Abstract
Garlic (Allium sativum) has been used for centuries as a prophylactic and therapeutic medicinal agent. Importantly, garlic has been suggested to have both cancer-preventive potential as well as significant enhancing effects on the immune system. While these observations are supported experimentally both in vitro and in vivo, the impact of garlic in assisting the immune system in the prevention of cancer still lacks experimental confirmation. Studies addressing the immunomodulatory effects of garlic reveal conflicting data as to pro- or anti-inflammatory responses depending on the particular experimental set-ups and the garlic preparation used (i.e. garlic extract versus chemically pure garlic compounds). Here we provide an overview of the chemistry of the major garlic organosulfur compounds, summarize the current understanding and propose a link between the immunomodulating activity of garlic and the prevention of cancer. We hypothesize that garlic rather elicits anti-inflammatory and anti-oxidative responses that aid in priming the organism towards eradication of an emerging tumor.
Keywords: Anti-inflammatory, anti-oxidant, cancer prevention, disulfide, garlic, immunomodulatory.
INTRODUCTION
The health effects of dietary garlic have been utilized throughout the centuries to offer protection against infections, heart disease and cancer. Crushed cloves are rich in sulfur containing compounds collectively termed garlic organosulfur compounds (OSCs) which are the active principles responsible for the biological activity of garlic. Regarding cancer, the face of garlic is multi-faceted being capable of eliciting both preventative and therapeutic effects. In cancer treatment, garlic organosulfur compounds have been shown to inhibit proliferation and induce apoptosis of cancer cells both in culture as well as in mouse xenograft models [1, 2]. Central to this effect are G2/M cell cycle arrest and activation of the mitochondrial-dependent caspase cascade [1, 2]. In cancer prevention, numerous epidemiological studies have demonstrated a link between garlic consumption and decreased risk of cancer especially cancer of the colon and stomach [3]. Some of these observed effects may be explained from the ability of garlic OSCs to inhibit tumorigenesis arising from exposure to chemical carcinogens as demonstrated in experimental animals [2, 4]. On the other hand, garlic OSCs are reported to exert an immunological effect by eliciting diverse immune responses depending on the particular experimental setting. We propose that garlic OSCs obtained through the diet, may aid in cancer prevention by shifting the overall balance from a tumor-mediated pro-inflammatory to a host-mediated anti-tumor response which may stimulate the immune system towards eradication of an emerging tumor.
THE ORGANOSULFUR COMPOUNDS IN CRUSHED GARLIC
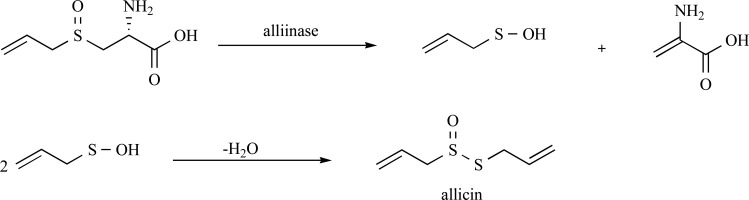
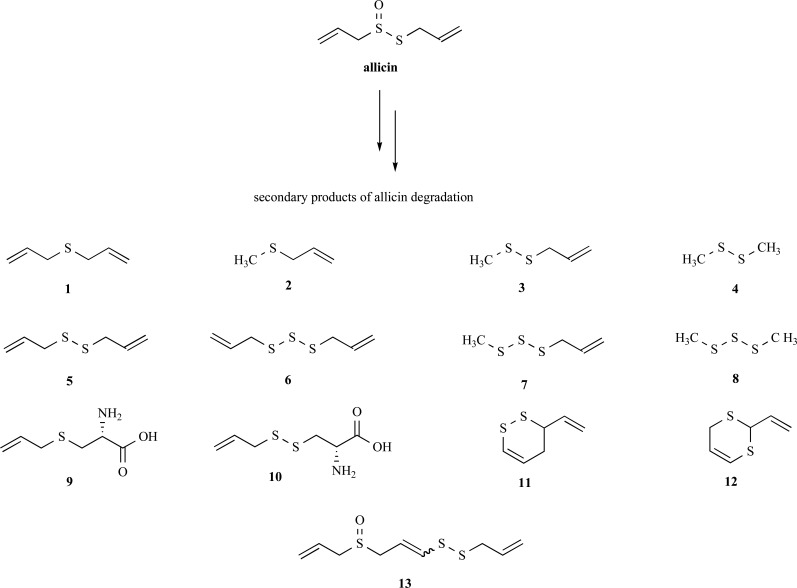
The beneficial health effects of garlic are attributed to the organosulfur compounds found in crushed cloves. The principle organosulfur compound in intact cloves is (+) S-allyl-L-cysteine sulfoxide (alliin) which is compartmentalized away from the vacuolar enzyme alliinase (EC 4.4.1.3). Upon damaging of the clove (i.e. by chewing or crushing), alliinase comes into contact with its substrate and an elimination reaction ensues to form 2-propenesulfenic acid, which self-condenses to form diallyl thiosulfinate (allicin) (Fig. 1). Allicin, therefore being a constituent of freshly crushed cloves, is an anti-microbial agent possibly generated by the plant as a defence against pathogen invasion. Allicin, however, is chemically unstable and degrades and rearranges over time to form a collection of second generation allyl sulfides and polysulfides (commonly termed garlic organosulfides), through pathways which were elegantly elucidated by Eric Block in the 1980’s [5]. These second generation organosulfides are constituents of fresh, but have increased concentrations in aged, garlic preparations. The chemical structures of the predominant second generation organosulfides are displayed in Fig. 2. Allicin is only found in fresh, raw garlic preparations in a reported amount of 3.1 mg.g-1 [6]. Garlic preparations vary in concentration of secondary organosulfur compounds, which can be isolated by steam distillation or oil extraction, sometimes with heat, to be (in micrograms per gram of garlic preparation) diallyl sulfide (DAS) 20-240; diallyl disulfide (DADS) 280-900; diallyl trisulfide (DATS) 40-200 and E/Z-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (E/Z-ajoene) 130-480 [7-10]. The other organosulfur compounds listed in Fig. 2 are only present in concentrations < 50 µg.g-1. As can be seen from above, the exact amount of compound in a preparation is subject to high variability. In vivo, allicin is never detected after ingestion of garlic supporting experimental evidence that allicin is both chemically and metabolically unstable. It is likely that the ingested allicin rapidly degrades into the second generation organosulfur compounds (as observed in vitro in garlic preparations), as well as participates in the rapid thiolation of plasma proteins (discussed in the next section). This has been demonstrated in the very rapid first pass effect observed for allicin in a perfused rat liver [11]. In this experiment, only allicin metabolites were detected after the first pass and these were identified to be DADS, ajoene, the vinyl diithins and allyl mercaptan. In addition, no allicin has been detected in blood after oral ingestion of a large amount of raw garlic (25g) [12]. Three studies have identified the organosulfur compounds present in the breath after ingestion of raw garlic by either GC-MS [13, 14] or proton-transfer-reaction mass spectrometry [15]. Again, only allicin metabolites were detected and these are collectively listed to be: DADS, AMS, AMDS, DAS, DADS, DATS, dimethyl sulfide, acetone and allyl mercaptan. It is therefore likely that the second generation allicin products DAS, DADS, DATS and EZ-ajoene are responsible for the biological activity of garlic with the minor constituents, due to their lower concentrations, playing a lesser role.
