8+ T cells within a polyclonal response by a CD27-CD70-dependent pathway is depicted here. Upon activation with cognate antigen, the progeny of a naive CD8+ T cell progressively downregulates CD27 while reciprocally upregulating CD70 in the context of IL-2 signaling. Physiologic inhibition of PI3K-Akt-mTOR pathway results in reacquisition of CD27 amidst a population of highly differentiated CD70-expressing cells, thereby triggering the CD27-dependent expansion of T cells that occurs with minimal effector differentiation. Antigen-induced cell death (AICD) further favors the outgrowth of CD-27-dependent pathway. Availability of CD70 negatively regulates CD-27-dependent cell expansion; constitutive CD70 results in autoimmune pathology.
IL-2 has long been considered the ‘T-cell growth factor’ and initially thought to play a non-redundant role in mediating clonal expansion of T cells (40-42). Ligation of the TCR on CD8+ T cells induces expression of the IL-2 gene and concomitant expression of the IL-2Ra chain (CD25), which enables high-affinity paracrine and autocrine binding of IL-2 to its receptor, thereby encouraging clonal expansion in a coordinated fashion (43). In addition to inducing clonal expansion, IL-2 signaling also plays a non-redundant role in driving effector differentiation to cytotoxic T lymphocytes (CTLs) (44-45). The physiologic coupling of T-cell expansion and effector differentiation is therefore largely attributed to the effects of IL-2 signaling (Fig. 2A).
Subsequent findings in IL-2-deficient F5 TCR-transgenic mice, however, showed that administration of antigenic peptide induced clonal expansion in the absence of CTL activity (46), thereby raising the possibility that clonal expansion without effector differentiation can occur in stimulated CD8+ T cells.
Uncoupling IL-2-driven proliferation and differentiation in CD8+ T cells
The abundance of IL-2 in the immune microenvironment could potentially drive the majority of CD8+ T cells to terminal differentiation. The observation that a subset of CD8+ T cells maintains a minimally differentiated state therefore demonstrates that the immune system has a mechanism for controlling cytokine-driven effector differentiation. There are at least two ways in which a polyclonal CD8+ T-cell response could undergo expansion without effector differentiation amidst IL-2 signaling. First, there may be IL-2-independendant mechanisms that result in CD8+ T-cell clonal expansion that do not result in concomitant effector differentiation. This will be further discussed in the section on CD27-dependent CD8+ T-cell clonal expansion. Another potential mechanism by which a population of CD8+ T cells can regulate differentiation is to control signals induced by IL-2 signaling in a cell-intrinsic manner. That is, if a subset of polyclonal CD8+ T cells were able to selectively inhibit the differentiation program driven by IL-2 but not the molecular machinery involved in cellular proliferation, it would potentially uncouple CD8+ T-cell expansion from terminal differentiation.
Cell intrinsic uncoupling of IL-2-mediated expansion and differentiation Active repression of selective pathways of IL-2 signaling may prevent effector differentiation while allowing for expansion and production of progeny capable of developing cytolytic function. A potential means for cell autonomous regulation of IL-2 signaling was initially suggested by studies of germinal center B cells (47). Ectopic expression of the transcriptional repressor, B-cell lymphoma 6 protein (BCL-6), prevented IL-2 from inducing the differentiation of B cells into immunoglobulin (IgM)-secreting plasma cells. Notably, BCL-6 overexpression enabled B cells to undergo iterative cycles of expansion and somatic hypermutation of Ig genes. The effect of BCL-6 was mediated by repression of signal transducer and activator of transcription (Stat3)-dependent expression of Blimp-1, the master regulator of plasma cell differentiation (48).
