Abstract
Purpose.
To examine whether calpain inhibition following retinal detachment would prolong autophagy and result in reduced photoreceptor apoptosis.
Methods.
Retinal detachments were created in Brown-Norway rats by subretinal injection of 1% hyaluronic acid and simulated in vitro by Fas-receptor activation of 661W cells, a cone cell line. Protein levels of LC3 and autophagy-related gene 5 (Atg5), both of which are involved in the creation of the autophagosome, were assayed by Western blot. Calpain 1, the protease responsible for Atg5 cleavage and transitioning photoreceptors from autophagy to apoptosis, activity was monitored by α-spectrin cleavage. Various calpain inhibitors were added either to the subretinal space or cell culture media. Apoptosis was assessed in vitro by caspase-8 activity assays and in vivo via TUNEL assays. Cell counts were assessed in vivo at 2 months following detachment.
Results.
Following retinal detachment or Fas-receptor activation of 661W cells, there was an increase in Atg5 and LC3-II that peaked at 3 days and decreased by 7-days postdetachment. Calpain 1 activity level peaked at 7 days and was associated with decreased autophagy. Calpain inhibition led to increased autophagy, a decrease in caspase-8 activation, reduced TUNEL-positive photoreceptors, and increased photoreceptor cell survival.
Conclusions.
Our data suggest that calpain activation, which peaks at 7-days postdetachment, is a key step in triggering photoreceptors to shift from cell survival to death. Prolonging autophagy through calpain inhibition leads to significantly reduced photoreceptor apoptosis and increased cell survival.
Keywords: retinal detachment, autophagy, apoptosis
This study examines calpain inhibition following retinal detachment and its effect on prolonging autophagy, and reducing photoreceptor apoptosis in an experimental model of retinal detachment.
Introduction
Separation of photoreceptors from the underlying RPE is a common pathologic feature seen in many retinal diseases. In cases of rhegmatogenous or tractional retinal detachment, we have reliable ways of achieving anatomic re-apposition of the retina to the RPE. In other disease processes, such as exudative retinal detachments and AMD, achieving the anatomic connection is more difficult and the separation is often chronic. Despite significant advances in the surgical and medical management of retina–RPE separation, patients still often have significant visual loss, largely through death of photoreceptors.1,2 Ways of increasing photoreceptor survival during periods of retina–RPE separation has the potential to be extremely beneficial in improving visual outcomes.
Using an experimental model of retinal detachment, we have shown that retina–RPE separation results in the activation of the Fas-receptor and downstream caspase cascade, leading to apoptotic death of the photoreceptors.3–5 Fas-mediated apoptosis also appears to play a role in photoreceptor cell death in more complex diseases, such as AMD.6 In our experimental system, inhibition of Fas-receptor activation results in a significant increase in the number of photoreceptors that survive prolonged periods of retina–RPE separation.5,7
An interesting paradox exists, however, with regards to the rate of photoreceptor cell death in cases of retina–RPE separation. Even though the RPE is the sole source for photoreceptor nutritional support, and apoptosis is activated within hours after separation, many of the photoreceptors can survive for an extended period of time. This is clinically apparent as eyes with retinal detachments affecting the central macula can be repaired with significant recovery of vision.8,9 This suggests that antiapoptotic signals are also activated shortly after detachment and serve to prolong photoreceptor survival. Several of these mechanisms have been described, including IL-6 activation and autophagy.10,11 Clinical data, however, show that visual recovery significantly decreases if the reattachment occurs after 7 days of retina–RPE separation, suggesting that the cells survive for a limited period of time before death prevails.8,9
Autophagy, a form of cellular recycling upregulated during times of metabolic stress, also undergoes upregulation following retinal detachment.11 Its activation is, at least in part, Fas dependent, just like apoptosis. Inhibition of autophagy, either pharmacologically or via knockout of the autophagy-related gene 5 (Atg5) gene, leads to elevated and more rapid apoptosis. Therefore, the same signal, Fas, activates both apoptosis and autophagy at the time of photoreceptor–RPE separation. Autophagy inhibits apoptosis, at least temporarily, until the cells eventually die.11 The cellular signals that transition the photoreceptors from autophagy to apoptosis, especially those active at 7-days postdetachment, are currently unknown.
