Abstract
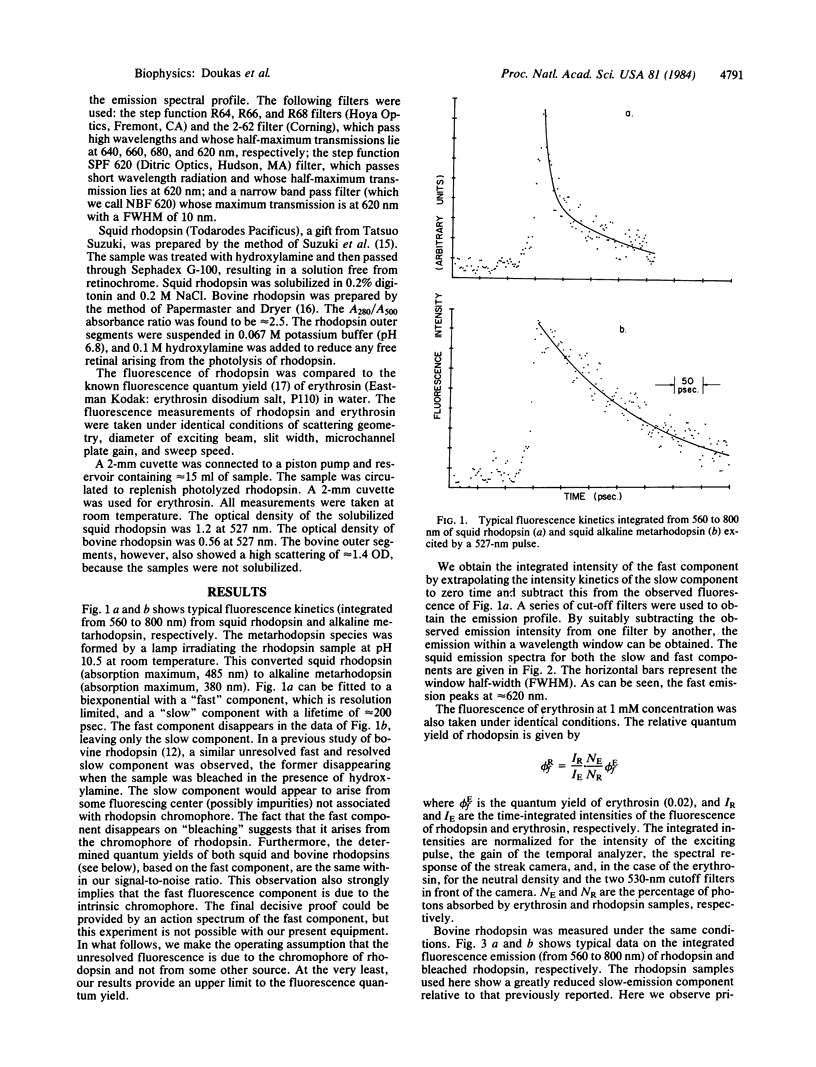
The fluorescence quantum yields (phi f) for bovine and squid rhodopsins are determined. Both pigments yield similar results, with an average value for phi f of 1.2 (+/- 0.5) X 10(-5). Since the estimated radiative lifetime of rhodopsin is 5 nsec, the rate constant of the process that competes with fluorescence must be on the order of 0.1 psec. Given the large quantum yield for isomerization of rhodopsin's retinal chromophore, this process is likely to correspond to the motion along retinal's C11-C12 torsional coordinate that leads to cis-trans isomerization. An empirical excited-state potential energy curve along this coordinate is derived. It is shown that subpicosecond torsional motion to highly twisted nonfluorescing regions of the potential is possible and, in fact, likely. Our results require the existence of a barrier-less excited-state potential energy curve and suggest that cis-trans isomerization occurs in less than 1 psec.