The frequent parallels between pathways regulating activation and differentiation in B and T cells at least suggested that BCL-6 may play a role in uncoupling IL-2-mediated expansion and effector differentiation in T cells. The hypothesis that BCL-6 could actively repress effector differentiation in CD8+ T cells has been evaluated in both gain-of-function BCL-6 transgenic mice and loss-of-function BCL-6−/− mice (49-50). Mice with a BCL-6 transgene regulated by a lck proximal promoter showed enhanced primary expansion after infection with vaccinia/OVA and a twofold increase in OVA-specific CD8+ T cells 10 weeks after infection, suggesting that suppression of IL-2-induced differentiation may promote the capacity for homeostatic proliferation. Loss-of-function studies with BCL-6−/− mice, however, did not compromise expansion in CD8+ T cells, though interpretation of the data is problematic because disruption of BCL-6 resulted in a spontaneous inflammatory disease thought to be mediated by macrophages (51).
IL-2 signaling activates several downstream pathways, including Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, the phosophoinosited-3-kinase (PI3K)-AKT pathway, and the mitogen-activated protein kinase (MAPK) pathway (52). Coordination of these pathways by IL-2 signaling serves to couple effector differentiation and clonal expansion (Fig. 2A). A better understanding of the regulatory mechanisms governing IL-2 signaling could enable selective modulation of pathways involved in cell proliferation and effector differentiation.
Cell extrinsic uncoupling of IL-2 signaling: CD27-dependent CD8+ T-cell clonal expansion
Engagement of the tumor necrosis factor (TNF) receptor superfamily CD27 by its ligand CD70 affects the quality and magnitude of T-cell responses in a variety of infectious, autoimmune, and tumor models (53). CD27-deficient mice were shown to have impaired primary and secondary expansion of CD8+ T cells in response to an influenza challenge (54). CD27 was also found to be necessary for the long-term response of T cells to polyomaviral infection (55). More recently, the possibility that CD27 promotes cellular expansion in the absence of IL-2 was supported by the finding that repeatedly stimulating IL-2−/− CD8+ T cells with antigen and a recombinant form of soluble CD70 cause marked clonal expansion despite high expression of CD62L and a notable absence of effector differentiation (56). The effect of CD27 resulted in increased cell cycling and survival that was mediated, in part, by enhanced expression of IL-7Rα on T cells. In contrast to IL-2-expanded cells, antigen-induced cell death (AICD) was not as prevalent in cells expanded by a CD27-dependent mechanism. Taken together, these findings suggest that CD27 may mediate the generation of a self-renewing subset of antigen-experienced CD8+ T cells that avoid terminal differentiation and senescence driven by IL-2.
CD70 is the only known ligand for CD27 which engenders a unique capacity to regulate the timing and location of CD27-CD70 interactions. CD27 and its ligand, CD70, have long been observed to promote cell expansion in vitro (57). CD27 is expressed on naive CD8+ T cells, and after a transient increase in expression, is downregulated after T cells have undergone several rounds of division (58-59). CD27 expression is progressively lost with differentiation, while CD70 expression is reciprocally increased on T cells and is largely observed on terminally differentiated T cells (60-61). Although CD70 is also expressed by activated dendritic cells, B cells, and CD4+ T cells (62), recent studies using a transgenic mouse line in which T cells constitutively express CD70 (CD2-CD70 Tg mice) suggest that T cell-T cell interactions may be especially important in the timing of CD27-CD70 interactions. In contrast to transgenic mouse lines in which CD70 is constitutively expressed on dendritic cells (CD19-CD70-Tg and CD11c-CD70-Tg), a particularly intriguing finding of CD2-CD70 Tg mice is they have been observed to generate more effector T cells over time without compromising the naive T-cell pool (63-64).
Though this hypothesis remains to be confirmed, Fig. 2B illustrates how ligation of CD27-CD70 on adjacent T cells may endow a self-renewing property to a population of clonally expanding T cells, even in a milieu of high concentrations of IL-2 that drives the bulk of cells towards terminal differentiation and senescence. This may at least partially account for the capacity of CD8+ T cells to self-renew and provide a continual source of CTL in the context of persistent antigen stimulation.
