Abstract
Purpose.
To investigate monthly and seasonal variations in the progression of myopia in children enrolled in the Correction of Myopia Evaluation Trial (COMET).
Methods.
An ethnically diverse cohort of 469 myopic 6- to <12 year-old children was randomized to single vision or progressive addition lenses and followed for 3 years with 98.5% retention. Progression of myopia was measured semiannually by noncycloplegic autorefraction (Nidek ARK 700A) and annually by cycloplegic autorefraction, with the former measurements used in these analyses. The semiannual progression rate was calculated as (change in spherical equivalent refraction between two consecutive semiannual visits/number of days between the two visits) times 182.5. Months were categorized as the midpoint between two visit dates. Seasons were classified as winter (October through March) or summer (April through September). The seasonal difference was tested using a linear mixed model adjusting for demographic variables (age, sex, ethnicity), baseline refraction, and treatment group.
Results.
Data from 358 children (mean [±SD] age = 9.84 ± 1.27 years; mean myopia = −2.54 ± 0.84 diopters [D]) met the criteria for these analyses. Myopia progression varied systematically by month; it was slower in April through September than in the other months. Mean progression in winter was −0.35 ± 0.34 D and in summer was −0.14 ± 0.32 D, a statistically significant difference (0.21 D, P < 0.0001). The same seasonal pattern was found by age, sex, ethnicity (except in the small sample of Asians), lens type, and clinical center.
Conclusions.
The slower progression of myopia found in summer is likely related to children's spending more time outdoors and fewer hours in school. The data have clinical implications regarding the time of year and the frequency with which myopic children have eye examinations and the need for precise timing of visits in clinical trials testing new myopia treatments. (ClinicalTrials.gov number, NCT00000113.)
Keywords: myopia, refractive error, children's vision
Myopia progression in children was found to vary systematically by month; it was slower in April through September than in the other months. These data have implications for both mechanisms of eye growth and clinical management of myopia.
Introduction
The prevalence of myopia has increased dramatically over the past few decades, suggesting a role for environmental factors.1,2 The environmental factor cited most frequently in the past was excessive near work and schooling (reviewed in Ref. 3). Recently, differences in outdoor activity, especially light exposure, have emerged as a potential key factor to account for differences in the refractive errors of children, including the reduced prevalence and incidence of myopia in children with more outdoor activity.4–12
A study of Chinese children living either in Sydney or in Singapore provides evidence for the role of outdoor light exposure in refractive development.13 The Sydney children had a significantly lower prevalence of myopia, and the most significant lifestyle factor associated with the difference in prevalence was time spent outdoors (13.5 hours per week in Sydney versus 3.05 hours per week in Singapore). Additional support for the influence of light on myopia progression comes from a recent paper by Cui et al.,11 who found less myopia progression and axial elongation in Danish children tested over 6-month periods that included longer summer (versus shorter winter) days. Even though information on each child's actual amount of outdoor light exposure was not collected, the assumption was that children in Denmark spend more time outdoors in the summer than in the winter and therefore the children tested in a 6-month period that included the summer had more outdoor light exposure.
The combination of low levels of near work and long periods of time outdoors is associated with less myopia in children.8,14 These factors also may account for the reported slower progression of myopia and/or axial elongation in the summer compared to the school year.15–19 Children in the United States typically are in school from late August or early September until May or June and then have a long summer vacation, usually with a significant increase in outdoor activity and decrease in near work compared to the school year.5 Indoor hours devoted to school/studying and outdoor activity hours vary dramatically between winter and summer for most children. For example, weekly hours of studying by myopic children in Boston, Massachusetts, decreased from 10.8 during the school year to 1.3 in the summer, while outdoor activity hours increased from 8.3 during school to 19.4 in the summer.5 It should be noted that these hours do not include time in school, which for children in Boston averaged 30 hours per week. Therefore, available hours for outdoor activity are more limited during the school year.
The previous studies investigating seasonal progression of myopia have reported results from relatively small numbers of children in the United States, southern China, and Japan.15–19 The Correction of Myopia Evaluation Trial (COMET) recruited 469 myopic children for a study of spectacle lens treatments for myopia control, with recruitment occurring throughout the year.20 Progression of myopia in COMET was found to be faster in younger children; in girls; and in Asian, Hispanic, and white children compared to African Americans.21 However, progression of myopia by month or season of the year was not investigated and is the purpose of the current study. The large dataset allows for a more fine-grained analysis of myopia progression by time of year than in previous reports.
