Abstract
BACKGROUND
Clinical trials have shown that amlodipine reduces cardiovascular events at a rate that is not predicted by changes in brachial arterial pressure alone. These findings may be explained, in part, by the pleiotropic effects of amlodipine on endothelial cell (EC) function. In this study, we elucidated the effect of amlodipine on nitric oxide (NO) bioavailability and cytotoxic peroxynitrite (ONOO–) and blood pressure (BP).
METHODS
Spontaneously hypertensive rats (SHRs) were treated with vehicle or amlodipine (5mg/kg/day) for 8 weeks and compared with untreated, baseline rats. NO and ONOO– release from aortic and glomerular ECs were measured ex vivo using amperometric nanosensors following maximal stimulation with calcium ionophore. BP was measured using the tail-cuff method.
RESULTS
As compared with baseline, vehicle treatment had reduced aortic endothelial NO release from 157±11nM to 55±6nM and increased ONOO– from 69±7nM to 156±19nM. The NO/ONOO– ratio, a comprehensive measurement of eNOS function, decreased from 2.3±0.3 to 0.3±0.1. Compared with vehicle, amlodipine treatment restored NO to 101±3nM, decreased ONOO– to 50±4nM, and increased the NO/ONOO– ratio to 2.0±0.2, a level similar to baseline. Similar changes were observed for glomerular ECs. Mean arterial blood pressure increased from 149±3mm Hg (baseline) to 174±1mm Hg (vehicle). Amlodipine slightly, but significantly, decreased mean arterial blood pressure to 167±3mm Hg vs. vehicle treatment.
CONCLUSIONS
Amlodipine increased NO bioavailability and decreased nitroxidative stress in SHRs with EC dysfunction disproportionately to BP changes. These direct, vascular effects of amlodipine on EC function may contribute to reduced risk for atherothrombotic events as observed in clinical trials.
Keywords: amlodipine, blood pressure, endothelial cells, hypertension, nitric oxide, peroxynitrite, spontaneously hypertensive rats.
Endothelial dysfunction is characterized by reduced nitric oxide (NO) bioavailability, resulting in elevated vascular resistance and hypertension.1 Endothelial NO is mainly generated from the conversion of l-arginine to l-citrulline by endothelial nitric oxide synthase (eNOS), a process that involves multiple cofactors. In spontaneously hypertensive rats (SHRs), a pronounced loss of NO in both arterial and glomerular endothelial cells has been observed despite an increase in levels of eNOS.2–4 The basis for the paradoxical loss of NO despite increased enzyme levels is the production of O2 – by uncoupled eNOS and other oxidases. In particular, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase represents major sources of O2 – in animal models of hypertension and other models of cardiovascular risk.5 Even in rats that were made hypertensive by aortic banding, changes in eNOS uncoupling were observed with an associated loss of NO bioavailability.6 Superoxide produced by eNOS or NADPH oxidase can be scavenged by NO to form peroxynitrite (ONOO–).7 Peroxynitrite is a main component of nitroxidative stress. The ratio of NO concentration to ONOO– concentration is a good indicator of the status of the redox environment: cytoprotective vs. cytotoxic in dysfunctional endothelial cells.8
Amlodipine has been shown to increase the release of NO in intact human arteries, cardiac tissue, and isolated cells through eNOS-dependent pathways.9 The ability of amlodipine to stimulate eNOS was not reproduced by other calcium channel blockers (CCBs) in human arteries but related to a novel kinin-dependent pathway.9,10 Indeed, the inactive enantiomer of amlodipine was also able to stimulate eNOS despite little activity as an l-type CCB.10,11 In addition to activation of eNOS, amlodipine enhances vasodilation through modulation of excitation-contraction mechanisms in smooth muscle cells while reducing oxidative stress.9 In a clinical study, it was shown that both amlodipine and S(-)-amlodipine can improve endothelial function in hypertensive patients.12 Also, an amlodipine-based regimen reduced cardiovascular events compared with other antihypertensive regimens in a manner that correlated with reduced central aortic pressures and enhanced vasodilation.13
This study was conducted to evaluate the effects of amlodipine on endothelial NO release and simultaneous release of ONOO– in a well-established model of hypertension using direct measurements with amperometric nanosensors. It was hypothesized that amlodipine may stimulate NO release and reduce ONOO–-related nitroxidative stress in SHRs before changes in blood pressure (BP). To test this hypothesis, we measured NO and ONOO– release in aortic and glomerular endothelium from SHRs after amlodipine vs. vehicle treatments.
