Abstract
BACKGROUND
Elevated central pressures and arterial stiffness are associated with increased peripheral resistance and higher sympathetic nervous system activity. Additionally, consumption of a meal is known to be sympathoexcitatory. However, the acute effects of a meal on aortic wave reflection and stiffness are unknown. Therefore, we tested the hypothesis that aortic wave reflection and stiffness would increase after a meal.
METHODS
We examined these effects using high-fidelity radial arterial pressure waveforms and carotid–femoral pulse wave velocity measured noninvasively by applanation tonometry before and 60 and 180 minutes after ingestion of a liquid mixed meal (Ensure; 40% of daily energy expenditure) in 17 healthy adults (9 men/8 women; aged 29±2 years). Additionally, we measured sympathetic activity by microneurography at baseline and up to 60 minutes after the meal.
RESULTS
Although sympathetic activity increased after the meal, both peripheral and central pressures were reduced at 180 minutes from baseline (all P < 0.05). Contrary to our hypothesis, augmentation index (14% ± 3% vs. 2% ± 3% vs. 8% ± 3%), augmentation index normalized for heart rate (8% ± 3% vs. −3% ± 3% vs. 3% ± 3%), augmented pressure (5±1mm Hg vs. 1±1mm Hg vs. 3±1mm Hg), and pulse wave velocity (7.1±0.2 m/s vs. 6.7±0.2 m/s vs. 6.7±0.1 m/s) were substantially reduced at 60 and 180 minutes after the meal (all P < 0.05).
CONCLUSIONS
Taken together, our results suggest that a liquid mixed meal acutely decreases central hemodynamics and arterial stiffness in healthy adults, which may be a result of meal-related increases in insulin and/or visceral vasodilation.
Keywords: aortic pressure, blood pressure, hypertension, mixed meal, pulse wave velocity, sympathetic nerve activity, wave reflection.
Central hemodynamics, which includes the pressure exerted on the heart and brain, commonly differ from peripheral pressures and are strongly influenced by arterial stiffness and wave reflections.1 Additionally, elevated central hemodynamics, central (aortic) arterial stiffness, and indices of aortic wave reflection are associated with increased cardiovascular risk.1 The central arterial pressure wave is composed of a forward traveling wave generated by left ventricular ejection and a later-arriving reflected wave from the periphery.2 Increased arterial stiffness causes an elevation in pulse wave velocity (PWV), which causes early return of reflected waves from the periphery back to the heart and thus augments ascending aortic systolic and pulse pressures and left ventricular afterload.2 Sympathetic nervous system activity, measured by muscle sympathetic nerve activity (MSNA), has been shown to be independently related to PWV in healthy humans.3,4 Furthermore, elevations in sympathetic neural outflow appear to have a stiffening influence on arterial mechanical properties and may contribute to myocardial hypertrophy and vascular remodeling.3,5–7
Consumption of a meal is associated with an increase in MSNA.8 The postprandial state has been shown to be proatherogenic,9 and it is possible that the increase in sympathetic activity after a meal contributes to increased arterial stiffness in conduit arteries and increased cardiac work; however, the relationship between acute food intake, MSNA, central pressures, and indices of arterial stiffness in humans has not been studied. In this context, we tested the hypothesis that aortic wave reflection and stiffness and central pressures would increase after consumption of a mixed meal. Healthy, young adults were studied to determine the acute effects of a liquid mixed meal in a population without elevated central hemodynamics and levels of arterial stiffness or confounding influences of cardiovascular risk factors.
METHODS
A total of 17 young, healthy adults (9 men/8 women; aged 29±2 years; body mass index: 24.1±0.5kg/m2) completed the study. Subjects completed written, informed consent. Studies were performed after an overnight fast, and subjects refrained from exercise, alcohol, and caffeine for 24 hours. All subjects followed a 3-day standardized balanced diet that supplied 100% of estimated total daily energy expenditure before the study. All study protocols were approved by the Mayo Clinic Institutional Review Board.