Methods
Details of the study design and main outcome of the clinical trial have been published previously and are briefly summarized here.20–22 The COMET study enrolled 469 6- to <12-year-old children with myopia between −1.25 and −4.50 diopters (D), with half randomized to single vision lenses (SVLs), the conventional treatment for myopia, and half to progressive addition lenses (PALs). Study visits were scheduled every 6 months for the first 5 years. The main outcome after 3 years (the length of the clinical trial) was a 0.20 D treatment benefit of PALs, with 98.5% retention.20 Four colleges/schools of optometry participated in COMET; they were located in Boston, Massachusetts; Birmingham, Alabama; Houston, Texas; and Philadelphia, Pennsylvania. The institutional review boards at all participating centers approved the research protocols, and the research followed the tenets of the Declaration of Helsinki. Informed consent from the parents was obtained after verbal and written explanation of the study, and assent was obtained from the children.
The ARK 700A (Nidek, Gamagori, Japan) autorefractor/autokeratometer was used to take five consecutive, reliable refraction readings, which were then averaged. Noncycloplegic autorefraction measurements were used in the present analyses because they were taken every 6 months in contrast to the main outcome measure of myopia progression by cycloplegic autorefraction, which was taken only annually. The mean (±SD) difference between the cycloplegic and noncycloplegic measurements at baseline was 0.19 ± 0.22 D.22
Right eye data from children with seven semiannual visits over 3 years and with measurements within ±35 days of their targeted visit dates were used. The semiannual progression rate was calculated as: (change in spherical equivalent refraction [SER] between two consecutive semiannual visits/number of days between the two visits) × 182.5. Months were categorized as the midpoint between two visit dates; for example, January includes progression from October to April. Seasons were classified as winter (October through March) or summer (April through September). The seasonal difference was tested using a linear mixed model adjusting for demographic variables (age, sex, and ethnicity), baseline refraction, and treatment group.
Results
Data from 358 of 469 participants (76.33%) met the criteria for this study. Baseline characteristics of included and excluded children were similar, as shown in the Table. The mean (±SD) age of the children used in these analyses was 9.84 ± 1.27 years, and their mean myopia was −2.54 ± 0.84 D. The group was ethnically diverse, and 53% were female. Two hundred twelve of the children had summer visits followed by winter, while 146 had winter visits first.
Table.
Participant Characteristics in the First Year