Naive CD27+CD8+ T cells may largely expand through an IL-2-dependent mechanism because there is limited CD70 ligand available in the early phase of the polyclonal response (it is mostly expressed in highly terminally-differentiated T cells) (60). With increasing effector differentiation driven by IL-2, expression of CD27 is downregulated and CD70 is progressively upregulated (61). Expression of CD27 is highly dynamic and has been shown to be expressed on central memory T cells (32). The PI3K-AKT-mTOR pathway has recently been observed to play a central role in sensing and integrating cues in the immune microenvironment such as availability of growth factors, amino acids, and glucose (65). It has been observed that limiting glucose or inhibition of mTOR or Akt can induce expression of CD27 on a small subset of antigen-experienced T cells (66-67), thereby triggering a competing pathway of expansion within a polyclonal response of CD8+ T cells that does not result in terminal differentiation and senescence. Because CD27-mediated clonal expansion limits cell death from AICD with repeated antigen stimulation and IL-2 exposure, this favors the outgrowth of T cells driven by CD27-mediated expansion. Limiting concentration of antigen and IL-2 with clearance of tumor or chronic infection further favors outgrowth of CD27-dependent population relative to IL-2 dependent population. Finally, bridling the expansion of CD27-depedent T cells to avoid limitless proliferation that could result in autoimmune pathology or T-cell malignancy is ensured by decreasing availability of CD70-expressing T cells as they are extinguished in the absence of IL-2 and antigen.
Importance of CD27 in the context of T-cell-based therapy for cancer
Manipulation of the CD70-CD27 axis has enhanced T-cell-mediated control of tumors in preclinical mouse models (63, 68). Peripheral CD8+ T cells in patients with metastatic melanoma express relatively low levels of CD70 and high levels of CD27 (61). In vitro stimulation by agonistic CD3/CD28 and high-dose IL-2 (3000 international units/ml) results in upregulation of CD70 expression and loss of CD27 in CD8+ T cells. The same reciprocal loss of CD27 and gain of CD70 in CD8+ T cells was observed in vivo in patients with metastatic melanoma receiving systemic IL-2 (61). Withdrawal of IL-2 in vitro reversed the pattern, such that an increase in CD27 was observed as this would allow the outgrowth of the CD27-dependent pathway. When TILs were cultured with a blocking antibody to CD70, it reduced cell expansion by 40%, presumably because this effectively terminates any expansion resulting from the CD27-dependent pathway. Finally, the size of the CD27+CD8+ T-cell subset in TILs excised from patients with metastatic melanoma was found to be highly associated with the ability to mediate tumor regression after adoptive transfer into patients (4, 69).
Clinical approaches to uncouple cell expansion and effector differentiation
Both ACT methods using genetically modified T cells or TILs rely on ex vivo expansion of cells to large numbers which invariably drives cells toward terminal differentiation. The identification of a means for expanding antigen-specific CD8+ T cells without causing replicative senescence after adoptive transfer may increase the efficacy of adoptive T cell therapy for cancer. Here we will discuss potential ways to expand T cells in vitro while preserving a minimally differentiated phenotype (Fig. 3).
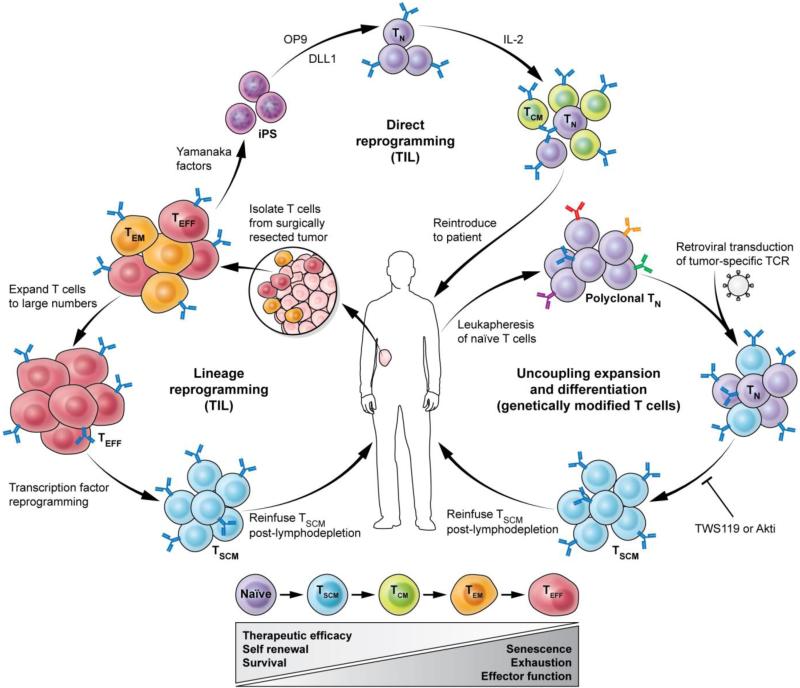
Fig. 3. Multi-pronged approach to obtain therapeutic levels of minimally differentiated tumor-specific CD8+ T cells.