Studies were performed in the clinical research unit at the Mayo Clinic starting at 7:00 am. Subjects were studied at rest in the supine position. A 20-gauge, 5-cm catheter was placed in the brachial artery of the left arm under sterile conditions after local anesthesia for measurements of blood pressure (BP) and for arterial blood samples. Heart rate (HR) was measured from an electrocardiogram.
The assessment of arterial wave reflection characteristics was performed noninvasively using the SphygmoCor system (AtCor Medical, Sydney, Australia) as described previously.2 Briefly, high-fidelity radial artery pressure waveforms were recorded by applanation tonometry of the radial pulse in the right wrist using a pencil-type micromanometer (Millar Instruments, Houston, TX). The radial BP and waveforms were calibrated from the systolic and diastolic brachial artery BP. A validated, generalized transfer function was used to generate the corresponding aortic pressure waveform. The generalized transfer function has been validated using both intra-arterially10,11 and noninvasively12 obtained radial pressure waves.
Pulse wave analysis of the aortic pressure waveform provided the following key variables of interest: (i) aortic BPs; (ii) aortic augmentation index (AIx; the reflected wave amplitude divided by pulse pressure expressed as a percentage); (iii) AIx adjusted for HR (AIx@75 bpm); (iv) augmented pressure (AP; the amplitude of the reflected wave, defined as the difference between the first (forward wave) and second systolic shoulders of the aortic systolic BP); and (v) wasted left ventricular pressure energy (Ew; the component of extra myocardial oxygen requirement attributable to early systolic wave reflection). Ew can be estimated as ((π/4)×(augmented pressure ×Δtr)×1.333), where 1.333 is the conversion factor from mm Hg/s to dyne × cm2 × s and Δtr is the systolic duration of the reflected wave. Only high-quality recordings, defined as an in-device quality index of >80% (derived from an algorithm including average pulse height variation, diastolic variation, and the maximum rate of rise of the peripheral waveform) were accepted for analysis. In general, 2–3 measurements were performed to get 2 measurements with an acceptable quality index.
To determine central PWV, pressure waveforms were recorded at the carotid and femoral arteries using the SphygmoCor PWV Vx system (AtCor Medical). Pressure waveforms were gated with simultaneous electrocardiogram and used to calculate PWV between the 2 sites. Carotid–femoral PWV (cfPWV) was calculated by determining the delay between the appearance of each pressure waveform foot in the carotid and femoral sites.13 The distance between recording sites was adjusted for parallel transmission in the aorta and carotid by correcting for the distance between the supra-sternal notch and the carotid, and these corrected distances were divided by the respective foot-to-foot transmission delays to give PWV. All pulse wave analysis variables were recorded by a single investigator on the right side of the subject and are reported as the mean of 2 applanation tonometry measurements for each individual.
MSNA was recorded in the peroneal nerve posterior to the fibular head with a tungsten microelectrode, as described previously.14 Sympathetic bursts in the integrated neurogram were identified by a custom-manufactured, semiautomated analysis program,15,16 and burst identification was controlled visually by a single investigator. The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle. MSNA was analyzed and expressed as both burst incidence (bursts per 100 heartbeats) and frequency (bursts per minute).
Beat-to-beat stroke volume was calculated from the brachial arterial pulse pressure wave by model flow analysis. Model flow computes an aortic waveform based on nonlinear pressure–volume, pressure–compliance, and pressure–characteristic impedance equations, incorporating age, sex, height, and body mass.17 Cardiac output (CO) was calculated as the average stroke volume measured over 5 minutes multiplied by the HR measured over the same 5-minute period.
Arterial blood was drawn from the brachial arterial catheter for analysis of glucose, insulin, norepinephrine, and epinephrine. Samples were analyzed by standard assays in the Immunochemical Core Laboratory of the clinical research unit.
After instrumentation, the microelectrode for microneurography was placed. Once a satisfactory site for measurement of MSNA was located, 15 minutes of baseline data were recorded with the subject resting quietly. Subsequently, a liquid mixed meal (Ensure; Abbot Laboratories, Columbus, OH) supplying 40% of each individual’s daily resting energy expenditure (58% carbohydrates, 28% fat, and 14% protein) was consumed by the subject over a period of 5 minutes. The average weight of the liquid mixed meal consumed by our subjects was 449±17 grams, and the total average energy was 661±25 kcals for each study day. MSNA was measured throughout the study or until a satisfactory recording was lost (roughly 60 minutes after the meal in most subjects). Blood samples were drawn and applanation tonometry measurements were conducted at baseline (before the meal) and at 60 minutes and 180 minutes after the meal.