|
Characteristic/Variable |
Included, n = 358 |
Excluded, n = 111 |
P |
||
|
n (%) |
Mean (SD) |
n (%) |
Mean (SD) |
||
| Sex | |||||
| Male | 167 (46.6) | 56 (50.5) | 0.48 | ||
| Female | 191 (53.4) | 55 (49.5) | |||
| Ethnicity | |||||
| Asian | 32 (8.9) | 4 (3.6) | 0.29 | ||
| African American | 91 (25.4) | 32 (28.8) | |||
| Hispanic | 55 (15.4) | 13 (11.7) | |||
| Caucasian | 163 (45.5) | 55 (49.5) | |||
| Mixed and other | 17 (4.7) | 7 (6.3) | |||
| Treatment | |||||
| PAL | 176 (49.2) | 59 (53.2) | 0.46 | ||
| SVL | 182 (50.8) | 52 (46.8) | |||
| Age, y, at baseline | 9.84 (1.27) | 9.82 (1.36) | 0.90 | ||
| Baseline SE myopia (D) | −2.54 (0.84) | −2.66 (0.82) | 0.20 | ||
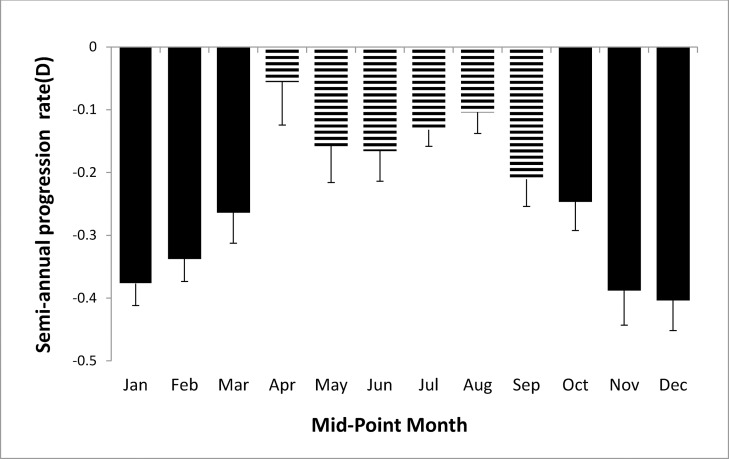
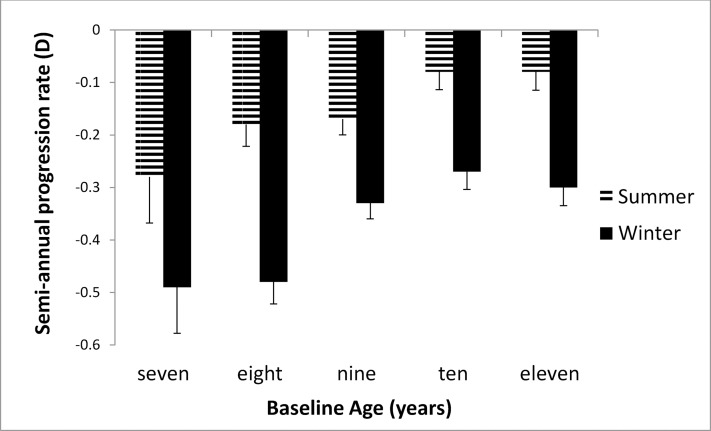
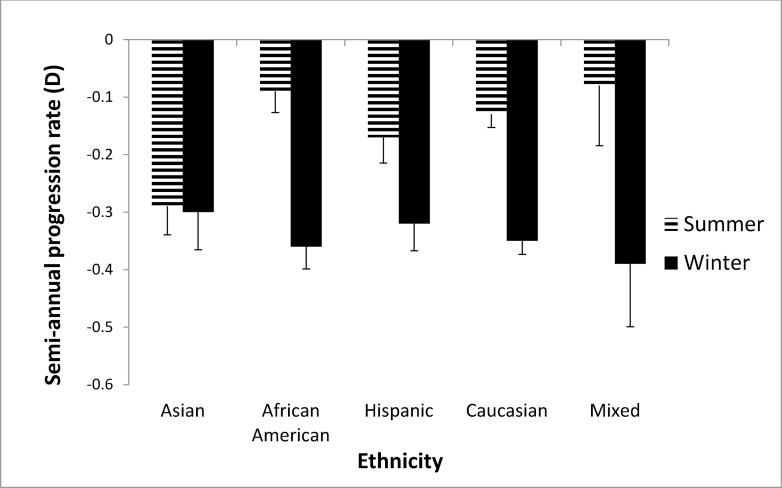
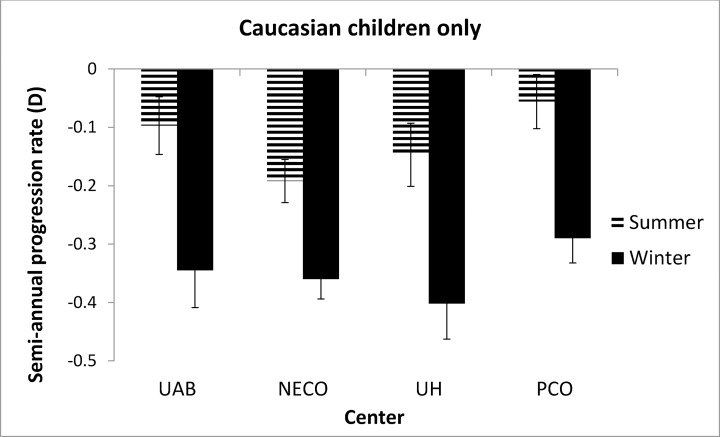
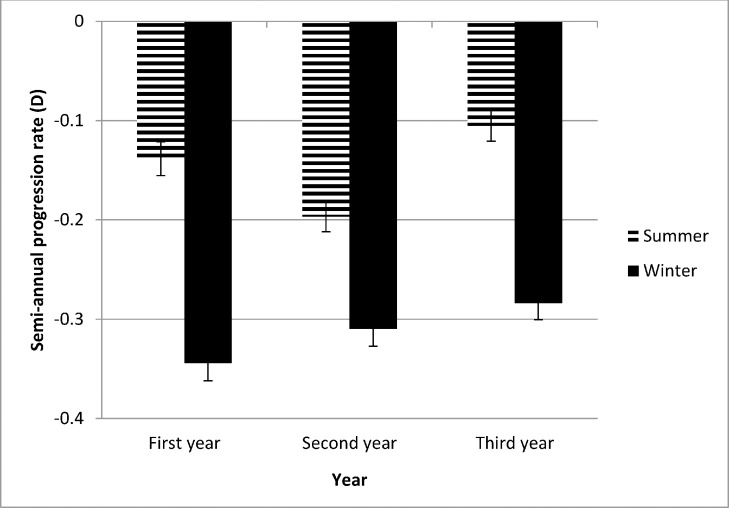
Figure 1 shows the semiannual progression rate by month in the first year of the study. The progression varied systematically by month; it was slower in April through September than in the other months. Mean progression in winter (dark bars in Fig. 1) was −0.35 ± 0.34 D and in summer was −0.14 ± 0.32 D, a statistically significant difference (0.21 D, P < 0.0001). As shown in Figure 2, the seasonal progression did not vary by age (interaction between age groups and season, P = 0.54). In addition, similar seasonal patterns were found for all ethnic groups except Asians (Fig. 3), with borderline significance for the interaction between ethnicity and season (P = 0.08). Because ethnic groups were not balanced across the four clinical centers, the data by center are shown only for Caucasian children in Figure 4, with similar seasonal patterns found at all centers. Seasonal differences also were found when the data were analyzed by sex and by lens type, SVLs or PALs (data not shown). The overall seasonal difference was slightly higher for children with first visits in the winter (0.24 D difference) than in the summer (0.18 D difference), but not statistically significant (P = 0.30). Figure 5 shows that a similar seasonal pattern of myopia progression was found in the second and third years of the study, with statistically significant differences (P < 0.0001 for each year).
Figure 1.
Semiannual progression rate by midpoint month in the first year of COMET. Winter months are shown by dark bars and summer months by hatched bars.
Figure 2.
Semiannual progression rate in winter and summer stratified by age in the first year of COMET. Winter months are shown by dark bars and summer months by hatched bars.
Figure 3.
Semiannual progression rate in winter and summer stratified by ethnicity in the first year of COMET. Winter months are shown by dark bars and summer months by hatched bars.
Figure 4.