Depicted here is direct reprogramming of terminally differentiated T cells (Teff) isolated from tumor-infiltrating lymphocytes (TILs) to generate induced pluripotent stem (iPS) cells by ectopic co-expression of the Yamanaka factors OCT4, sex determining region Y (SRY) BOX 2 (SOX2), and Kruppel-like factor 4 (KLF4) with or without MYC. TIL-derived iPS cells can be subsequently redifferentiated into Tn cells by coculture with OP9 cells expressing Notch ligand Delta-like 1 (DL1). Lineage reprogramming of Teff cells may be accomplished by forced expression of transcription factors that are differentially expressed in Tn or Tscm. Uncoupling of expansion and effector differentiation using small molecules such as inhibitors of Akt (Akti) is a third approach to obtain an adequate yield of minimally differentiated anti-tumor T cells for ACT. TWS119 is an inhibitor GSK3β, glycogen synthase 3β. Tscm, stem cell memory; Tcm, central memory; Tem, effector memory; Teff, short-lived effector.
We propose three general strategies. The first strategy relies on effective uncoupling of the IL-2-dependent physiologic process that links cell expansion and effector differentiation. Recent identification of signaling pathways critical to programs of cellular proliferation and effector differentiation now enables modulation of these signaling pathways to grow cells and limit differentiation.
The second strategy takes advantage of the recent observation that CD8+ T-cell expansion can occur in an IL-2-independent manner, thereby avoiding the differentiating effects of this cytokine. The finding that the CD27-dependent pathway can mediate significant clonal expansion in vitro suggests that it might be feasible to develop protocols for generating adequate numbers of relatively undifferentiated T cells that retain replicative function and a capacity for effector differentiation after adoptive transfer.
The third strategy is to use conventional methods to culture TILs or PBMCs for ACT. After cells have expanded to yield an adequate number for ACT therapy, regenerative medicine techniques such as lineage or direct reprogramming can be used to restore a minimally differentiated phenotype.
Cytokine or pharmacologic uncoupling of IL-2 dependant expansion and effector differentiation
Several pharmacologic agents have recently been identified that selectively target components of IL-2 signaling or developmental and metabolic pathways in activated T cells that are critical in directing cell expansion and differentiation programs. Inhibition of these pathways by small molecules such as inhibitors of mTORC1, p70S6K, AMPK, PI3K, and GSK-3β can minimize effector differentiation of activated CD8+ T cells (70-76) and may improve the efficacy of ACT (35-36, 67, 73-74). To date, these agents have had limited clinical utility, however, because they appear to arrest both differentiation and proliferation of antigen-experienced CD8+ T cells, thereby providing insufficient cell yield for adoptive transfer. Therefore, there is a critical need to identify pharmacological lead compounds that effectively uncouple cell expansion from effector differentiation to provide a therapeutic yield of minimally differentiated anti-tumor T cells.