Data were collected at 250 Hz, stored on a computer, and analyzed offline with signal processing software (WinDaq; DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was calculated as the time integral over the pulse pressure. CO was calculated as the average stroke volume over a 5-minute period multiplied by the HR measured over the same time period. Total peripheral vascular resistance (TPR) was calculated as MAP/CO. MAP, HR, stroke volume, CO, and TPR were averaged from the last 5 minutes of the 15-minute MSNA baseline period and from 5-minute periods during applanation tonometry measurements 60 minutes and 180 minutes after the meal.
Group data are expressed as means ± SEM. Aortic wave reflection characteristics, carotid–femoral PWV, MSNA, central pressures, hemodynamic variables, and blood samples were analyzed and compared between time points by repeated measures analysis of variance using SigmaStat software version 12.0 (Systat Software Inc., San Jose, CA). Significance was set a priori at P < 0.05.
RESULTS
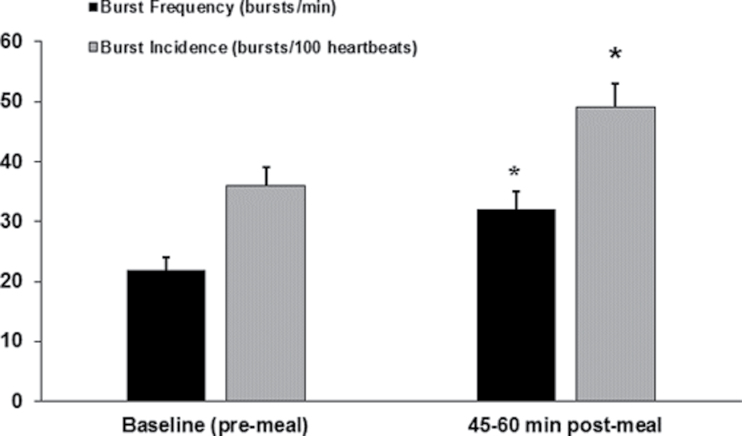
All 17 subjects completed the study protocol; however, MSNA data was only obtained on 13 subjects. Burst frequency and burst incidence increased from baseline at 45–60 minutes after the meal (22±2 vs. 32±3 bursts per minute, respectively; 36±3 vs. 49±4 bursts per 100 heartbeats, respectively; both P < 0.05) (Figure 1). At 60 minutes after the meal, HR and CO were elevated (P < 0.05) but all central and peripheral pressures were similar to baseline (Table 1). However, TPR was reduced from baseline at both 60 and 180 minutes after the meal (all P < 0.05). Additionally, 180 minutes after the meal, MAP and brachial and aortic systolic and diastolic BPs were reduced from baseline (all P < 0.05). MAP, brachial systolic BP, and brachial pulse pressure were also further attenuated at 180 minutes when compared with 60 minutes after the meal (all P < 0.05).