Semiannual progression rate in winter and summer in white children stratified by clinical center in the first year of COMET. Winter months are shown by dark bars and summer months by hatched bars.
Figure 5.
Semiannual progression rate in winter and summer over the first 3 years of COMET. Winter months are shown by dark bars and summer months by hatched bars.
Discussion
The large number of myopic children in COMET and their ethnic diversity allow for more detailed analyses of progression of myopia by time of year than in previous studies. Monthly differences in myopia progression in children are reported here for the first time, with greater progression in winter compared to summer months. Exposure to higher light levels and reduced academic pressures in the summer are likely to account for the slowed progression over the summer months found in the current study. The present data are consistent with results of a recent study by Cui et al.,11 who found less myopia progression and axial elongation in Danish children tested over 6-month periods that included summer days. It should be noted that the current method of averaging across 6 months and attributing the data to the most central month, used in this and some other studies, likely underestimates the actual monthly differences.
In the present study, seasonal differences in progression of myopia were found when the data were stratified by age, sex, treatment group, and ethnicity, except possibly for Asian children (P value for interaction between ethnicity and seasonal progression was of borderline significance). While reasons to account for the latter result are not known, one possibility is that these children have heavy educational commitments during both the school year and the summer, resulting in limited time outdoors year round, similar to the low number of outdoor hours shown for Chinese children living in Singapore.13 Similar seasonal progression patterns also were shown at the four COMET clinical centers, two in the Northeast and two in the southern United States. These locations have different hours of daylight depending on the time of year, resulting in a range of differences in daylight hours between the longest and shortest days of the year, from a high of 6.12 hours in Boston, which has the shortest winter days and longest summer days, to 5.41 hours in Philadelphia, 4.27 hours in Birmingham, and 3.49 hours in Houston (downloaded on October 9, 2013 from http://www.timeanddate.com/worldclock/sunrise.html [in the public domain]). In addition to the different hours and intensity of light depending on the time of year, the climate at each location also has to be considered. Children are less likely to be outside on summer days in Houston or Birmingham, locations with high heat and humidity, than on summer days in Boston or Philadelphia.