There is also a growing body of evidence that common γc cytokines, such as IL-15 and IL-21, can sustain T-cell proliferation without excessive promotion of differentiation into effector T cell subsets characterized by exhaustion and senescence. T cells cultured in IL-15 resemble Tcm in phenotype, gene expression, metabolism, and have greater anti-tumor function in mice compared to T cells cultured in IL-2 (28, 77-79). Another common γc cytokine, IL-21, modulates the differentiation of activated T cells and results in development of a population of cells characterized by a Tscm phenotype (80-83). In mouse T cells, IL-21 caused a dose-dependent inhibition of the acquisition of antigen-experienced markers such as CD44 while preserving expression of CD62L, Tcf7, and Lef1 (80). Human T cells cultured in IL-21 retain the ability to release IL-2 and prevent the loss of CD45RA, CD28, CD27, IL7R-α, and CD62L—markers associated with a minimally differentiated phenotypes (82-83). In a mouse model of melanoma, T cells cultured in IL-21 demonstrated profoundly enhanced anti-tumor activity compared to cells grown in other common γc cytokines (80).
CD27-dependent expansion of CD8+ T cells
The use of methods for ex vivo expansion of TILs or PBMCs that are largely γc cytokine-independent could potentially provide a sufficiently large number of cells for adoptive transfer that are minimally differentiated. As discussed in this review, the recently identified CD27-dependant pathway of T-cell expansion has therapeutic potential to enhance the efficacy of ACT. Translation to the bedside seems fairly feasible and may be especially relevant in the context of genetically modified naive T cells with specificity against tumor antigen. Peripheral PBMCs can be isolated with plasmaphersis and purified into naive CD8+ T cells that have high expression of CD27. Transduction of these cells with a tumor-specific TCR such as NY-ESO would confer specificity against melanomas and synovial cell sarcomas expressing this antigen. In vitro culture with recombinant soluble CD70 could mediate expansion in this CD27-enriched population of CD8+ T cells without causing concomitant differentiation. Further preclinical studies using ACT models are necessary to evaluate if this is a feasible approach to improve the efficacy of ACT for patients with advanced cancer.
Lineage or direct reprogramming of CD8+ T cells
The capacity to induce pluripotency in somatic cells by ectopic expression of transcription factors, direct reprogramming, has revitalized the field of regenerative medicine. Several mature cells types have been reprogrammed into induced pluripotent stem cells (iPSCs): keratinocytes (84), fibroblasts (85), hepatocytes (86), and human peripheral blood cells (87). T lymphocytes have also been reprogrammed into iPSCs (88) and subsequently differentiated into T-cell lineages by coculturing iPS cell-derived T cells with OP9 cells expressing Notch ligand Delta-like 1 (DL1) (89). In vitro stimulation of iPS cell-derived T lymphocytes resulted in secretion of IL-2 and IFNγ. Furthermore, T-cell-derived iPSCs maintain the rearranged variable (V), diversity (D), and joining regions (J) of the TCR chains (89).
A two-step method of direct reprogramming and subsequent differentiation to naive or memory T cells is an appealing approach to revitalize exhausted and senescent T cells to improve the efficacy of ACT. In a recent study by Vizcardo et al. (90-91), it was demonstrated that TIL excised from a patient with metastatic melanoma can be successfully transduced with Yamanaka factors and reprogrammed to iPSC as evidenced by ESC-like morphology, capacity for teratoma formation, endogenous expression of OCT3/4, SOX2, KLF4, and c-MYC, and disappearance of T-cell markers CD3 and CD8. Subsequent co-culture with DL1-expressing OP9 feeder cells resulted in redifferentation to CD8+ T cells (92). When cultured with antigen-presenting cells and challenged with the cognate antigen MART-1, reprogrammed cells produced IFNγ, demonstrating functional integrity.
Although this was a remarkable demonstration that regenerative medicine techniques such as direct reprogramming can be successfully applied to terminally differentiated TILs, further work needs to evaluate the anti-tumor efficacy of iPSC-derived CD8+ T cells. There are several other obstacles in translating this technology to the bedside. The reprogramming process can induce genetic and epigenetic abnormalities in iPSCs including point mutations (93), copy number variations (94), an aberrant methylome (95), and chromosomal aneuploidy (96). Especially concerning is the potential for functional mutations in oncogenes or tumor suppressors and malignant transformation (97). In an attempt to reduce the risk of genetic and epigenetic disruption, several methods such as refined and DNA-free reprogramming methods are actively being explored (98-100).