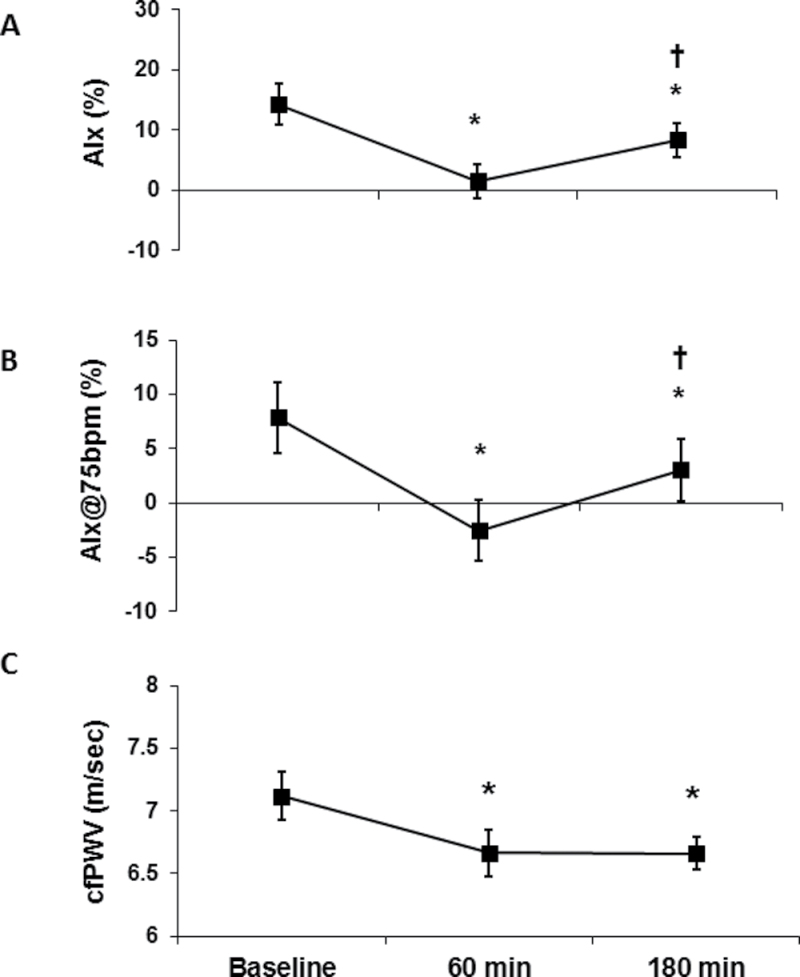
Figure 2.
Acute effects of a liquid mixed meal on indices of aortic wave reflection and pulse wave velocity. (a) Augmentation index (AIx), (b) augmentation index normalized for a heart rate of 75 bpm (AIx@75bpm), and (c) carotid–femoral pulse wave velocity (cfPWV) at baseline (before the meal), 60 minutes and 180 minutes after consumption of a liquid mixed meal. Data are mean ± SEM. *P < 0.05 vs. baseline; † P < 0.05 180 minutes vs. 60 minutes.
Table 1.
Hemodynamics and waveform characteristics
| Characteristic | Baseline | 60min postmeal | 180min postmeal |
|---|---|---|---|
| HR, bpm | 62±2 | 66±2* | 65±2 |
| MAP, mm Hg | 85±2 | 85±2 | 80±2*,** |
| Brachial SBP, mm Hg | 114±2 | 116±2 | 108±2*,** |
| Brachial DBP, mm Hg | 66±2 | 64±2 | 61±2* |
| Brachial PP, mm Hg | 48±2 | 52±2 | 47±1** |
| Aortic SBP, mm Hg | 100±2 | 96±2 | 93±2* |
| Aortic DBP, mm Hg | 67±2 | 65±2 | 62±2* |
| Aortic PP, mm Hg | 33±2 | 32±1 | 31±1 |
| CO, L/min | 5.8±0.3 | 6.3±0.3* | 6.0±0.3 |
| TPR, mm Hg/L/min | 15.2±0.7 | 13.8±0.6* | 13.7±0.6* |
| Augmented pressure, mm Hg | 5±1 | 1±1* | 3±1*,** |
| ED, ms | 330±4 | 323±5 | 315±6* |
| Ejection % | 34±1 | 36±1* | 34±1** |
| PP amplification, % | 148±5 | 166±4* | 155±4** |
| Ew, dyne × cm2 × s | 1,053±282 | 210±185* | 525±181* |
Data are mean ± SEM.
Abbreviations: CO, cardiac output; DBP, diastolic blood pressure; ED, ejection duration; Ew, wasted energy; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; TPR, total peripheral resistance.
*P < 0.05 vs. baseline; **P < 0.05 180 minutes vs. 60 minutes.