METHODS
Animals and materials
Male SHRs aged 7–9 weeks and weighing 250±20g were obtained from inbred colonies (Harlan Laboratories, Indianapolis, IN). SHRs were maintained in an environment that provided free access to food (commercial standard pellets for rodents) and water during the study. Calcium ionophore A23187 (CaI) was purchased from Sigma-Aldrich (St. Louis, MO). Amlodipine was provided by Pfizer (New York, NY).
Treatment groups
Rats were assigned either to vehicle or 5mg/kg/day amlodipine and compared with baseline rats (euthanized and tested at start of study). Six to 8 animals were included in each experimental group. For the drug-treated groups, amlodipine or vehicle alone (sterile water) was administered orally. After completion of the various treatments, rats were euthanized and tissue samples were obtained immediately for further experimentation. All procedures were conducted in accordance with standard and accepted Institutional Animal Care and Use Committee guidelines.
NO and ONOO– nanosensors
Concurrent measurements of NO and ONOO– were performed with a tandem of electrochemical nanosensors combined into one working unit. Their design was based on previously developed and chemically modified carbon-fiber technology.14,15 Each of the nanosensors was made by depositing a sensing material on the tip of a carbon fiber (length 4–5 µm; diameter 200–300nm). The fibers were sealed with nonconductive epoxy and electrically connected to copper wires with conductive silver epoxy. Conductive films of polymeric nickel(II)tetrakis(3-methoxy-4-hydroxyphenyl)porphyrin and polymeric manganese(III)-[2,2]paracyclophenylporphyrin were used for the NO and ONOO– sensors, respectively.
The amperometric method (with a response time of 0.1ms) provided a quantitative signal (current) that was directly proportional to changes (from basal levels) in NO or ONOO– concentration. Amperometric measurements were performed with a Gamry Reference 600 dual potentiostat (Gamry Instruments, Warminster, PA). Basal NO or ONOO– levels were measured by differential pulse voltammetry in separate experiments. The differential pulse voltammetry current at the peak potential for NO and ONOO– was directly proportional to the local concentrations of these compounds in the immediate vicinity of the sensor. For each set of analyses, linear calibration curves (current vs. concentration) were constructed for each sensor from 50nM to 400nM before and after measurements with aliquots of NO and ONOO– standard solutions.
Aortic NO and ONOO– measurements
Rats were euthanized with sodium thiopental (150mg/kg intraperitoneally), and aortic ring segments were isolated and immobilized on Sylgord film in an organ chamber containing Hank’s balanced salt solution at 37 ºC (pH 7.4). All measurements of NO and ONOO– were performed on intact endothelial cells (6–8 rats per experimental group, 3–4 measurements per rat). The module of NO and ONOO– nanosensors was positioned near the surface of individual endothelial cells using a remote-controlled micromanipulator (Sensapex, Oulu, Finland) and a microscope fitted with a CD camera (AmScope, Irvine, CA).
The sensors had a high reproducibility of measurement (±8%) at a constant distance (5±2 μm) from the surface of the endothelial cell. After establishing a background current, CaI was injected into the organ chamber using a nanoinjector (World Precision Instruments, Sarasota, FL). Rapid changes in current (proportional to the concentrations of NO or ONOO–) were observed after the addition of CaI and were monitored continuously.
Glomerular NO and ONOO– measurements
Immediately after rats were euthanized as described above, the kidneys were removed, cut into 100-µm sections, and transferred to an organ chamber containing Hank’s balanced salt solution (37 ºC; pH 7.4). The NO/ONOO– nanosensor module was positioned 5±2 μm from the surface of a glomerular endothelial cell (cortical zone). We included 6–8 rats per experimental group and performed 3–4 measurements per rat. All other aspects of NO and ONOO– measurement were performed as described for aortic endothelial cells above.
Measurement of BP
BP was measured using the CODA noninvasive BP system (Kent Scientific Corporation, Torrington, CT). All animals were acclimated to this procedure for 3 days before measurement to minimize stress-induced variations in BP. We included 6–8 rats per experimental group and performed 7–8 measurements per rat.
Statistical analyses
Data are presented as mean ± SEM. The significance of differences between results from independent experimental conditions was tested using either the two-tailed Student t test (measurements of NO and ONOO– release, including ratio calculations, from various treatments and diets) or 1-way analysis of variance with Student–Newman–Keuls multiple comparisons post hoc analysis. A value of P < 0.05 was considered significant.