Lineage reprogramming by forced expression of transcription factors can induce a cell to undergo dedifferentiation, transdifferentiation, or transdetermination, and may represent a safer and more efficient alternative to nuclear reprogramming because it does not require a pluripotent intermediate. Experimentally, hepatocytes have been converted to pancreatic cells (101), pancreatic exocrine cells to β-cells (102), fibroblasts into neurons (102) and cardiomyocytes (103), and B cells into macrophages (104). This approach may be adapted to efficiently revitalize exhausted and senescent T cells by enforced expression of transcription factors differentially expressed in naive or memory-like T cells.
We propose three applications of regenerative medicine to uncouple cell expansion and effector differentiation in ACT (Fig. 3). Lineage reprogramming of CD8+ T cells may be accomplished by transduction of transcription factors that are highly expressed in naive T cells and downregulated in terminally differentiated effector cells. Candidate transcription factors for reprogramming can be developed using microarray data of T-cells isolated in distinct states of differentiation [an example is available in supplementary data of reference (36)]. Concern for malignant transformation when using lentivirus or retrovirus vectors may be circumscribed by using non-integrating sendai or adenovirus and emerging technologies such as the piggyback transposon system (105).
Arresting differentiation of naive T cells transduced with a TCR specific for a cancer stem cell antigen is another approach relying on principles of regenerative medicine to preserve proliferative capacity in the transferred population. As discussed above, pharmacologic agents that target key differentiation pathways can modulate the program of differentiation and expand subsets of T cells with greater antitumor immunity. Arresting differentiation with an inhibitor of Akt, for example, may uncouple expansion and terminal differentiation to improve antitumor immunity.
Conclusions
Cell-based immunotherapy is a potentially curative therapy for patients with advanced cancer. One of the main limitations to improving the efficacy of ACT is the capacity to expand T cells to therapeutic yield while limiting effector differentiation. The capacity to uncouple T-cell expansion from effector differentiation has been a long-standing goal of ACT, because response to therapy is correlated with both a large number of transferred cells and cells that have a minimally differentiated phenotype. Herein we have reviewed the biologic mechanisms involved in linking T-cell expansion and differentiation and proposed clinical approaches to effectively uncouple this process to improve the efficacy of ACT for patients with advanced cancer.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, and Center for Cancer Research. JG Crompton also acknowledges funding support from the Wellcome Trust Translational Medicine and Therapeutics Programme.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Klebanoff CA, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon DT, Carr JM, Telaranta A, Carrasco MJ, Thaventhiran JE. The rationale for the IL-2-independent generation of the self-renewing central memory CD8+ T cells. Immunol Rev. 2006;211:104–118. doi: 10.1111/j.0105-2896.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT. The expansion and maintenance of antigen-selected CD8(+) T cell clones. Adv Immunol. 2007;96:103–139. doi: 10.1016/S0065-2776(07)96003-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;7:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;1:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Xu JQ, Ward E. Cancer Statistics, 2010. Ca-a Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 8.Winter C, Albers P. Testicular germ cell tumors: pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2011;7:43–53. doi: 10.1038/nrendo.2010.196. [DOI] [PubMed] [Google Scholar]
- 9.Besser MJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 10.Radvanyi LG, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itzhaki O, et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 12.Robbins PF, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton L, Simon R, Brereton HD, Bogden AE. Predicting the course of Gompertzian growth. Nature. 1976;264:542–545. doi: 10.1038/264542a0. [DOI] [PubMed] [Google Scholar]
- 14.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Rocha B, Tanchot C. The Tower of Babel of CD8+ T-cell memory: known facts, deserted roads, muddy waters, and possible dead ends. Immunol Rev. 2006;211:182–196. doi: 10.1111/j.0105-2896.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 17.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 18.Segel GB, Cokelet GR, Lichtman MA. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood. 1981;57:894–899. [PubMed] [Google Scholar]