Similar to our hemodynamic findings, indices of aortic wave reflection were reduced (Table 1). Notably, AIx and AIx@75 bpm were reduced at 60 and 180 minutes after the meal when compared with baseline (AIx: 14% ± 3% vs. 2% ± 3% vs. 8% ± 3%, respectively; AIx@75bpm: 8% ± 3% vs. −3% ± 3% vs. 3% ± 3%, respectively; all P < 0.05) (Figure 2a,b) and, although still reduced at 180 minutes compared with baseline, were higher when compared with 60 minutes after the meal (AIx: 8% ± 3% vs. 2% ± 3%, respectively; AIx@75bpm: 3% ± 3% vs. −3% ± 3%, respectively; all P < 0.05). AP and Ew were reduced at 60 and 180 minutes from baseline (all P < 0.05) (Table 1), and ejection duration was reduced 180 minutes after the meal when compared with baseline (P < 0.05). However, ejection percentage and pulse pressure amplification were augmented at 60 minutes compared with baseline, yet attenuated at 180 minutes compared with 60 minutes (all P < 0.05). Lastly, AP was augmented at 180 minutes when compared with 60 minutes after the meal (P < 0.05). cfPWV was also reduced at both 60 and 180 minutes after the meal when compared with baseline (6.7±0.1 vs. 6.7±0.2 vs. 7.1±0.2cm/s, respectively; P < 0.05 for both); however, there was no difference in cfPWV between 60 and 180 minutes after the meal (Figure 2c).
Figure 1.
Muscle sympathetic nerve activity (MSNA) before the meal and 45–60 minutes after consumption of a liquid mixed meal. Burst frequency measured in bursts/minute; burst incidence measured in bursts/100 heart beats. Data are mean ± SEM. *P < 0.05 vs. baseline.
Glucose, insulin, and norepinephrine were elevated at 60 minutes compared with baseline (P < 0.05) (Table 2); however, epinephrine was reduced at 60 minutes after the meal (P < 0.05). Glucose and insulin were both reduced at 180 minutes compared with 60 minutes (P < 0.05) but were still elevated above baseline (P < 0.05). Norepinephrine also remained elevated at 180 minutes compared with baseline (P < 0.05).
Table 2.
Blood results
| Arterial sample | Baseline | 60min postmeal | 180min postmeal |
|---|---|---|---|
| Glucose, mg/dl | 94±2 | 142±2* | 108±4*,** |
| Insulin, mg/dl | 4±1 | 56±6* | 19±2*,** |
| Epinephrine, mg/dl | 29±4 | 20±2* | 26±4 |
| Norepinephrine, mg/dl | 134±12 | 179±14* | 167±14* |
Data are mean ± SEM.
*P < 0.05 vs. baseline; **P < 0.05 180 minutes vs. 60 minutes.
Previously, we have shown that indices of aortic wave reflection are related to MSNA but that the relationship is different between men and women.18 Additionally, vasoconstrictor responsiveness to sympathetic stimulation is different between sexes.19–21 Therefore, we explored potential sex differences in our data; however, we found none. Although women had higher baseline levels of AIx and AIx@75 bpm, there were no sex differences in the response to the meal.
DISCUSSION
Our most salient findings from this study are that consumption of a liquid mixed meal acutely reduces central hemodynamics, indices of aortic wave reflection, and central arterial stiffness in healthy, young adults. Because consumption of a meal has been shown to be sympathoexcitatory, we hypothesized that arterial stiffness and central hemodynamics would be elevated due to their known association with MSNA.3,22 However, we found that healthy adults demonstrated reductions in measures of arterial stiffness and indices of aortic wave reflection and attenuations in both peripheral and central pressures up to 180 minutes after a meal. The mechanisms for the favorable effects of an acute meal on vascular function observed in this study are unclear but may be related to increased levels of insulin or postprandial visceral vasodilation.