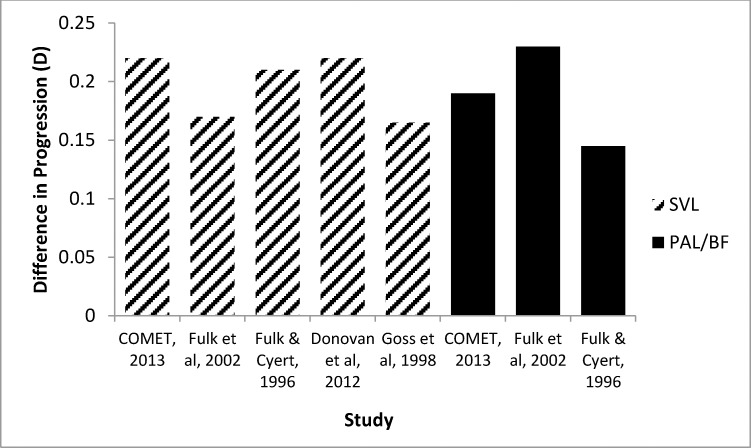
Similar seasonal differences in myopia progression have been reported in studies conducted in various places in the United States and also in different locations and latitudes around the world (and therefore with different photoperiods), as shown in Figure 6. To illustrate this point, in Guangzhou, China, the location of a recent study by Donovan et al.,15 seasonal differences in hours of daylight are small (10:52 hours of daylight in mid-January and 13:25 in mid-July, as reported in the paper) compared to most study locations in the United States, and yet the summer–winter differences in myopia progression are similar. The results presented in Figure 6 suggest that light is not likely to be the only factor affecting myopia progression.
Figure 6.
Comparison of COMET seasonal progression of myopia (winter–summer) with other studies using either single vision lenses (hatched bars) and/or PALs/bifocals (dark bars).
Results of animal studies point to a role for light in protecting eyes from axial elongation and the development of myopia. Experiments on chicks, tree shrews, and infant monkeys showed that higher ambient light levels were associated with slower development of form-deprivation myopia and, in chicks and tree shrews, were associated with a slower rate of myopia development in response to negative-lens wear.23–27 The higher light levels did not prevent negative lens–induced myopia from reaching full compensation; however, a negative lens provides a refractive target to which animal eyes respond. Although human emmetropization is known to be regulated by defocus, underaccommodation does not provide a fixed target and, in this one respect, may be analogous to the situation imposed by form deprivation in animals.27 Thus, the slowed responses of both negative lens–induced myopia and form-deprivation myopia during exposure to higher light intensities provide support for a light-based explanation for the observed monthly and seasonal differences in myopia progression in children.
It is difficult to reconcile the present results and others suggesting that progression of myopia is modulated seasonally with the absence of an association between outdoor activity or near work and myopia progression using questionnaire data in the CLEERE study.28 Possible reasons to account for the latter result include a limited range of outdoor activity shown by the myopic children, data collection only for activities done in the school year, the combination of sports and outdoor activity in the questionnaire, and possible statistical limitations.
Strengths of the current study include the large, ethnically diverse sample of myopic children with standardized measurements of refractive error taken at regular intervals. Limitations include the use of noncycloplegic autorefraction measurements, which were taken every 6 months, rather than cycloplegic autorefraction, which was done only annually, though in this group of myopic children these measurements were very similar. In addition, axial length was measured only annually in COMET, so those data could not be included in the seasonal analyses.