RESULTS
Effects of amlodipine on BP
Systolic, diastolic, and mean arterial BP levels were elevated in SHRs at baseline and further increased during the course of the study (Table 1). Mean arterial BP was 149±3mm Hg and increased to 174±1mm Hg after 8 weeks with vehicle treatment. SHRs treated with amlodipine had measurements of systolic and diastolic BPs of 210±2mm Hg and 147±3mm Hg, respectively, which were not significantly different from vehicle treatments. However, a mean arterial BP of animals treated with amlodipine was 167±3mm Hg, slightly but significantly different from vehicle-treated animals.
Table 1.
Body weight and blood pressure data collected from rats examined in this study
| Treatment group | Body weight, g | SBP, mm Hg | DBP, mm Hg | MABP, mm Hg |
|---|---|---|---|---|
| SHRs, 0wk (baseline) | 309±4 | 186±3 | 131±3 | 149±3 |
| SHRs, vehicle treatment 8 wk | 398±6* | 214±1* | 154±1* | 174±1* |
| SHRs, amlodipine treatment 8 wk | 418±5*,** | 210±2* | 147±3* | 167±3*,** |
Values are reported as mean ± SEM (body weight: 6–8 rats per group; systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MABP): 6–8 rats per group, 7–8 measurements per rat). *P < 0.05 vs. baseline group; **P < 0.05 vs. rats treated with vehicle for 8 weeks (Student–Newman–Keuls multiple comparisons test; overall analysis of variance: body weight data: P < 0.0001, F = 142.02; SBP data: P < 0.0001; F = 55.352; DBP data: P < 0.0001, F = 20.760; MABP: P < 0.0001, F = 33.765). Abbreviation: SHRs, spontaneously hypertensive rats.
Effects of amlodipine on NO and ONOO– release from aortic endothelial cells
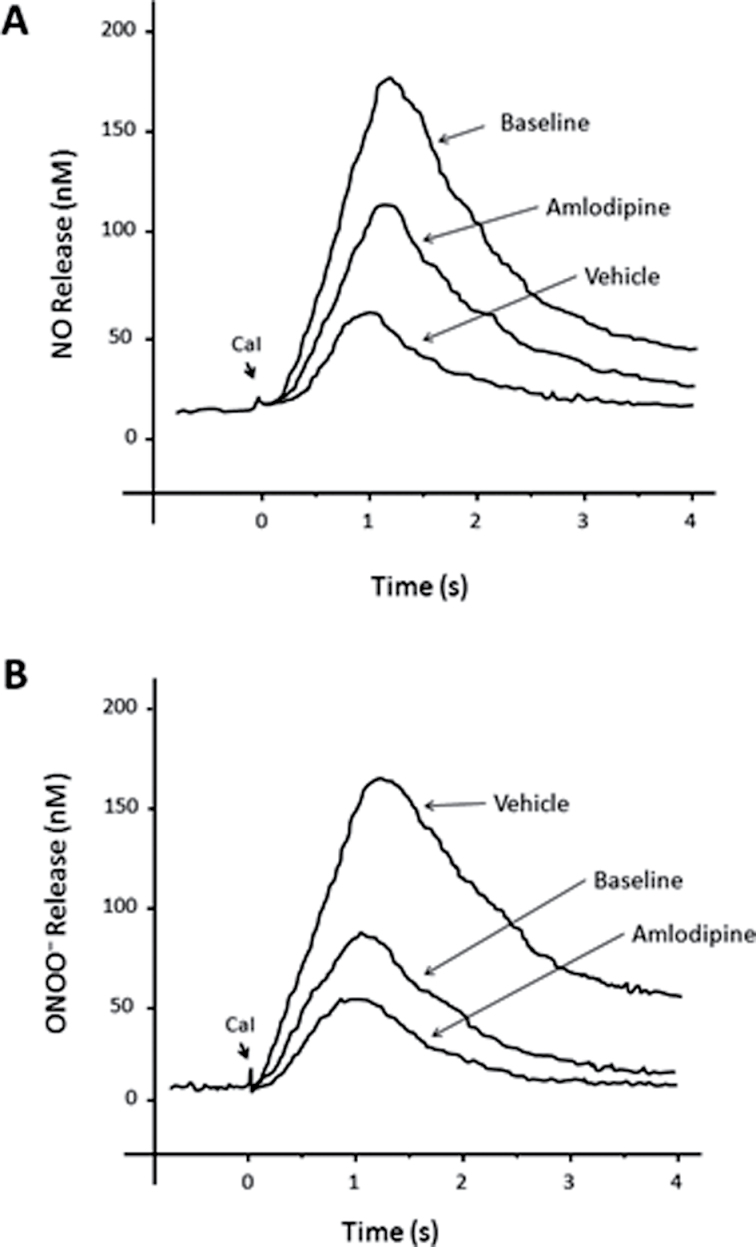
Figure 1 shows the amperograms of NO and ONOO– release, recorded after stimulation of aortic ECs with CaI. A rapid increase of both NO and ONOO– was observed, reaching a maximal concentration after about 1 second. Thereafter, 1 second of gradual decay in NO and ONOO– concentration was recorded. The maximal NO and ONOO– concentrations calculated from these measurements are shown in Figure 2. The aim of this experiment was to elucidate the effects of treatment with amlodipine on the relative changes in NO and ONOO– release in the aortic endothelium of SHRs after maximal stimulation of eNOS. In the absence of an exogenous eNOS agonist such as calcium, basal concentrations of NO (15±5nM) and ONOO– (5±4nM) were detected near the endothelial surface; these levels increased significantly after stimulation with CaI.