There are a number of studies exploring the cardiovascular responses to various macronutrient meals in healthy adults, and of particular interest is the role of insulin in mediating these responses.23,24 Specifically, the carbohydrate content of a meal, which results in distinct changes in the magnitude of postprandial glucose and insulin, has been shown to result in different reductions in AIx in healthy, postmenopausal women, with greater reductions as a result of a high-carbohydrate, high-fat meal as opposed to a low-carbohydrate, high-fat meal.25 Greenfield et al.25 also demonstrated that the observed reductions in AIx and central pressures in insulin-resistant, postmenopausal women after high- and low-carbohydrate, high-fat meals were similar to those in insulin-sensitive subjects. Insulin is greatest after high-carbohydrate meals and has been shown to stimulate vasodilation in the skeletal muscle and to result in greater increases in superior mesenteric artery flow than increases due to protein, fat, or water intake.26 Therefore, it appears that the 14-fold increase in insulin concentration we found in this study is likely involved in driving the peripheral vasodilation and reductions in central and peripheral pressures. In support of this idea, Westerbacka et al.24 demonstrated that physiological doses of insulin significantly decreased aortic wave reflection in healthy men before any effects on forearm blood flow, peripheral pressures, or TPR, which would indicate that insulin likely influences the distensibility of large to medium conduit arteries before arterioles. These findings are consistent with our observation of marked reductions 60 minutes after a meal in aortic wave reflection indices (AIx and AP) and cfPWV. However, because PWV is heavily influenced by distending pressure, the attenuation in cfPWV could be partially mediated by the slight decreases in aortic systolic pressure after a meal (Table 1).
The reductions in central hemodynamics and indices of aortic wave reflection may also result from substantial postprandial visceral vasodilation. Along these lines, ingestion of a meal results in intestinal hyperemia with significant increases in superior mesenteric artery blood flow velocity and volume.27 During both ingestion and absorption of a meal, distinct cardiovascular processes occur that result in increased sympathetic nervous system activity, CO, BP, HR, and splanchnic and renal vascular resistance, with concurrent increases in blood flow throughout the gastrointestinal tract.28 This increased blood flow to the digestive organs in the postprandial state results in compensatory reductions in flow to other organs, including skeletal muscles.28
Interestingly, these hemodynamic changes appear to be dependent on the direct effects of specific macronutrients. Potentially because of the effects of hyperinsulinemia on measures of aortic wave reflection and PWV, a high-carbohydrate meal has also been shown to result in different cardiovascular and regional vascular responses when compared with a high-fat meal. Specifically, a high-carbohydrate meal has been associated with greater increases in glucose, insulin, and norepinephrine, as well as more immediate increases in superior mesenteric artery blood flow.29 Kearney et al.30 have also demonstrated that a physiological insulin infusion combined with a high-fat meal resulted in significantly greater decreases in TPR and superior mesenteric artery vascular resistance than those that resulted from a high-fat diet alone. A mixed meal has been shown to result in significant decreases in TPR and leg vascular resistance, with parallel increases in cardiac index and calf blood flow.31 Similarly, we found a significant reduction in TPR at 60 and 180 minutes after the meal compared with baseline, whereas CO and HR were augmented 60 minutes after the meal. Moreover, consumption of a mixed meal was found to result in postprandial hemodynamic changes most similar to hyperinsulinemia, when compared with hyperglycemia and hypertriglyceridemia.31
There are several experimental considerations in this study. First, this study was part of a larger invasive study examining the thermic effect of food, and therefore, we do not have a control group for comparison. Although we recognize the importance of considering the macronutrient content of the meal and the actual form of the meal itself for comparison (i.e., liquid, solid, mixed), previous studies have examined the effects of liquid water intake on measures of MSNA, BP, and central hemodynamics. Ahuja et al.23 found that in healthy adults, consumption of food plus water, when compared with water consumption alone, resulted in significantly greater drops in peripheral pressure (diastolic BP, MAP) and central hemodynamics (central BP and pulse pressure, AP, and AIx) and a greater rise in HR. Additionally, water ingestion has been shown to increase MSNA, resulting in peripheral vasoconstriction with little to no effect on arterial pressure.32 Although these studies and data mentioned previously,25,30 which have examined similar measures with various macronutrient content meals, have been completed, we believe the integration of MSNA, arterial stiffness, central hemodynamics, and arterial blood sampling contribute important methodological understanding of the acute arterial consequences after a mixed meal. Additionally, we did not control for menstrual cycle in our female subjects. Vascular responsiveness has been shown to be influenced by menstrual cycle phase, with notable differences in central pressures and augmentation index.33 This could have introduced substantial variability in our measurements in young women and could have potentially masked any sex differences.