Fig. (1).
The enzymatic conversion of alliin to 2-propenesulfenic acid which then undergoes self-condensation to produce allicin.
Fig. (2).
The predominant secondary products of allicin degradation in crushed garlic. (1) diallyl sulfide (DAS), (2) allyl methyl sulfide (AMS), (3) allyl methyl disulfide (AMDS), (4) dimethyl disulfide (DMDS), (5) diallyl disulfide (DADS), (6) diallyl trisulfide (DATS), (7) allyl methyl trisulfide (AMTS), (8) dimethyl trisulfide (DMTS), (9) S-allyl cysteine (SAC), (10) S-allyl mercaptocysteine (SAMC), (11) 2-vinyl-2,4-dihydro-1,3-dithiin, (12) 3-vinyl-3,4-dihydro- 1,2-dithiin, (13) E/Z-4,5,9-trithiadodeca-1,6,11-triene 9-oxide (E/Z-ajoene). Note that compounds 9 and 10 are the only water soluble compounds.
The garlic organosulfides are all sulfur-rich containing either a sulfide, disulfide or polysulfide functional group in their backbone which is likely the pharmacophore responsible for their biological activity. In support of this, the disulfide bond in ajoene was found to be important for both anti-microbial and anti-cancer activities where removal of the disulfide to the sulfide rendered the analog inactive in both cases [16, 17]. Disulfides and polysulfides can be considered as thiol oxidising agents, able to thiolate cysteine residues in proteins to form mixed disulfides. In a thiol-disulfide exchange reaction, a disulfide RSSR reacts with a thiol R’SH to form a mixed disulfide RSSR’ and a thiol RSH. For this reaction to occur, a thiolate anion first needs to be generated. In the cell, cysteine normally has a pKa significantly higher than the physiological range; however, active site cysteines in enzymes usually provide an unusually basic microenvironment in which cysteine dissociates more readily to form a thiolate. Experimentally, this reaction has been demonstrated to occur by the ajoene thiolation of glutathione reductase at the active site Cys58 to form the modified protein CH2=CH-CH2-SO-CH2-CH=CH-S-Cys58-GR revealing the vinyl sulfur to be the preferred site of enzyme thiol sulfur attachment [18]. We have demonstrated the feasibility of this sulfenylating reaction through the formation of S-allylmercaptocysteine from the reaction between cysteine and ajoene in solution although this reaction was found to proceed via regioselective attack at the non-vinyl sulfur [19]. In another cell free system, DADS has been demonstrated to alkylate β-tubulin at cysteine residues Cys-12β and Cys-354β in the protein [20] lending support to the hypothesis that garlic organosulfur compounds induce apoptosis in cancer cells by destabilizing the microtubule network [21]. The biological chemistry of garlic organosulfur compounds probably involves more than a simple thiolation reaction with an enzyme as this should be reversible in cells which contain 2-10 millimolar concentrations of cytosolic glutathione [22, 23] compared to the micromolar concentrations [23] of garlic compounds which are required to bring about a biological effect. In the absence of oxidative stress, these cells should overcome the effects of micromolar concentrations of garlic organosulfur compounds. The biological chemistry of garlic likely involves various types of oxidation, radical generation, protein modification and enzyme inhibition reactions.