The current data showing differences in myopia progression by time of year have clinical implications, suggesting that it is important to consider the time of year and the frequency with which myopic children have eye examinations. Children in the United States typically get eye examinations at the end of summer before school starts or at the beginning of the school year when the child fails a school vision screening. New spectacles or contact lenses in the autumn and more rapid progression occurring at the same time mean that many children could be undercorrected soon after school starts, and undercorrection has been shown to increase myopia progression by a small amount compared to full correction.29,30 Therefore, it would be optimal for myopic children, especially those younger than 9 years of age and/or with two myopic parents (the groups with faster progression),21 to be examined more frequently than once a year as is now recommended. Parents and teachers should encourage more outdoor play and less near work (though not to the point of affecting school performance), especially during the school year when myopia progression is greater. With respect to clinical trials testing new treatments for myopia control, researchers should consider carefully the time of year for recruitment of patients and the duration of the study. Finally, it is important to note that the current results are relevant to strategies for the prevention of high myopia and its attendant complications. More time spent outdoors and less time indoors doing near work may slow axial elongation and prevent high myopia, thereby reducing the risk of developing sight-threatening conditions such as retinal detachments and myopic retinopathy.
Acknowledgments
Supported by National Eye Institute/National Institutes of Health Grants EY11756, EY11754, EY11805, EY11752, EY11740, and EY11755.
Disclosure: J. Gwiazda, None; L. Deng, None; R. Manny, None; T.T. Norton, None
Appendix
COMET Study Group
Study Chair's Office.
New England College of Optometry, Boston, Massachusetts. Jane Gwiazda (Study Chair/Principal Investigator); Thomas Norton (Consultant); Li Deng (Biostatistician, 6/10–present); Kenneth Grice (Study Coordinator, 9/96–7/99); Christine Fortunato (Study Coordinator, 8/99–9/00); Cara Weber (Study Coordinator, 10/00–8/03); Alexandra Beale (Study Coordinator, 11/03–7/05); David Kern (Study Coordinator, 8/05–8/08); Sally Bittinger (Study Coordinator, 8/08–4/11); Debanjali Ghosh (Study Coordinator, 5/11–7/13); Rosanna Pacella (Research Assistant, 10/96–10/98).
Coordinating Center.
Department of Preventive Medicine, Stony Brook University Health Sciences Center; Stony Brook, New York. Leslie Hyman (Principal Investigator); M. Cristina Leske (Co-principal Investigator until 9/03); Mohamed Hussein (Co-Investigator/Biostatistician until 10/03); Li Ming Dong (Co-investigator/Biostatistician, 12/03–5/10); Melissa Fazzari (Co-investigator/Biostatistician, 5/11–4/12); Wei Hou (Co-investigator/Biostatistician, 10/12–present); Elinor Schoenfeld (Epidemiologist until 9/05); Lynette Dias (Study Coordinator, 6/98–present); Rachel Harrison (Study Coordinator, 4/97–3/98); Wen Zhu (Senior Programmer until 12/06); Qinghua Zhang (Data Analyst, 4/06–present); Ying Wang (Data Analyst, 1/00–12/05); Ahmed Yassin (Data Analyst, 1/98–1/99); Elissa Schnall (Assistant Study Coordinator, 11/97–11/98); Cristi Rau (Assistant Study Coordinator, 2/99–11/00); Jennifer Thomas (Assistant Study Coordinator, 12/00–4/04); Marcela Wasserman (Assistant Study Coordinator, 5/04–7/06); Yi-ju Chen (Assistant Study Coordinator, 10/06–1/08); Sakeena Ahmed (Assistant Study Coordinator, 1/09–6/11); Leanne Merill (Assistant Study Coordinator, 10/11–8/13); Lauretta Passanant (Project Assistant, 2/98–12/04); Maria Rodriguez (Project Assistant, 10/00–6/13); Allison Schmertz (Project Assistant, 1/98–12/98); Ann Park (Project Assistant, 1/99–4/00); Phyllis Neuschwender (Administrative Assistant until 11/99); Geeta Veeraraghavan (Administrative Assistant, 12/99–4/01); Angela Santomarco (Administrative Assistant, 7/01–8/04); Laura Sisti (Administrative Assistant, 4/05–10/06); Lydia Seib (Administrative Assistant, 6/07–present).
National Eye Institute, Bethesda, Maryland. Donald Everett (Project Officer).
Clinical Centers.