Figure 1.
Typical amperogram showing the change of nitric oxide (NO) (a) or peroxynitrite (ONOO–) (b) concentration release from an aortic endothelial cell. NO and ONOO– were measured with nanosensors after stimulation with calcium ionophore (CaI, 1μM).
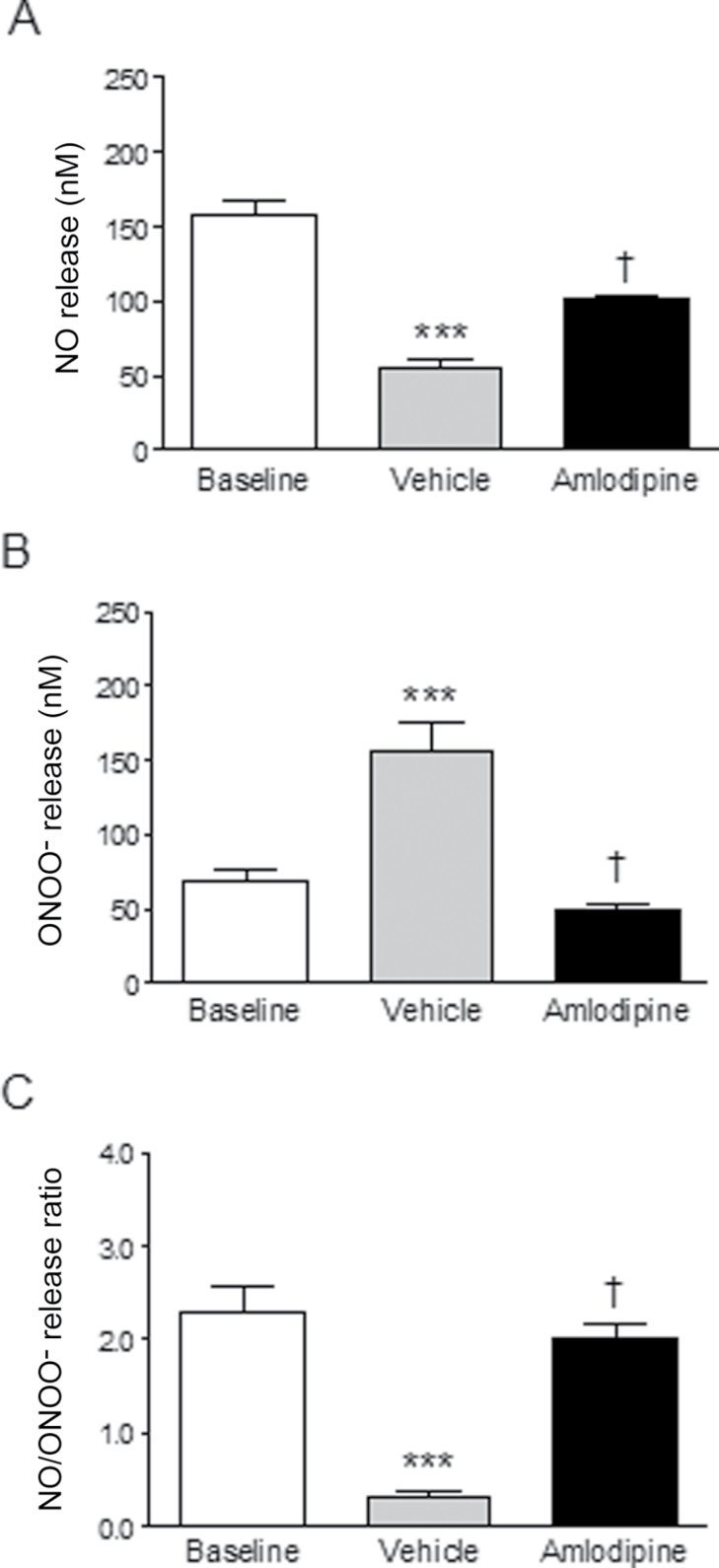
Figure 2.