EPIDEMIOLOGICAL AND EXPERIMENTAL EVIDENCE FOR CANCER CHEMOPREVENTION BY GARLIC
Over the last thirty years there have been numerous reports linking the etiology of cancer to the consumption of Allium vegetables. Some of the first evidences came from case-control studies in China [24] and Italy [25] where consumption of Allium vegetables was found to be negatively correlated with incidence of gastric cancer. Since then there have been in excess of 20 additional publications documenting the outcome of various case-control and cohort studies. These studies cover populations from China, Europe, South America and the United States and have looked at the incidence of many cancers but predominantly stomach and colorectal cancers. There are four systematic reviews or meta-analyses which critically scrutinise the vast amount of literature on the subject. Fleischauer and Arab critically reviewed nineteen articles documenting garlic consumption to the incidence of stomach, colorectal, head and neck, prostate, breast and lung cancers reported for the period 1966 - 1998 [3]. Site specific case-control studies of stomach and colorectal cancers were analyzed to suggest a protective effect for high intake of raw and / or cooked garlic with cohort studies confirming this inverse relationship for colorectal cancer. However, garlic supplementation as opposed to dietary intake was not found to be related to cancer risk, as analysed by four cohort studies and one case-control study, from two distinct populations. Kim and Kwon performed their own analysis of the published epidemiological literature for the period 1955 - 2007 using the US Food and Drug Administration’s evidence-based review system [26]. Nineteen studies were identified and evaluated to conclude that limited evidence exists to support a relation between garlic consumption and reduced risk of colon, prostate, oesophageal, larynx, oral, ovary or renal cancers with no credible evidence for gastric, breast, lung or endometrial cancers. A systematic meta-analysis by Ngo et al., [27] of 7 colorectal studies confirmed an inverse association with cancer risk. Zhou et al., conducted a meta-analysis of 21 cohort and case-control studies published for the period 1966 - 2010. The relative risks from individual studies were pooled using a random effects model and performed a dose-response, heterogeneity and publication bias analysis to conclude that consumption of high levels of Allium vegetables reduces the risk of gastric cancer. Taken together, critical review of the epidemiological literature supports a link between the consumption of Allium vegetables including garlic and decreased risk of colorectal and gastric cancers.
Numerous studies in the 1980s and 1990s investigated the cancer chemopreventative activity of garlic OSCs against chemically-induced cancers in experimental animals. Research into this topic has culminated in over 40 publications that have been comprehensively reviewed [2, 4]. In these investigations, a mouse or rat is pre-treated with the garlic extract or pure garlic compound (topically or orally) followed by tumor induction by a chemical carcinogen. Some of the chemical carcinogens that have been used for this purpose include nitrosamines [28, 29], azides [30, 31], aromatic hydrocarbons including benzo[a]pyrene [32] and anthracenes [33] which are administered either topically, by intraperitoneal injection or orally. The outcome of these studies, excluding one [34] claims a reduction in tumor size and tumor number in the garlic pre-treated animals. More specifically and as expected, the pure garlic compounds DADS and SAC were found to exert an increased effect relative to the extract [35]. There are several lines of evidence to suggest that these observed effects may be related to the ability of garlic OSCs to inhibit phase I enzymes (such as the cytochrome P450-dependent monooxygenases [36, 37]) which are responsible for activation of a number of carcinogens; as well as to enhance detoxification processes by inducing expression of phase II enzymes to include glutathione S-transferases [38], quinone reductase [39] and epoxide hydrolase [40].
Free radicals are able to induce damaging effects which may lead to tumorigenesis. Garlic extracts and pure garlic organosulfur compounds are reported to have radical scavenging and anti-oxidant activity as demonstrated by their ability to inhibit lipid peroxidation [41]. It has been hypothesized that increased activity of anti-oxidant enzymes namely catalase [42], superoxide dismutase [43] and glutathione peroxidase [42, 44], as well as increased glutathione content [42, 45, 46] may be responsible for these observed effects. On the other hand, garlic OSCs are reported to induce ROS in cultured cancer cells in a dose-dependent manner and this effect has been linked to the therapeutic action of garlic. Some studies have demonstrated a link between ROS generation and apoptosis induction in cancer cells [47-49] although other studies have failed to find this link [50]. The contrasting effects of ROS generation versus ROS scavenging by the individual garlic organosulfur compounds may be explained from the redox chemistry of the thiol-disulfide exchange or through homolytic cleavage of the disulfide to generate a reactive thiyl radical. It is still debated whether there is an overall generation of ROS that is linked to the therapeutic action of garlic against growing tumor cells. It is possible that ROS as well as Reactive Nitrogen Species (RNS) are implicated in the immunomodulating activity of garlic by acting as signal transducer in the immune defence mechanism involved in the killing of malignant cells. However, excessive levels (for example due to inflammatory reactions, see section below) can damage DNA and proteins leading to impaired cellular functions and an enhanced inflammatory response.
THE IMMUNOMODULATORY EFFECTS OF GARLIC
Although the cancer-preventative properties of garlic may be ascribed in part to the anti-carcinogenic and anti-oxidant activities of garlic, they might, at least in part be explained by underlying effects on the immune system. While acute inflammatory responses promote the elimination of growing tumor cells, chronic inflammation and the presence of inflammatory cells, particularly tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), at tumor sites is strongly associated with specific malignancies and influences both tumor initiation as well as tumor promotion and progression [51-53]. Indeed, inflammation is considered a double-edged sword in neoplasia as the immune cells exert rather paradoxical effects during cancer development: they play important roles both in host-mediated anti-tumor immunity as well as in tumor-mediated immunosuppressive activity [52-54]. A delicate balance between these immune responses determines the overall outcome of the carcinogenic process and depends on different mediators released by host inflammatory cells, cancer cells and cancer-associated cells in the tumor micro-environment [55]. This is best illustrated by activated macrophages and neutrophils that are able to kill tumor cells, but can also release reactive oxygen species, angiogenic, proliferative, pro-inflammatory as well as immunosuppressive substances [56].
The complex role of the different cytokines that are produced upon innate immune activation and inflammation exerts opposing activities in tumor suppression and progression and is thought to be stage and cancer cell type dependent. Generally, activation of the NFκB signaling pathway and expression of pro-inflammatory cytokines such as IL6, IL1β, TNFα and IL17 promote tumor development while pro-apoptotic and anti-inflammatory cytokines such as TRAIL, IL10 and TGF-β inhibit carcinogenesis depending on the particular tumor microenvironment [54]. As mentioned above, the generation of ROS and RNS is another important immune defence mechanism in killing of malignant cells. Indeed, both oxidative stress and overexpression of the inducible form of nitric oxide synthase (iNOS) are common phenomena during chronic inflammatory conditions and play fundamental roles in the initiation of cancer, promotion of tumor growth and progression [57, 58]. As signal transducers, ROS and RNS can stimulate NFκB which leads to the expression of numerous genes encoding proteins in immune function [59]. Macrophages represent the typical source for both oxidative burst in immune responses as well as for iNOS. iNOS expression can be transcriptionally upregulated (via NFκB signaling) by pro-inflammatory cytokines such as IFN, IL1, IL2 and TNFα, as well as LPS and hypoxia; it can be downregulated by anti-inflammatory cytokines such as TGFβ and IL10, the tumor suppressor gene p53 and NO (nitric oxide) itself [60].