One potential candidate regulator of the shift from autophagy to apoptosis is calpain activation. In other cell systems, the cleavage of Atg5, an essential component in the activation of autophagosome formation, by calpain 1, a member of a group of calcium-dependent, nonlysosomal cysteine proteases, leads cells to abandon autophagy and enter apoptosis.12,13 Calpain inhibitors have been extensively studied and have shown to be neuroprotective in a number of models, including retinal hypoxia,14 photoreceptor degeneration,15 optic neuritis and other optic nerve injuries,16–18 cataracts,19 traumatic brain injury,20 and glutamate excitotoxicity.21
In this study, we test the hypothesis that calpain activation after retina–RPE separation results in decreased autophagy and increased photoreceptor apoptosis. We tested this hypothesis in our well-established in vivo model of retinal detachment and in an in vitro cell culture model. Our findings show that Atg5 undergoes upregulation within 1 day after detachment, similar to other autophagy markers. Calpain 1 reaches peak activity levels at 7-days postdetachment, where autophagy decreases. Calpain inhibitors prolong the period of autophagy, which leads to decreased apoptosis and prolonged cell survival. Our data suggest a novel way of prolonging autophagy and decreasing apoptosis following retina–RPE separation.
Methods
Experimental Model of Retinal Detachment
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines established by the University Committee on Use and Care of Animals of the University of Michigan. Detachments were created in adult male Brown-Norway rats (300–400 g; Charles River Laboratories, Wilmington, MA), as described previously. Briefly, rodents were anesthetized with a 50:50 mix of ketamine/xylazine, and pupils were dilated with topical phenylephrine (2.5%) and tropicamide (1%). A 20-gauge microvitreoretinal blade was used to create a sclerotomy 2-mm posterior to the limbus, carefully avoiding lens damage. A subretinal injector (Glaser, 32-gauge tip; BD Ophthalmic Systems, Franklin Lakes, NJ) was introduced through the sclerotomy into the vitreous cavity and then through a peripheral retinotomy into the subretinal space. Sodium hyaluronate (10 mg/mL, Healon OVD; Abbott Medical Optics, Uppsala, Sweden) was slowly injected to detach the neurosensory retina from the underlying RPE. In all experiments, approximately one-third to one-half of the neurosensory retina was detached. Detachments were created in the left eye. The right eye served as the control, with all the steps of the procedure performed except for introduction of the subretinal injector and injection of the sodium hyaluronate. In some experimental eyes, subretinal injection of either Bafilomycin-A (Baf-A, 50 μg; Enzo Life Sciences, Plymouth Meeting, PA), 0.2 μg calpeptin (Santa Cruz Biotechnology, Santa Cruz, CA) or vehicle (dimethyl sulfoxide [DMSO]) was performed at the time of retina–RPE separation. In these experiments, the subretinal sodium hyaluronate was injected as described earlier, immediately followed by additional subretinal injection of the specific agent delivered in a 5-μL volume.
Cell Culture
The 661W photoreceptor cell line was generously provided by Muayyad Al-Ubaidi (Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The 661W cell line was maintained in Dulbecco's modified Eagle's medium (Gibco Company, Grand Island, NY) containing 10% fetal bovine serum, 300 mg/L glutamine, 32 mg/L putrescine, 40 μL/L β-mercaptoethanol, and 40 μg/L of both hydrocortisone 21-hemisuccinate and progesterone. The media also contained penicillin (90 units/mL) and streptomycin (0.09 mg/mL). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Western Blot Analysis
Retinas from experimental eyes with detachment (detached portion of retina only) and control eyes without detachment were dissected from the RPE–choroid, homogenized, and lysed in buffer containing 10 mM HEPES (pH 7.6), 0.5% IgEPal, 42 mM KCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 5 mM MgCl2, and one tablet of protease inhibitors per 10 mL buffer (Complete Mini; Roche Diagnostics GmbH, Madison, WI). Retinas from three animals were pooled to create one sample for each individual experiment. 661W cells were plated at 150,000 cells per well in six-well culture dishes in growth media. After 24 hours, the cells were washed one time with warmed PBS solution and then placed in growth media without fetal bovine serum. Depending on experimental conditions, 500 ng/mL Fas activating antibody (BD Biosciences, San Jose, CA) and various calpain inhibitors including MDG-28170 (Sigma, St. Louis, MO), MG-101 (Sigma), calpeptin (Santa Cruz Biotechnology), or equal volume of DMSO was added. Calpain inhibitors were added 1-hour prior to the addition of Fas activating antibody. The cells were incubated for various lengths of time and then lysed and homogenized in lysis buffer (as above). The homogenates were incubated on ice and centrifuged at 22,000 g at 4°C for 60 minutes. The protein concentration of the supernatant was then determined (DC Protein Assay kit; Bio-Rad Laboratories, Hercules, CA). The protein samples were separated on SDS-PAGE (Tris-HCl Ready Gels; Bio-Rad Laboratories). After electrophoretic separation, the proteins were transferred onto polyvinylidene fluoride membranes (Immobilon-P; Millipore, Billerica, MA). Membranes were then immunoblotted with antibodies according to the manufacturer's instructions. The following antibodies were used: LC3b (Cell Signaling, Beverly, MA), Atg5 (Abgent, San Diego, CA), Atg12 (Cell Signaling), α-spectrin (Santa Cruz Biotechnology), and actin (Sigma). Band intensities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Caspase Assay and Cell Viability
Caspase-8 activity was measured by a commercially available luminescent cleavage assay kit (Promega, Madison, WI). The 661W cells were seeded in 96-well plates at 2000 cells/well for 24 hours before treatment. Cells were treated with 500 ng/mL Fas-agonistic Jo2 monoclonal antibody and various concentrations of calpeptin or equal volume of DMSO. Caspase-8 activity was measured at 48 hours by incubating the cells with the proluminescent substrate in 96-well plates according to the manufacturer's instructions. Controls included untreated cells and wells with no cells. Luminescence was measured in a plate reader luminometer (Veritas; Turner Biosystems, Sunnyvale, CA).