Comparative effects of amlodipine treatment on nitric oxide (NO) (a) and peroxynitrite (ONOO–) (b) release from aortic endothelial cells isolated from study animals. Maximal concentrations of NO and ONOO– were measured from single endothelial cells after stimulation with calcium ionophore. The NO/ONOO– was calculated as the ratio of maximal concentration of NO and ONOO– (c). Values are mean ± SEM (6–8 rats per group, 3–4 measurements per rat). ***P < 0.001 vs. baseline; † P < 0.001 vs. vehicle treatment (Student–Newman–Keuls multiple comparisons test; overall analysis of variance: NO data: P < 0.0001, F = 55.773; ONOO– data: P < 0.0001, F = 24.974; NO/ONOO– ratio data: P < 0.0001, F = 33.417).
As compared with baseline measurements, there was a pronounced loss in stimulated NO release in the SHRs over 8 weeks. The maximal release of NO decreased by about 290%, from 157±11nM to 55±6nM (P < 0.001), with a concomitant increase in levels of ONOO– by 230%, from 69±7nM to 156±19nM (P < 0.001). The NO/ONOO– ratio, a comprehensive measurement of eNOS function, decreased by >7-fold (from 2.3±0.3nM to 0.3±0.1nM) in endothelial cells after 8 weeks (P < 0.001). Treatment with amlodipine reversed endothelial dysfunction, as evidenced by increased NO release, while decreasing nitroxidative stress. Amlodipine treatment was associated with increased aortic NO release by 184%, to 101±3nM (P < 0.001), and the NO/ONOO– ratio increased by >6-fold (to 2.0±0.2) in endothelial cells (P < 0.001), reaching a level observed for baseline levels.
Effects of amlodipine on NO and ONOO– release from glomerular endothelial cells
As compared with baseline measurements, there was a pronounced loss in glomerular NO bioavailability in the SHRs over 8 weeks (Figure 3). The maximal release of NO decreased by about 250%, from 72±4nM to 29±3nM (P < 0.001), with a concomitant increase in levels of ONOO– by 165%, from 66±5nM to 108±10nM (P < 0.001). The NO/ONOO– ratio, a comprehensive measurement of eNOS function, decreased by >3-fold (from 1.1±0.1 to 0.3±0.1) in glomerular endothelial cells after 8 weeks (P < 0.001). As observed in aortic endothelial cells, treatment with amlodipine increased glomerular NO release while decreasing nitroxidative stress. Amlodipine treatment increased glomerular NO release to 69±3nM (P < 0.001) and the NO/ONOO– ratio increased by >5-fold (from 0.3±0.1 to 1.5±0.2) in endothelial cells (P < 0.001).
Figure 3.
Comparative effects of amlodipine treatment on nitric oxide (NO) (a) and peroxynitrite (ONOO–) (b) release from glomerular endothelial cells isolated from study animals. The drug treatment was compared with baseline levels and vehicle treatments. Maximal concentration of NO and ONOO– were measured from single endothelial cells after stimulation with calcium ionophore. The NO/ONOO–was calculated as the ratio of maximal concentration of NO and ONOO– (c). Values are mean ± SEM (6–8 rats per group, 3–4 measurements per rat). *P < 0.05 and ***P < 0.001 vs. baseline; † P < 0.001 vs. vehicle treatment (Student–Newman–Keuls multiple comparisons test; overall analysis of variance: NO data: P < 0.0001, F = 43.353; ONOO– data: P < 0.0001, F = 19.514; NO/ONOO– ratio data: P < 0.0001, F = 30.197).