Immune responses are regulated by intrinsic and extrinsic factors; but diet also plays an important role in the proper functioning of the immune system [54]. There are numerous studies addressing the effect of garlic on the immune system. However, dependent on the particular experimental set-ups and the garlic preparation used (such as fresh garlic juice, garlic powder extract or chemically purified compounds), these studies revealed conflicting data as to garlic being able to stimulate pro- or anti-inflammatory responses as outlined below.
There is evidence that garlic acts as an immune booster by actively strengthening the host immune system within the tumor microenvironment against the immunosuppressive activity of an emerging tumor, the relevant literature has been summarized in Table 1. Aged garlic extract was found to stimulate lymphocyte proliferation, macrophage phagocytosis, macrophage and lymphocyte infiltration into tumors, as well as the release of cytokines such as IL2, IL12, TNFα and IFNγ that suggests the induction of a Th1-type cellular immune response [61-63]. It was further shown to enhance NK cell number and activity [64], to increase the number of white blood cells [65], to increase NO and the anti-tumoral agent IFNα [66]. However, there are a number of reports indicating that garlic rather inhibits Th1 response and shifts towards a Th2 response. Stimulation of the anti-inflammatory cytokine IL10 was observed in LPS-stimulated human whole-blood cultures by garlic extracts while monocyte production of TNFα, IL1α, IL6 and IL8 and T-cell IFNγ, IL2 and TNFα was significantly suppressed [67]. This was confirmed on LPS-stimulated human placental explants: garlic extracts were found to stimulate the production of IL10 while decreasing TNFα and IL6 production [68]. Although garlic powder extract also inhibited LPS-induced TNFα and IL1β in human whole blood by reducing the pro-inflammatory activity of NFκB, IL10 secretion was not affected [69]. Chang et al., found that garlic oil derivatives differentially suppressed the production of NO and prostaglandin E2 (PGE2) in activated macrophages [70], resulting in an overall anti-inflammatory effect. On that note, the anti-oxidative effects of garlic have been suggested to be due to the prevention of intracellular glutathione depletion and to the removal of peroxides; also, inhibition of NFκB activation seems to be central to these effects [71].
Table 1.
Selected studies on the immunomodulatory effects of garlic.
| Functional Component | Concentration | Experimental System | Reported Effect | Ref. |
|---|---|---|---|---|
| ajoene | 5-40 μM | Human leukemic cells | Induction of apoptosis by stimulation of peroxide production and NFκB activation | [47] |
| AGE | 500 mg daily for 6 months | Inoperable colorectal, liver or pancreatic cancer patients | Increase in NK cell number and activity | [64] |
| DAS, DADS, AMS | 5 x 20 mg daily | BALB/c mice | Enhanced number of white blood cells and antibody titre | [65] |
| Fresh garlic | 2g (once or daily for 7 days) | Healthy human volunteers | Increase in plasma NO and IFNα levels | [66] |
| Garlic extract | 0.1-10 μg/ml | Whole blood and PBMCs | Decreased production of IL12, TNFα, IL1α, IL6, IL8, IFNγ, IL2; increase of IL10 | [67] |
| Garlic extract | 10-1000 μg/ml | Normal placental and preeclamptic explants | Inhibition of IL6 and TNFα production; IL10 increase (10μg/ml garlic extract) or IL10 decrease (1000μg/ml garlic extract) | [68] |
| Garlic powder extract DADS |
10-100 mg/l 1-100 μmol/l |
LPS-induced human whole blood | Reduction of NFκB activity and production of IL1β and TNFα | [69] |
| DAS DADS AMS |
1-10 μM 0.1-0.5 μM 2-20 μM |
LPS-stimulated RAW 264.7 macrophages | Reduction of iNOS expression and NO production; Reduction of COX2 expression and PGE2 release (only DAS) | [70] |
| AGE SAC |
0.1-5 g/l 0.1-20 mmol/l |
Ox-LDL induced injury in endothelial cells | Prevention of membrane damage, loss of cell viability and lipid peroxidation by prevention of intracellular GSH depletion and NFκB activation | [71] |
| Alliin | 0.05-3 μg/ml | LPS-induced PMBCs | Increase in IL1β and TNFα; Decrease in IL6 production | [73] |
| DADS, DATS | 50-400 μM | LPS-stimulated RAW 264.7 macrophages | Reduction of iNOS expression, NO and peroxide production; Inhibition of NFκB activation | [74] |
| ajoene allicin |
IC50 2.5-5 μM IC50 15-20 μM |
LPS-stimulated RAW 264.7 macrophages | Reduction of iNOS expression and NO accumulation | [75] |
| DAS DADS AMS |
1-10 μM 0.1-0.2 μM 2-20 μM |
LPS-stimulated RAW 264.7 macrophages | Inhibition of TNFα, IL1β, IL6, IL10 production; Inhibition of NO and prostaglandin PGE2 release Inhibition of TNFα, IL10 and NO release; Increase of IL1β and IL6 Inhibition of TNFα, Increase of IL10, Decrease of NO release |
[76] |
| ajoene | IC50 2.4-3.4 μM | LPS-stimulated RAW 264.7 macrophages | Inhibition of COX2 enzyme activity and PGE2 release | [77] |
Studies that used purified garlic compounds describe allicin as an inhibitor of NFκB activation, iNOS and TNFα production [72], while alliin was found to inhibit IL6 but increased IL1β and TNFα [73]. Both DADS and DATS decreased NO and cellular peroxide production in LPS-activated RAW264.7 macrophages by suppression of NFκB-mediated iNOS expression [74]. This effect was also seen when allicin and ajoene were studied: both compounds inhibited the expression of iNOS in activated macrophages [75]. The purified garlic derivative DAS was shown to inhibit both pro-and anti-inflammatory cytokines (TNFα, IL1β, IL6, IL10) in LPS-stimulated RAW264.7 macrophages which was closely associated with the suppression of NO and PGE2 production [76]. DADS enhanced IL1 β and IL6, but suppressed IL10 and TNFα, while AMS decreased NO production and TNFα but enhanced IL10 [76]. Moreover, COX2, an enzyme that is induced under similar pathophysiological conditions as iNOS and is implicated in the pathogenesis of various inflammatory diseases as well as carcinogenesis, was found to be inhibited by ajoene in activated macrophages [77].