- 19.Harris TH, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens TC, Peacock JH. Cell yield and cell survival following chemotherapy of the B16 melanoma. Br J Cancer. 1978;38:591–598. doi: 10.1038/bjc.1978.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budhu S, et al. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. J Exp Med. 2010;207:223–235. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudley ME, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 31.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117:1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinrichs CS, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger C, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 41.Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 42.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 43.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 44.Yu A, et al. Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol. 2003;170:236–242. doi: 10.4049/jimmunol.170.1.236. [DOI] [PubMed] [Google Scholar]
- 45.D'Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 46.Kramer S, et al. Thymic selection and peptide-induced activation of T cell receptor-transgenic CD8 T cells in interleukin-2-deficient mice. Eur J Immunol. 1994;24:2317–2322. doi: 10.1002/eji.1830241009. [DOI] [PubMed] [Google Scholar]
- 47.Reljic R, Wagner SD, Peakman LJ, Fearon DT. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med. 2000;192:1841–1848. doi: 10.1084/jem.192.12.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaffer AL, et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 49.Ichii H, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 50.Ichii H, et al. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int Immunol. 2007;19:427–433. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- 51.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 52.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 53.Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 54.Hendriks J, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 55.Kemball CC, et al. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J Immunol. 2006;176:1814–1824. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- 56.Carr JM, et al. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. Proc Natl Acad Sci U S A. 2006;103:19454–19459. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 58.Hamann D, et al. Evidence that human CD8+CD45RA+CD27- cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 59.de Jong R, et al. Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. J Immunol. 1991;146:2488–2494. [PubMed] [Google Scholar]
- 60.Tesselaar K, et al. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 61.Huang J, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176:7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong H, et al. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. J Immunol. 2012;188:3829–3838. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 64.van Gisbergen KP, et al. Protective CD8 T cell memory is impaired during chronic CD70-driven costimulation. J Immunol. 2009;182:5352–5362. doi: 10.4049/jimmunol.0802809. [DOI] [PubMed] [Google Scholar]
- 65.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roberts DJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell DJ, Jr., Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;1:11ps12. doi: 10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 73.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butler MO, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 83.Albrecht J, et al. IL-21-treated naive CD45RA+ CD8+ T cells represent a reliable source for producing leukemia-reactive cytotoxic T lymphocytes with high proliferative potential and early differentiation phenotype. Cancer Immunol Immunother. 2011;60:235–248. doi: 10.1007/s00262-010-0936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 86.Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51:1810–1819. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loh YH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seki T, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260:1–5. doi: 10.1016/j.cellimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Vizcardo R, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 91.Crompton JG, Rao M, Restifo NP. Memoirs of a reincarnated T cell. Cell Stem Cell. 2013;12:6–8. doi: 10.1016/j.stem.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmitt TM, uniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 93.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hussein SM, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 95.Lister R, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayshar Y, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Baker DE, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 98.Lohle M, et al. Differentiation efficiency of induced pluripotent stem cells depends on the number of reprogramming factors. Stem Cells. 2012;30:570–579. doi: 10.1002/stem.1016. [DOI] [PubMed] [Google Scholar]
- 99.Pera MF. Stem cells: Low-risk reprogramming. Nature. 2009;458:715–716. doi: 10.1038/458715a. [DOI] [PubMed] [Google Scholar]
- 100.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meivar-Levy I, et al. Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein beta. Hepatology. 2007;46:898–905. doi: 10.1002/hep.21766. [DOI] [PubMed] [Google Scholar]
- 102.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 117:663–676. doi: 10.1016/s0092-8674(04)00419-2. 20040. [DOI] [PubMed] [Google Scholar]
- 105.Patel M, Yang S. Advances in reprogramming somatic cells to induced pluripotent stem cells. Stem Cell Rev. 2010;6:367–380. doi: 10.1007/s12015-010-9123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]