DISCUSSION
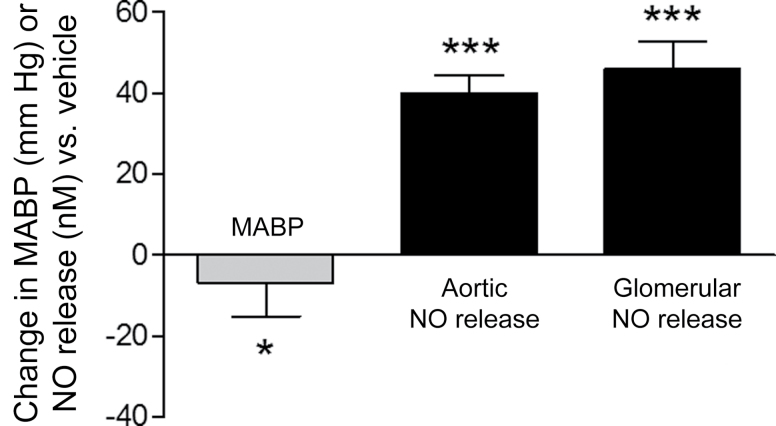
The key finding from this study is that treatment of hypertensive rats with amlodipine dramatically improved NO release from aortic and glomerular endothelial cells. The increase in NO release with amlodipine treatment was associated with a large and disproportionate decrease in nitroxidative stress, as reflected by ONOO– production. The NO/ONOO– ratio, an indicator of eNOS coupling efficiency, increased in aortic and glomerular endothelium >5-fold with amlodipine treatment compared with vehicle. The increases in NO release and the NO/ONOO– ratio were not directly proportional to a small change in BP (Figures 2–4). These findings support the hypothesis that amlodipine has direct effects on endothelial activity and NO metabolism in hypertension that cannot be predicted by peripheral measurements of BP. Because the endothelial cells do not have high levels of l-type calcium channels, these findings support a potent antioxidant effect with amlodipine. This effect is potentiated by increased NO bioavailability independently of changes in calcium mobilization.
Figure 4.
Comparative effects of amlodipine on change in mean arterial blood pressure (MABP) vs. nitric oxide (NO) release from aortic and glomerular endothelial cells as compared with vehicle treatment. Values are mean ± SEM. *P = 0.0179 and ***P < 0.001 vs. vehicle treatment (unpaired, 2-tailed Student t test).
The eNOS protein is a tightly regulated electron transport enzyme that is uncoupled by alterations in the availability of substrate (l-arginine and/or oxygen) and/or cofactors such as tetrahydrobiopterin (BH4).5 Uncoupling of eNOS is also associated with increased concentrations of asymmetrical dimethyl-arginine and the production of superoxide that leads to the conversion of NO to ONOO–, a powerful pro-oxidant that interferes with the expression and activity of eNOS. Recouplings of eNOS with l-arginine, BH4, and antioxidants are mechanisms by which amlodipine may have favorable effects on eNOS when administered alone or in combination with other modulators of this enzyme. In particular, we have previously observed an additional increase in NO release from isolated human endothelial cells when amlodipine was combined with a statin.16 A reduction in ONOO– production with amlodipine treatment by >50% is also evidence for antioxidant actions.9,17 By direct scavenging of free radicals through resonance stabilization mechanisms, amlodipine may reduce the generation of ONOO– from NO and O2 –. These findings do not exclude the possibility that other approaches to reducing oxidative stress may enhance the actions of amlodipine, such as oxidase inhibitors or other potent chain-breaking antioxidants.
Endothelial dysfunction is characterized by attenuated NO bioavailability resulting in elevated vascular resistance and reduced sensitivity to normal stimuli of vasodilation, such as shear stress and acetylcholine.1 This abnormality is an early event in atherogenesis and causally related to accelerated rates of oxidative stress. Aging, vascular injury, and metabolic disorders contribute to reduced NO bioavailability.18,19 SHRs are characterized by low levels of functional NO, despite increased eNOS enzyme levels, due to excessive O2 – generation that reacts with NO to form ONOO–.20 The eNOS uncoupling in SHRs was shown to be reversed with inhibitors of NADPH oxidase and augmentated with l-arginine or the cofactor BH4.4 These findings are consistent with studies linking endothelial dysfunction in hypertension to excessive O2 – production through eNOS uncoupling and oxidase activity.21,22 Thus, renal and cardiac microvascular disturbances in hypertension are also closely related to a loss in NO bioavailability.23
Certain antihypertensive agents improve vasodilation through enhanced NO bioavailability by either increasing endogenous production through enzymatic mechanisms or by stimulating direct release by its redox congeners in a spontaneous fashion.24 Inhibitors of the renin-angiotensin system, such as angiotensin-converting enzyme (ACE) inhibitors enhance NO release by inhibiting NADPH oxidase while preventing ACE-mediated degradation of bradykinin, an agonist of endothelial-dependent NO release. Amlodipine improves endothelial-dependent release of NO through a novel kinin-related mechanism along with antioxidant effects.9,10,25 In coronary microvessels isolated from canine cardiac tissue, amlodipine caused a dose-dependent release of nitrite, the oxidation product of NO.10 The effects of amlodipine on both nitrite release and the NO-dependent regulation of cardiac oxygen consumption were reduced with antagonists of eNOS such as l-NG-monomethyl arginine. Under identical conditions, other dihydropyridine- and non dihydropyridine -type CCBs, such as nifedipine and diltiazem, respectively, failed to reproduce these effects. In addition to activation of eNOS, amlodipine enhances vasodilation by increasing the antioxidant capacity and relaxation of vascular smooth muscle cells.9