Full text
PDF

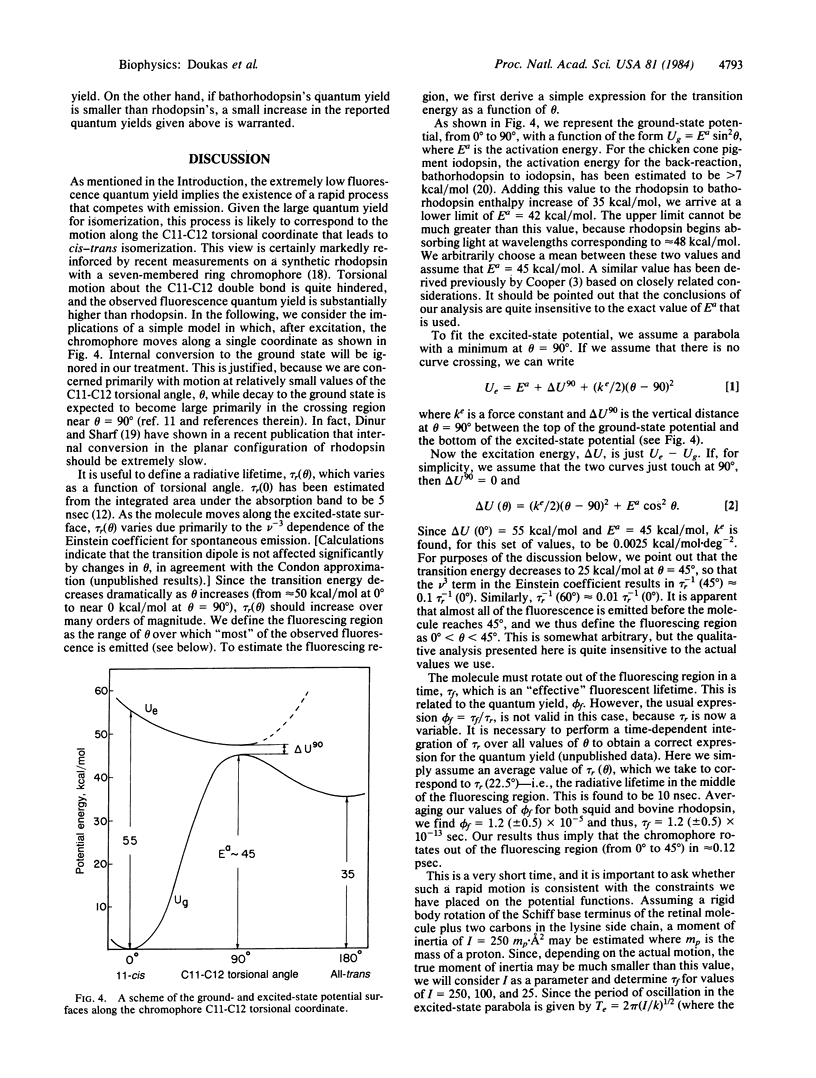

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge R. R., Hubbard L. M. Molecular dynamics of trans-cis isomerization in bathorhodopsin. Biophys J. 1981 Jun;34(3):517–534. doi: 10.1016/S0006-3495(81)84865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R. Photophysics of light transduction in rhodopsin and bacteriorhodopsin. Annu Rev Biophys Bioeng. 1981;10:315–354. doi: 10.1146/annurev.bb.10.060181.001531. [DOI] [PubMed] [Google Scholar]
- Buchert J., Stefancic V., Doukas A. G., Alfano R. R., Callender R. H., Pande J., Akita H., Balogh-Nair V., Nakanishi K. Picosecond kinetic absorption and fluorescence studies of bovine rhodopsin with a fixed 11-ene. Biophys J. 1983 Sep;43(3):279–283. doi: 10.1016/S0006-3495(83)84351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Lu P. Y., Alfano R. R. Fluorescence relaxation kinetics from rhodopsin and isorhodopsin. Biophys J. 1981 Aug;35(2):547–550. doi: 10.1016/S0006-3495(81)84810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- Huppert D., Rentzepis P. M., Kliger D. S. Picosecond and nanosecond isomerization kinetics of protonated 11-cis retinylidene Schiff bases. Photochem Photobiol. 1977 Feb;25(2):193–197. doi: 10.1111/j.1751-1097.1977.tb06897.x. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Monger T. G., Alfano R. R., Callender R. H. Photochemistry of rhodopsin and isorhodopsin investigated on a picosecond time scale. Biophys J. 1979 Jul;27(1):105–115. doi: 10.1016/S0006-3495(79)85205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Uji K., Kito Y. Studies on cephalopod rhodopsin: photoisomerization of the chromophore. Biochim Biophys Acta. 1976 Apr 23;428(2):321–338. doi: 10.1016/0304-4165(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]