University of Alabama at Birmingham School of Optometry, Birmingham, Alabama. Wendy Marsh-Tootle (Principal Investigator); Katherine Weise (Optometrist, 9/98–present); Marcela Frazier (Optometrist, 1/10–present); Catherine Baldwin (Primary Optician and Clinic Coordinator, 10/98–6/13); Carey Dillard (Clinic Coordinator and Optician,10/09–6/13); Kristine Becker (Ophthalmic Consultant, 7/99–3/03); James Raley (Optician, 9/97–4/99); Angela Rawden (Back-up Optician, 10/97–9/98); Nicholas Harris (Clinic Coordinator, 3/98–9/99); Trana Mars (Back-up Clinic Coordinator, 10/97–3/03); Robert Rutstein (Consulting Optometrist until 8/03). New England College of Optometry, Boston, Massachusetts. Daniel Kurtz (Principal Investigator until 6/07); Erik Weissberg (Optometrist, 6/99–present; Principal Investigator since 6/07); Bruce Moore (Optometrist until 6/99); Elise Harb (Optometrist, 8/08–present); Robert Owens (Primary Optician until 6/13); Sheila Martin (Clinic Coordinator until 9/98); Joanne Bolden (Coordinator, 10/98–9/03); Justin Smith (Clinic Coordinator, 1/01–8/08); David Kern (Clinic Coordinator, 8/05–8/08); Sally Bittinger (Clinic Coordinator, 8/08–4/11); Debanjali Ghosh (Clinic Coordinator, 5/11–8/13); Benny Jaramillo (Back-up Optician, 3/00–6/03); Stacy Hamlett (Back-up Optician, 6/98–5/00); Laura Vasilakos (Back-up Optician, 2/02–12/05); Sarah Gladstone (Back-up Optician, 6/04–3/07); Chris Owens (Optician, 6/06–9/09; Patricia Kowalski (Consulting Optometrist until 6/01); Jennifer Hazelwood (Consulting Optometrist, 7/01–8/03). University of Houston College of Optometry, Houston, Texas. Ruth Manny (Principal Investigator); Connie Crossnoe (Optometrist until 5/03); Karen Fern (Consulting Optometrist until 8/03; Optometrist since 9/03); Heather Anderson (Optometrist, 1/10–present); Sheila Deatherage (Optician until 3/07); Charles Dudonis (Optician until 1/07); Sally Henry (Clinic Coordinator until 8/98); Jennifer McLeod (Clinic Coordinator, 9/98–8/04; 2/07-5/08); Mamie Batres (Clinic Coordinator, 8/04–1/06); Julio Quiralte (Back-up Coordinator, 1/98–7/05); Giselle Garza (Clinic Coordinator, 8/05–1/07); Gabynely Solis (Clinic Coordinator, 3/07–8/11); Joan Do (Clinic Coordinator, 4/12–8/13); Andy Ketcham (Optician, 6/07–9/11). Pennsylvania College of Optometry, Philadelphia, Pennsylvania. Mitchell Scheiman (Principal Investigator); Kathleen Zinzer (Optometrist until 4/04); Karen Pollack (Clinic Coordinator, 11/03–6/13); Timothy Lancaster (Optician until 6/99); Theresa Elliott (Optician until 8/01); Mark Bernhardt (Optician, 6/99–5/00); Daniel Ferrara (Optician, 7/00–7/01); Jeff Miles (Optician, 8/01–12/04); Scott Wilkins (Optician, 9/01–8/03); Renee Wilkins (Optician, 01/02–8/03); Jennifer Nicole Lynch (Optician & Back-up Coordinator, 10/03–9/05); Dawn D'Antonio (Optician, 2/05–5/08); Lindsey Lear (Optician, 5/06–1/08); Sandy Dang (Optician, 1/08–2/10); Charles Sporer (Optician, 3/10–10/11); Mary Jameson (Optician, 10/11–6/13); Abby Grossman (Clinic Coordinator, 8/01–11/03); Mariel Torres (Clinic Coordinator, 7/97–6/00); Heather Jones (Clinic Coordinator, 8/00–7/01); Melissa Madigan-Carr (Coordinator, 7/01–3/03); Theresa Sanogo (Back-up Coordinator, 7/99–3/03); JoAnn Bailey (Consulting Optometrist until 8/03).
Data and Safety Monitoring Committee.
Robert Hardy (Chair); Argye Hillis; Donald Mutti; Richard Stone; Carol Taylor.