Treatment of hypertensive subjects with either CCBs or RAS inhibitor reduced the central aortic augmentation index, consistent with a reduction in arterial stiffness and improved vessel wall elasticity.26,27 In healthy volunteers, infusion of angiotensin II significantly increased the augmentation index, whereas infusion of the vasodilator nitroglycerin reduced the augmentation index.28 In a crossover study of untreated hypertensive patients, bisoprolol produced a greater reduction in brachial systolic BP than other antihypertensives but increased the central aortic augmentation index.29 In elderly patients with elevated systolic BP, nonvasodilating β-blockers increased the augmentation index and augmented aortic systolic BP, whereas CCBs and ACE inhibitors lowered these parameters.30
The use of a RAS inhibitor combination with amlodipine has been supported by clinical studies. In the Conduit Artery Function Evaluation, patients with hypertension were assigned to a CCB/ACE inhibitor regimen (amlodipine plus perindopril) or β-blocker/diuretic regimen (atenolol plus bendroflumethiazide) to achieve BP targets.13 Both antihypertensive treatment approaches produced similar reductions in brachial BP, but the CCB/ACE inhibitor regimen reduced central aortic pressure to a greater extent that correlated with reductions in clinical events.13 Results from the Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension study have also provided clinical support for the combination of a RAS inhibitor (ACE inhibitor) and amlodipine in reducing the rate of cardiovascular events as compared with a diuretic combination that again does not influence central aortic pressures.31 These agents may have complimentary effects on endothelial function and vasodilation that improve cardiovascular outcomes that cannot be predicted only by changes in brachial arterial BP changes.
The results of this study provide new insights into the role of amlodipine on endothelial function in animals with hypertension. Hypertensive animals demonstrated significant reductions in NO release and increased nitroxidative stress reflected by high ONOO– production. Amlodipine reversed these changes, as evidenced by increased NO release with concomitant reductions in ONOO– levels. The vascular benefits observed with amlodipine may be attributed to improved eNOS coupling efficiency associated with reduced oxidative stress through various enzymatic and nonenzymatic pathways. These direct, vascular effects of amlodipine treatment may contribute to reduced risk for atherothrombotic events beyond BP control.
DISCLOSURE
This study was supported, in part, by an investigator- initiated research grant to R.P.M. from Pfizer, which also provided the drug used in this study. The other authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Marvin White Endowment Fund at Ohio University. A special thanks to Pfizer for providing the drug used in this study and to Collin Arocho and Paula Hale for their assistance in the preparation of this manuscript. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (grant C06 RR-014575-01) from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1. Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990; 323:22–27 [DOI] [PubMed] [Google Scholar]
- 2. Mason RP, Kubant R, Jacob RF, Walter MF, Boychuk B, Malinski T. Effect of nebivolol on endothelial nitric oxide and peroxynitrite release in hypertensive animals: role of antioxidant activity. J Cardiovasc Pharmacol 2006; 48:862–869 [DOI] [PubMed] [Google Scholar]
- 3. McIntyre M, Hamilton CA, Rees DD, Reid JL, Dominiczak AF. Sex differences in the abundance of endothelial nitric oxide in a model of genetic hypertension. Hypertension 1997; 30:1517–1524 [DOI] [PubMed] [Google Scholar]
- 4. Mason RP, Kubant R, Jacob RF, Malinski P, Huang X, Louka FR, Borowiec J, Malinski T. Loss of arterial and renal nitric oxide bioavailability in hypertensive rats with diabetes: effect of beta-blockers. Am J Hypertens 2009; 22:1160–1166 [DOI] [PubMed] [Google Scholar]