THE INTERRELATIONSHIP OF GARLIC AND THE IMMUNE SYSTEM IN CARCINOGENESIS
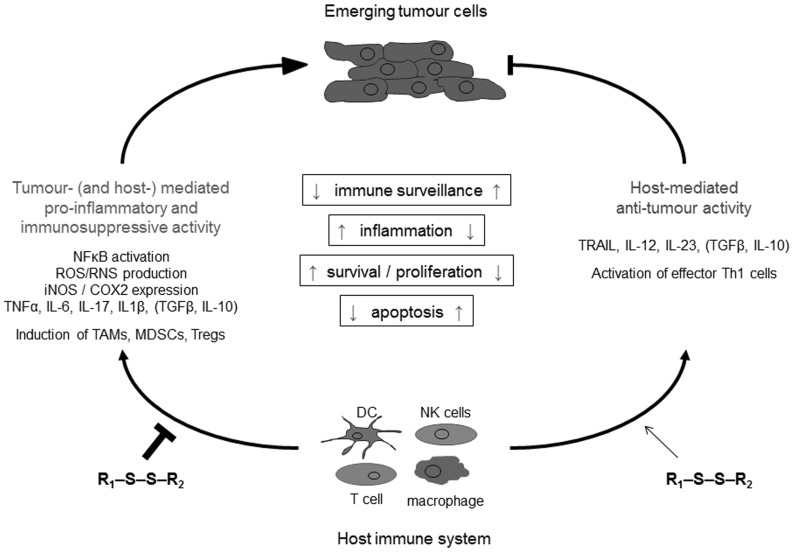
Based on the studies outlined above, it seems that the net effect of garlic elicits anti-inflammatory immune responses. This is particularly relevant in the context of the pro-inflammatory and immunosuppressive environment during chronic inflammation, a well-established factor facilitating carcinogenesis. Dampening of the tumor-mediated pro-inflammatory activity is likely induced by several active garlic compounds modulating the cytokine pattern in a way that leads to an overall inhibition of NFκB, the central regulator of pro-inflammatory gene expression and the molecular link between inflammation and tumor promotion and progression [69, 71, 78]. It could therefore be hypothesized that garlic acts as an immune modulator that can shift the balance from a pro-inflammatory and immunosuppressive environment to an enhanced anti-tumor response leading to suppression of an emerging tumor (Fig. 3).
Fig. (3).
Proposed model for the interrelationship between garlic organosulfur compounds and the immune system in carcinogenesis. In early tumorigenesis, a delicate balance exists between the tumor-mediated pro-inflammatory response of the emerging tumor and the host anti-tumor immunity. When the host-mediated anti-tumor activity is weaker than the tumor-mediated pro-inflammatory and immunosuppressive activity, tumor cells undergo immune escape and grow rapidly. By contrast, when host-mediated anti-tumor immunity is stronger than tumor-mediated pro-inflammatory and immunesuppressive activity, tumor cells are eliminated. We propose that long-term supplementation with dietary garlic organosulfur compounds contributes in shifting the balance from a pro-inflammatory to an anti-tumor response by dampening the pro-inflammatory response (left) and/or strengthening the anti-tumor immunity (right) towards tumor eradication. Note that the cytokines IL-10 and TGFβ can have dual effects in tumorigenesis depending on the context in a particular tumor microenvironment and are therefore listed in brackets.
Although garlic seems to have an overall anti-inflammatory and immune-boosting effect, the conflicting results outlined above strongly reflect the complexity of the system: the individual components of garlic might exert immunomodulatory effects that, at least partially, act through alterations in the production of various cytokines and immune modulators. Some of the conflicting data probably relates to the complex chemical composition of garlic which is related to the method of preparation [79]. Current limitations are that most studies have been carried out in vitro at a cellular level, and in vivo animal models are lacking. It is therefore of utmost importance to design future studies using well-defined systems and chemically pure active garlic compounds at defined concentrations, and to develop animal models to demonstrate the immunomodulatory effect of garlic in cancer prevention.
Many dietary bioactive food components interact with the immune system with the potential to reduce the risk of cancer [80]. We therefore hypothesize that the organosulfur compounds of garlic contribute to the prevention or reduction of the immuno-suppressive environment during chronic inflammation with the downstream consequence of assisting the host to escape tumor-mediated immune suppression and to elicit an anti-tumor immune response. Future studies including those in animal models are required to demonstrate the role of garlic supplementation in cancer prevention. Although the effects might be subtle, garlic should be considered as a dietary anti-inflammatory supplement that in the long term might lead to the reduced risk of certain types of cancers, an effect known for long-term usage of aspirin [81].