To our knowledge, this is the first study to examine the acute effects of a liquid mixed meal on measures of arterial stiffness and central hemodynamics in healthy young adults. Although we did not anticipate reductions in central pressures and arterial stiffness with a sympathoexcitatory response, our findings are consistent with the vasodilatory effects of systemic elevations in insulin, augmented mesenteric blood flow, and decreased TPR that occur after a meal. Our findings provide insight into the postprandial sympathetic–hemodynamic interactions and raise important questions regarding these interactions in aging adults and in patient populations with reduced endothelial function and cardiovascular disease. For example, a direct and proportional association exists between postprandial dysmetabolism and both coronary artery disease and cardiac events in diabetic and glucose-intolerant patients.34 The consumption of high-fat and high-glucose meals has also been shown to be associated with higher free radical formation, resulting in increased inflammation and endothelial dysfunction in both healthy adults and diabetic patients.35 Additionally. increased amplitude of reflected pressure waves to the heart is associated with an increased risk of experiencing adverse cardiovascular events,36–38 and increased arterial stiffening can lead to altered blood flow and augmentation of left ventricular and aortic pressure, resulting in increased cardiac work.39 Therefore, our findings imply liquid mixed meals may be beneficial for vascular health because of their association with reductions in measures of arterial stiffness and cardiac work. Aging, obesity, and various disease states (e.g., diabetes, atherosclerosis, hypertension) are all associated with reduced endothelial dysfunction, which appears to be further impaired by postprandial dysmetabolism. Therefore, future studies should address the impact of different types of meals on sympathetic neural activity, central pressures, and aortic wave reflection variables in both healthy young and aging individuals, in diabetics, and in those with endothelial dysfunction at risk for cardiovascular disease.
DISCLOSURES
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (K23 DK82424 to T.B.C.; P30 DK50456 to Minnesota Obesity Center; UL1 RR024150 to Mayo Clinic CTSA; and HL105467 to D.P.C.). We are grateful to the study volunteers for their participation. We also thank Shelly Roberts, Sarah Wolhart, Nancy Meyer, and Pam Engrav for their technical assistance and help with subject recruitment.
REFERENCES
- 1. Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 2007; 50:154–160 [DOI] [PubMed] [Google Scholar]
- 2. Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 2002; 17:543–551 [DOI] [PubMed] [Google Scholar]
- 3. Swierblewska E, Hering D, Kara T, Kunicka K, Kruszewski P, Bieniaszewski L, Boutouyrie P, Somers VK, Narkiewicz K. An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens 2010; 28:979–984 [DOI] [PubMed] [Google Scholar]
- 4. Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand 1997; 159:139–145 [DOI] [PubMed] [Google Scholar]
- 5. Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol 2001; 38:1711–1717 [DOI] [PubMed] [Google Scholar]
- 6. Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol 2000; 278:H1205–H1210 [DOI] [PubMed] [Google Scholar]
- 7. Grassi G. Sympathetic overdrive as an independent predictor of left ventricular hypertrophy: prospective evidence. J Hypertens 2006; 24:815–817 [DOI] [PubMed] [Google Scholar]
- 8. Jones PP, Van Pelt RE, Johnson DG, Seals DR. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J Clin Endocrinol Metab 2004; 89:5138–5144 [DOI] [PubMed] [Google Scholar]
- 9. Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979; 60:473–485 [DOI] [PubMed] [Google Scholar]
- 10. Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38:932–937 [DOI] [PubMed] [Google Scholar]
- 11. Oparil S, Levine RL, Chen SJ, Durand J, Chen YF. Sexually dimorphic response of the balloon-injured rat carotid artery to hormone treatment. Circulation 1997; 95:1301–1307 [DOI] [PubMed] [Google Scholar]
- 12. Gallagher D, Adji A, O’Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens 2004; 17:1059–1067 [DOI] [PubMed] [Google Scholar]
- 13. Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation 2002; 105:2955–2961 [DOI] [PubMed] [Google Scholar]