- 5. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006; 113:1708–1714 [DOI] [PubMed] [Google Scholar]
- 6. Bouloumié A, Bauersachs J, Linz W, Schölkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension 1997; 30:934–941 [DOI] [PubMed] [Google Scholar]
- 7. Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517 [DOI] [PubMed] [Google Scholar]
- 8. Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol 2012; 60:467–473 [DOI] [PubMed] [Google Scholar]
- 9. Mason RP, Marche P, Hintze TH. Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol 2003; 23:2155–2163 [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation 1998; 97:576–580 [DOI] [PubMed] [Google Scholar]
- 11. Zhang XP, Loke KE, Mital S, Chahwala S, Hintze TH. Paradoxical release of nitric oxide by an L-type calcium channel antagonist, the R+ enantiomer of amlodipine. J Cardiovasc Pharmacol 2002; 39:208–214 [DOI] [PubMed] [Google Scholar]
- 12. Si D, Yang C, Ni L, Li B, Ding M, He Y, Yang P. The effects of amlodipine and S(-)-amlodipine on vascular endothelial function in patients with hypertension. Am J Hypertens 2013; e-pub ahead of print 19 August 2013. [DOI] [PubMed] [Google Scholar]
- 13. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006; 113:1213–1225 [DOI] [PubMed] [Google Scholar]
- 14. Mason RP, Kalinowski L, Jacob RF, Jacoby AM, Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation 2005; 112:3795–3801 [DOI] [PubMed] [Google Scholar]
- 15. Malinski T, Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992; 358:676–678 [DOI] [PubMed] [Google Scholar]
- 16. Mason RP, Kubant R, Heeba G, Jacob RF, Day CA, Medlin YS, Funovics P, Malinski T. Synergistic effect of amlodipine and atorvastatin in reversing LDL-induced endothelial dysfunction. Pharm Res 2008; 25:1798–1806 [DOI] [PubMed] [Google Scholar]
- 17. Mason RP, Walter MF, Trumbore MW, Olmstead EG, Mason PE. Membrane antioxidant effects of the charged dihydropyridine calcium antagonist amlodipine. J Mol Cell Cardiol 1999; 31:275–281 [DOI] [PubMed] [Google Scholar]
- 18. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001; 38:274–279 [DOI] [PubMed] [Google Scholar]
- 19. Funovic P, Korda M, Kubant R, Barlag RE, Jacob RF, Mason RP, Malinski T. Effect of beta-blockers on endothelial function during biological aging: a nanotechnological approach. J Cardiovasc Pharmacol 2008; 51: 208–215 [DOI] [PubMed] [Google Scholar]
- 20. Kerr S, Brosnan MJ, McIntyre M, Raid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension role of the endothelium. Hypertension 1999; 33:1353–1358 [DOI] [PubMed] [Google Scholar]
- 21. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holand SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003; 111:1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74:1141–1148 [DOI] [PubMed] [Google Scholar]
- 23. Fujii H, Takiuchi S, Kawano Y, Fukagawa M. Putative role of asymmetric dimethylarginine in microvascular disease of kidney and heart in hypertensive patients. Am J Hypertens 2008; 21:650–656 [DOI] [PubMed] [Google Scholar]
- 24. Kojda G, Harrison DG. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571 [DOI] [PubMed] [Google Scholar]
- 25. Zhang X, Kichuk MR, Mital S, Oz M, Michler R, Nasjletti A, Kaley G, Hintze TH. Amlodipine promotes kinin-mediated nitric oxide production in coronary microvessels of failing human hearts. Am J Cardiol 1999; 84:27L–33L [DOI] [PubMed] [Google Scholar]
- 26. Chen CH, Ting CT, Lin SH, Hsu TL, Yin FC, Siu CO, Chous P, Wang SP, Chang MS. Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension 1995; 25:1034–1041 [DOI] [PubMed] [Google Scholar]
- 27. Hirata K, Vlachopoulos C, Adji A, O’Rourke MF. Benefits from angiotensin-converting enzyme inhibitor “beyond blood pressure lowering”: beyond blood pressure or beyond the brachial artery? J Hypertens 2005; 23:551–556 [DOI] [PubMed] [Google Scholar]
- 28. Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension 2001; 37:1429–1433 [DOI] [PubMed] [Google Scholar]
- 29. Deary AJ, Schumann AL, Murfet H, Haydock S, Foo RS, Brown MJ. Influence of drugs and gender on the arterial pulse wave and natriuretic peptide secretion in untreated patients with essential hypertension. Clin Sci 2002; 103:493–499 [DOI] [PubMed] [Google Scholar]
- 30. Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 2004; 17:118–123 [DOI] [PubMed] [Google Scholar]
- 31. Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ. ACCOMPLISH Trial investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428 [DOI] [PubMed] [Google Scholar]