Footnotes
See the appendix for the members of the COMET Study Group.
References
- 1. Vitale S, Sperduto RD, Ferris FL III. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009; 127: 1632–1639 [DOI] [PubMed] [Google Scholar]
- 2. Morgan I, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 5: 1739–1748 [DOI] [PubMed] [Google Scholar]
- 3. Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005; 24: 1–38 [DOI] [PubMed] [Google Scholar]
- 4. Sherwin J, Reacher M, Keogh R, Khawaja A, Mackey D, Foster P. The association between time spent outdoors and myopia in children and adolescents. Ophthalmology. 2012; 119: 2141–2151 [DOI] [PubMed] [Google Scholar]
- 5. Deng L, Gwiazda J, Thorn F. Children's refractions and visual activities in the school year and summer. Optom Vis Sci. 2010; 87: 406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002; 43: 3633–3640 [PubMed] [Google Scholar]
- 7. Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007; 48: 3524–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose K, Morgan I, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285 [DOI] [PubMed] [Google Scholar]
- 9. Dirani M, Tong L, Gazzard G, et al. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009; 93: 997–1000 [DOI] [PubMed] [Google Scholar]
- 10. Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012; 53: 2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui D, Trier K, Ribel-Madsen SM. Effect of day length on eye growth, myopia progression, and change of corneal power in myopic children. Ophthalmology. 2013; 120: 1074–1079 [DOI] [PubMed] [Google Scholar]
- 12. French AN, Ashby RS, Morgan I, Rose K. Time outdoors and the prevention of myopia. Exp Eye Res. 2013; 114: 58–68 [DOI] [PubMed] [Google Scholar]
- 13. Rose K, Morgan I, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008; 126: 527–530 [DOI] [PubMed] [Google Scholar]
- 14. French AN, Morgan I, Mitchell P, Rose K. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013; 120: 2100–2108 [DOI] [PubMed] [Google Scholar]
- 15. Donovan L, Sankaridurg P, Ho A, et al. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012; 89: 1196–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulk G, Cyert L. Can bifocals slow myopia progression? J Am Optom Assoc. 1996; 67: 749–754 [PubMed] [Google Scholar]
- 17. Fulk G, Cyert L, Parker D. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002; 79: 46–51 [DOI] [PubMed] [Google Scholar]
- 18. Goss D, Rainey B. Relation of childhood myopia progression rates to time of year. J Am Optom Assoc. 1998; 69: 262–266 [PubMed] [Google Scholar]
- 19. Fujiwara M, Hasebe S, Nakanishi R, Tanigawa K, Ohtsuki H. Seasonal variation in myopia progression and axial elongation: an evaluation of Japanese children participating in a myopia control trial. Jpn J Ophthalmol. 2012; 56: 401–406 [DOI] [PubMed] [Google Scholar]
- 20. Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003; 44: 1492–1500 [DOI] [PubMed] [Google Scholar]
- 21. Hyman L, Gwiazda J, Hussein M, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the Correction of Myopia Evaluation Trial. Arch Ophthalmol. 2005; 123: 977–987 [DOI] [PubMed] [Google Scholar]
- 22. Gwiazda J, Marsh-Tootle W, Hyman L, Hussein M, Norton TT; COMET Study Group. Baseline refractive and ocular component measures of children enrolled in the Correction of Myopia Evaluation Trial (COMET). Invest Ophthalmol Vis Sci. 2002; 43: 314–321 [PubMed] [Google Scholar]
- 23. Smith E, Hung L, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith E, Hung L, Arumugam B, Huang J. Negative-lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013; 54: 2959–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354 [DOI] [PubMed] [Google Scholar]
- 26. Ashby R, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253 [DOI] [PubMed] [Google Scholar]
- 27. Norton T, Siegwart J. Light levels, refractive development, and myopia – a speculative review. Exp Eye Res. 2013; 114: 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones-Jordan L, Sinnott L, Cotter S, et al. CLEERE Study Group Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Invest Ophthalmol Vis Sci. 2012; 53: 7169–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002; 42: 2555–2559 [DOI] [PubMed] [Google Scholar]
- 30. Adler D, Millodot M. The possible effect of undercorrection on myopic progression in children. Clin Exp Optom. 2006; 89: 315–321 [DOI] [PubMed] [Google Scholar]