ACKNOWLEDGEMENTS
We thank the Cancer Association of South Africa (CANSA) and the Polio Research Foundation (PRF) for financial support. We also thank Professor Timothy Egan and Professor Roger Hunter (Department of Chemistry, UCT) for proof reading this manuscript for proof reading the manuscript and Mr Ingo Jastram for assisting with the graphic art (Fig. 3).
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
LIST OF ABBREVIATIONS
- OSC
= Organosulfur compounds
- NFκB
= Nuclear Factor kappa B
- IL
= Interleukin
- TNF
= Tumor Necrosis Factor
- TRAIL
= TNF related apoptosis-inducing ligand
- TGF
= Transforming Growth Factor
- ROS
= Reactive Oxygen Species
- RNS
= Reactive Nitrogen Species
- iNOS
= (inducible) Nitric Oxide Synthase
- NO
= Nitric Oxide
- IFN
= Interferon
- LPS
= Lipopolysaccharide
- Th
= T helper cells
- NK
= Natural Killer cells
- PGE2
= Prostaglandin E2
- DAS
= Diallyl sulfide
- DADS
= Diallyl disulfide
- DATS
= Diallyl trisulfide
- AMS
= Allyl methyl sulphide
- SAC
= S-allyl cysteine
- SAMC
= S-allylmercaptocysteine
- COX2
= Cyclooxygenase-2
- AGE
= Aged Garlic Extract
- PBMCs
= Peripheral Blood Monocytes
- Ox-LDL
= Oxidized low-density lipoprotein
- GSH
= Glutathione
REFERENCES
- 1.Powolny A, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Fleischauer A, Arab L. Garlic and Cancer: A critical review of the epidemiologic literature. J. Nutr. 2001;131:1032S–1040S. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 4.Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable derived organosulfur componds: a review. Mut. Res. 2004;555:121–131. doi: 10.1016/j.mrfmmm.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Block E, Ahmad S, Catalfamo JL, Jain M K, Apitz-Castro R. The chemistry of alkyl thiosulfinate esters.9. Antithrombotic organosulfur compounds from garlic: structural mechanistic and synthetic studies. J. Am. Chem. Soc. 1986;108 (22): 7045–7055. [Google Scholar]
- 6.Block E, Naganathan S, Putman D, Zhao S-H. Allium Chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms): leek scallion shallot elephant (great headed) garlic, chive, and Chinese chive.Uniquely high allyl to methyl ratios in some garlic samples. J. Agric. Food. Chem. 1992;40:2418–2430. [Google Scholar]
- 7.Lee SN, Kim NS, Lee DS. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003;377(4): 749–756. doi: 10.1007/s00216-003-2163-z. [DOI] [PubMed] [Google Scholar]
- 8.Laakso I, Seppanen-Laakso T, Hiltunen R, Muller B, Jansen H, Knobloch K. Volatile garlic odor components: gas phases and adsorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Med. 1989;55 (3): 257–261. doi: 10.1055/s-2006-961998. [DOI] [PubMed] [Google Scholar]
- 9.Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis P A, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006;13 (1): 54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Naznin M, Akagawa M, Okukawa K, Maeda T, Morita N. Characterization of E- and Z-ajoene obtained from different varieties of garlics. Food Chem. 2008;106:1113–1119. [Google Scholar]
- 11.Egen-Schwind C, Eckard R, Kemper F H. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Med. 1991;58:301–305. doi: 10.1055/s-2006-961471. [DOI] [PubMed] [Google Scholar]
- 12.Lawson LD, Ransom DK, Hughes BG. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb. Res. 1992;65 (2): 141–156. doi: 10.1016/0049-3848(92)90234-2. [DOI] [PubMed] [Google Scholar]
- 13.Minami T, Boku T, Inada K, Morita I, Okazaki Y. Odor components of human breath after the ingestion of grated raw garlic. J. Food Sci. 1989;54 (3): 763–764. [Google Scholar]
- 14.Rosen RT, Hiserodt RD, Fukuda EK, Riuiz RJ, Zhou Z, Lech J, Rosen SL, Hartman TG. Determination of allicin s-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001;131:968S–971S. doi: 10.1093/jn/131.3.968S. [DOI] [PubMed] [Google Scholar]
- 15.Taucher J, Hansel A, Jordan A, Lindinger W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996;44:3778–3782. [Google Scholar]
- 16.Naganawa R, Iwata N, Ishikawa K, Fukuda H, Fujino T, Suzuki A. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 1996;62:4238–4242. doi: 10.1128/aem.62.11.4238-4242.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaschula CH, Hunter R, Stellenboom N, Caira MR, Winks S, Oqunleye T, Richards P, Cotton J, Zilbeyaz K, Wang Y, Siyo V, Ngarande E, Parker MI. Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012;50:236–254. doi: 10.1016/j.ejmech.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Gallwitz H, Bonse S, Martinez-Cruz A, Schlichting I, Schumacher K, Krauth-Siegel RL. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and trypanosoma cruzi trypanothione reductase: crystallographic kinetic and spectroscopic studies. J. Med. Chem. 1999;42 (3): 364–372. doi: 10.1021/jm980471k. [DOI] [PubMed] [Google Scholar]
- 19.Kaschula CH, Hunter R, Parker MI, Hassan HT, Stellenboom N, Cotton J, Zhai XQ. Synthesis and anti-proliferation efficacy of synthetic ajoene analogues on cancer cell-lines. Anti-Cancer Agents Med. Chem. 2011;11 (3): 260–266. doi: 10.2174/187152011795347450. [DOI] [PubMed] [Google Scholar]
- 20.Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfidesuppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of b-tubulin J. Biol. Chem. 2005;280 (50): 4147–4193. doi: 10.1074/jbc.M507127200. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Ciu J-R, Ye Y, Min J-M, Zhang L-H, Wang K, Gares M, Cros J, Wright M, Leung-Track J. Antitumor activity of Z-ajoene a natural compound purified from garlic: antimitotic and microtubule-interaction properties. Carcinogenesis. 2002;23 (4): 573–579. doi: 10.1093/carcin/23.4.573. [DOI] [PubMed] [Google Scholar]