- 14. Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 1977; 272:383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol 2005; 568:315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 2001; 531:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Power C, Kong PA, Crawford TO, Wesselingh S, Glass JD, McArthur JC, Trapp BD. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol 1993; 34:339–350 [DOI] [PubMed] [Google Scholar]
- 18. Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Relationship between muscle sympathetic nerve activity and aortic wave reflection characteristics in young men and women. Hypertension 2011; 57:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hachiya T, Hashimoto I, Saito M, Blaber AP. Peripheral vascular responses of men and women to lbnp. Aviat Space Environ Med 2012; 83:118–124 [DOI] [PubMed] [Google Scholar]
- 20. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 2011; 589:5285–5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 2000; 36:1233–1238 [DOI] [PubMed] [Google Scholar]
- 22. Casey DP, Curry TB, Joyner MJ, Charkoudian N, Hart EC. Acute beta-adrenergic blockade increases aortic wave reflection in young men and women: differing mechanisms between sexes. Hypertension 2012; 59:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahuja KD, Robertson IK, Ball MJ. Acute effects of food on postprandial blood pressure and measures of arterial stiffness in healthy humans. Am J Clin Nutr 2009; 90:298–303 [DOI] [PubMed] [Google Scholar]
- 24. Westerbacka J, Wilkinson I, Cockcroft J, Utriainen T, Vehkavaara S, Yki-Jarvinen H. Diminished wave reflection in the aorta. A novel physiological action of insulin on large blood vessels. Hypertension 1999; 33:1118–1122 [DOI] [PubMed] [Google Scholar]
- 25. Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Effect of postprandial insulinemia and insulin resistance on measurement of arterial stiffness (augmentation index). Int J Cardiol 2007; 114:50–56 [DOI] [PubMed] [Google Scholar]
- 26. Waaler BA, Eriksen M. Post-prandial cardiovascular responses in man after ingestion of carbohydrate, protein or fat. Acta Physiol Scand 1992; 146:321–327 [DOI] [PubMed] [Google Scholar]
- 27. Jager K, Bollinger A, Valli C, Ammann R. Measurement of mesenteric blood flow by duplex scanning. J Vasc Surg 1986; 3:462–469 [DOI] [PubMed] [Google Scholar]
- 28. Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res 2000; 93:182–196 [DOI] [PubMed] [Google Scholar]
- 29. Sidery MB, Macdonald IA, Cowley AJ, Fullwood LJ. Cardiovascular responses to high-fat and high-carbohydrate meals in young subjects. Am J Physiol 1991; 261:H1430–H1436 [DOI] [PubMed] [Google Scholar]
- 30. Kearney MT, Cowley AJ, Stubbs TA, Macdonald IA. Effect of a physiological insulin infusion on the cardiovascular responses to a high fat meal: evidence supporting a role for insulin in modulating postprandial cardiovascular homoeostasis in man. Clin Sci (Lond) 1996; 91:415–423 [DOI] [PubMed] [Google Scholar]
- 31. Fugmann A, Millgard J, Sarabi M, Berne C, Lind L. Central and peripheral haemodynamic effects of hyperglycaemia, hyperinsulinaemia, hyperlipidaemia or a mixed meal. Clin Sci (Lond) 2003; 105:715–721 [DOI] [PubMed] [Google Scholar]
- 32. Scott EM, Greenwood JP, Gilbey SG, Stoker JB, Mary DA. Water ingestion increases sympathetic vasoconstrictor discharge in normal human subjects. Clin Sci (Lond) 2001; 100:335–342 [PubMed] [Google Scholar]
- 33. Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010; 235:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bell DS, O’Keefe JH, Jellinger P. Postprandial dysmetabolism: the missing link between diabetes and cardiovascular events? Endocr Pract 2008; 14:112–124 [DOI] [PubMed] [Google Scholar]
- 35. Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 2004; 53:701–710 [DOI] [PubMed] [Google Scholar]
- 36. Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NR, Thom SA, Hughes AD. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial) substudy. J Am Coll Cardiol 2010; 56:24–30 [DOI] [PubMed] [Google Scholar]
- 37. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871 [DOI] [PubMed] [Google Scholar]
- 38. Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004; 109:184–189 [DOI] [PubMed] [Google Scholar]
- 39. Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens 2005; 18:3S–10S [DOI] [PubMed] [Google Scholar]