- 22.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30 (11): 1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaschula CH, Hunter R, Parker MI. Garlic-derived anti-cancer agents: structure and biological activity of ajoene. Biofactors. 2010;36:78–85. doi: 10.1002/biof.76. [DOI] [PubMed] [Google Scholar]
- 24.You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF, Jr., Wang TG. Allium vegetables and reduced risk of stomach cancer. J. Natl. Cancer Inst. 1989;81 (2): 162–164. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]
- 25.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, Marubini E, Puntoni R, Vindigni C, Fraumeni J, Blot W. A case-control study of gastric cancer and diet in Italy. Int. J. Cncer. 1989;44 (4): 611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 26.Kim JY, Kwon O. Garlic intake and cancer risk: An analysis using the food and drug administration's evidence-based review system for the scientific evaluation of health claims. Am. J. Clin. Nutr. 2009;89 (1): 257–264. doi: 10.3945/ajcn.2008.26142. [DOI] [PubMed] [Google Scholar]
- 27.Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. J. Nutr. 2007;137 (10): 2264–2269. doi: 10.1093/jn/137.10.2264. [DOI] [PubMed] [Google Scholar]
- 28.Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether diallyl sulfide. Cancer Res. 1988;48 (23): 6872–6875. [PubMed] [Google Scholar]
- 29.Wattenberg LW, Sparnins VL, Barany G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res. 1989;49 (10): 2689–2692. [PubMed] [Google Scholar]
- 30.Sparnins VL, Venegas PL, Wattenberg LW. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J. Natl. Cancer Inst. 1982;68 (3): 493–496. [PubMed] [Google Scholar]
- 31.Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res. 1993;53:3493–3498. [PubMed] [Google Scholar]
- 32.Singh A, Shukla Y. Antitumor activity of diallyl sulfide on polycyclic aromatic hydrocarbon-induced mouse skin carcinogenesis. Cancer Lett. 1998;131 (2): 209–214. doi: 10.1016/s0304-3835(98)00152-9. [DOI] [PubMed] [Google Scholar]
- 33.Mehta RG, Steele V, Kelloff GJ, Moon RC. Influence of thiols and inhibitors of prostaglandin biosynthesis on the carcinogen-induced development of mammary lesions in vitro. Anticancer Res. 1991;11:587–592. [PubMed] [Google Scholar]
- 34.Cohen LA, Zhao Z, Pittman B, Lubet R. S-allylcysteine a garlic constituent fails to inhibit N-methylnitrosourea-induced rat mammary tumorigenesis. Nutr. Cancer. 1999;35 (1): 58–63. doi: 10.1207/S1532791458-63. [DOI] [PubMed] [Google Scholar]
- 35.Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer Lett. 1996;102 (1-2): 199–204. doi: 10.1016/0304-3835(96)04160-2. [DOI] [PubMed] [Google Scholar]
- 36.Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, Yang CS. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem. Res. Toxicol. 1991;4 (6): 642–647. doi: 10.1021/tx00024a008. [DOI] [PubMed] [Google Scholar]
- 37.Park K A, Kweon S, Choi H. Anticarcinogenic effect and modification of cytochrome P450 2E1 by dietary garlic powder in diethylnitrosamine-initiated rat hepatocarcinogenesis. J. Biochem. Mol. Biol. 2002;35 (6): 615–622. doi: 10.5483/bmbrep.2002.35.6.615. [DOI] [PubMed] [Google Scholar]
- 38.Hatono S, Jimenez A, Wargovich MJ. Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase. Carcinogenesis. 1996;17 (5): 1041–1044. doi: 10.1093/carcin/17.5.1041. [DOI] [PubMed] [Google Scholar]
- 39.Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem. Biophys. Res. Commun. 1998;244 (3): 917–920. doi: 10.1006/bbrc.1998.8352. [DOI] [PubMed] [Google Scholar]
- 40.Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Antimutagenic activity of organosulfur compounds from Allium is associated with phase II enzyme induction. Mutat. Res. 2001;495 (1-2): 135–145. doi: 10.1016/s1383-5718(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 41.Rose P, Whiteman M, Moore P K, Zhun Zhu Y. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potenial therapeutic agents. Nat. Prod. Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 42.Sundaresan S, Subramanian P. Prevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by S-allylcysteine. Mol. Cell Biochem. 2008;310 (1-2): 209–214. doi: 10.1007/s11010-007-9682-4. [DOI] [PubMed] [Google Scholar]
- 43.Gudi VA, Singh SV. Effect of diallyl sulfide, a naturally occurring anti-carcinogen, on glutathione-dependent detoxification enzymes of female CD-1 mouse tissues. Biochem. Pharmacol. 1991;42 (6): 1261–1265. doi: 10.1016/0006-2952(91)90263-5. [DOI] [PubMed] [Google Scholar]
- 44.Perchellet JP, Perchellet EM, Abney NL, Zirnstein JA, Belman S. Effects of garlic and onion oils on glutathione peroxidase activity the ratio of reduced/oxidized glutathione and ornithine decarboxylase induction in isolated mouse epidermal cells treated with tumor promoters. Cancer Biochem. Biophys. 1986;8 (4): 299–312. [PubMed] [Google Scholar]
- 45.Ameen M, Musthapa MS, Abidi P, Ahmad I, Rahman Q. Garlic attenuates chrysotile-mediated pulmonary toxicity in rats by altering the phase I and phase II drug metabolizing enzyme system. J. Biochem. Mol. Toxicol. 2003;17 (6): 366–371. doi: 10.1002/jbt.10100. [DOI] [PubMed] [Google Scholar]
- 46.Pinto J T, Qiao C, Xing J, Rivlin RS, Protomastro ML, Weissler ML, Tao Y, Thaler H, Heston WD. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. 1997;66 (2): 398–405. doi: 10.1093/ajcn/66.2.398. [DOI] [PubMed] [Google Scholar]
- 47.Dirsch VM, Gerbes AL, Volmar AM. Ajoene a compound of garlic induces apoptosis in human promyleoleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kB. Mol. Pharmacol. 1998;53:402–407. doi: 10.1124/mol.53.3.402. [DOI] [PubMed] [Google Scholar]
- 48.Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, Park JW, Kim HR, Park BH. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem. Pharmacol. 2002;63 (1): 41–47. doi: 10.1016/s0006-2952(01)00860-7. [DOI] [PubMed] [Google Scholar]
- 49.Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63 (18): 5940–5949. [PubMed] [Google Scholar]
- 50.Kelkel M, Cerella C, Mack F, Schneider T, Jacob C, Schumacher M, Dicato M, Diederich M. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis. 2012;33 (11): 2162–2171. doi: 10.1093/carcin/bgs240. [DOI] [PubMed] [Google Scholar]
- 51.Yang J-Y, Della-Fera M, Hausman DB, Baile CA. Enhancement of ajoene-induced apoptosis by conjugated linoleic acid in 3T3-L1 adipocytes. Apoptosis. 2007;12:1117–1128. doi: 10.1007/s10495-006-0043-7. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 2006;16 (1): 38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol. 2012;22 (4): 307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117 (5): 1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144 (5): 646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol. Lett. 2003;90 (2-3): 103–122. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Mocellin S, Bronte V, Nitti D. Nitric oxide a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27 (3): 317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 58.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89 (6): 873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14 (11): 879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 60.Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003;384 (10-11): 1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 61.Lamm DL, Riggs DR. Enhanced immunocompetence by garlic: role in bladder cancer and other malignancies. J. Nutr. 2001;131 (3s ): 1067S–1070S. doi: 10.1093/jn/131.3.1067S. [DOI] [PubMed] [Google Scholar]
- 62.Kyo E, Uda N, Suzuki A, Kakimoto M, Ushijima M, Kasuga S, Itakura Y. Immunomodulation and antitumor activities of aged garlic extract. Phytomedicine. 1998;5 (4): 9. doi: 10.1016/S0944-7113(98)80064-0. [DOI] [PubMed] [Google Scholar]
- 63.Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. J. Nutr. 2001;131 (3s ): 1075S–1079S. doi: 10.1093/jn/131.3.1075S. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006;136 (3 Suppl ): 816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 65.Kuttan G. Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J. Ethnopharmacol. 2000;72 (1-2): 93–99. doi: 10.1016/s0378-8741(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 66.Bhattacharyya M, Girish GV, Karmohapatra SK, Samad SA, Sinha AK. Systemic production of IFN-alpha by garlic (Allium sativum) in humans. J. Interferon Cytokine Res. 2007;27 (5): 377–382. doi: 10.1089/jir.2006.0124. [DOI] [PubMed] [Google Scholar]
- 67.Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry . 2002;48 (4): 209–215. doi: 10.1002/cyto.10133. [DOI] [PubMed] [Google Scholar]
- 68.Makris A, Thornton CE, Xu B, Hennessy A. Garlic increases IL-10 and inhibits TNFalpha and IL-6 production in endotoxin-stimulated human placental explants. Placenta. 2005;26 (10): 828–834. doi: 10.1016/j.placenta.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 69.Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J, Kahane R, Vollmar AM. Garlic (Allium sativum L. modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J. Nutr. 2003;133 (7): 2171–2175. doi: 10.1093/jn/133.7.2171. [DOI] [PubMed] [Google Scholar]
- 70.Chang HP, Chen YH. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition. 2005;21 (4): 530–536. doi: 10.1016/j.nut.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 71.Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J. Nutr. 2001;131 (3s ): 1020S–1026S. doi: 10.1093/jn/131.3.1020S. [DOI] [PubMed] [Google Scholar]
- 72.Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005;25 (3): 613–621. doi: 10.1111/j.1478-3231.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 73.Salman H, Bergman M, Bessler H, Punsky I, Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int. J. Immunopharmacol. 1999;21 (9): 589–597. doi: 10.1016/s0192-0561(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 74.Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264. macrophages. J. Agric. Food Chem. 2006;54 (9): 3472–3478. doi: 10.1021/jf060043k. [DOI] [PubMed] [Google Scholar]
- 75.Dirsch VM, Kiemer AK, Wagner H, Vollmar AM. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis. 1998;139 (2): 333–339. doi: 10.1016/s0021-9150(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 76.Chang HP, Huang SY, Chen YH. Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J. Agric. Food Chem. 2005;53 (7): 2530–2534. doi: 10.1021/jf048601n. [DOI] [PubMed] [Google Scholar]
- 77.Dirsch VM, Vollmar AM. Ajoene a natural product with non-steroidal anti-inflammatory drug (NSAID)-like properties?. Biochem. Pharmacol. 2001;61 (5): 587–593. doi: 10.1016/s0006-2952(00)00580-3. [DOI] [PubMed] [Google Scholar]
- 78.Ho SE, Ide N, Lau BH. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 2001;8 (1): 39–46. doi: 10.1078/0944-7113-00005. [DOI] [PubMed] [Google Scholar]
- 79.Amagase H, Petesch B L, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131 (3s ): 955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 80.Ferguson LR, Philpott M. Cancer prevention by dietary bioactive components that target the immune response. Curr. Cancer Drug Targets. 2007;7 (5): 459–464. doi: 10.2174/156800907781386605. [DOI] [PubMed] [Google Scholar]
- 81.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J. Natl. Cancer Inst. 2007;99 (8): 608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]