Histology
At varying intervals after creation of the detachment, the animals were euthanatized, and the eyes were enucleated. Whole eyes were fixed overnight at 4°C in PBS with 4% paraformaldehyde (pH 7.4). The specimens were embedded in paraffin and sectioned at a thickness of 5 to 6 μm. For light microscopic analysis, paraffin sections were stained with 0.5% toluidine blue in 0.1% borate buffer. Terminal deoxynucleotidyl transferase dUTP nick end labeling staining was performed on the sections with a detection kit (ApopTag Fluorescein In Situ Apoptosis Detection Kit; Millipore) according to the manufacturer's instructions. In each experiment, three nonoverlapping high-power fields (40×) at the maximal height of the retinal detachment were selected per section. The total number of cells or TUNEL-positive cells in the outer nuclear layer (ONL) was measured and then averaged. For each experimental group, measurements were made on three eyes.
Data Analysis
Statistical analysis comparing groups was performed using two-tailed Student's t-tests assuming equal variance. Differences were considered significant at P less than or equal to 0.05.
Results
Atg5 Activity Following Detachment
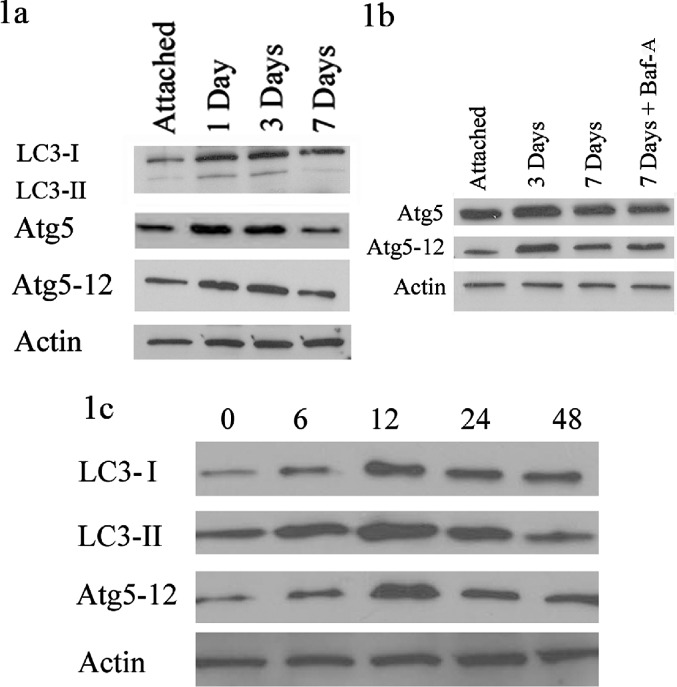
We compared Atg5 levels to LC3, an essential component of the autophagosome complex that has already been well characterized in our system.11 LC3 migrates as two bands on polyacrylamide gel electrophoresis, LC3-I, its inactive form, and LC3-II, its lapidated form. LC3-II is a marker of autophagy and is indicative of autophagosome formation. Similar to prior results, LC3-II protein levels were elevated following detachment, peaking at approximately 1 to 3 days (Fig. 1a). When looking at full length Atg5 and the Atg5-12 complex, the majority state of the protein covalently bound to Atg12, their levels were also elevated at 1 and 3 days, with a 59% (n = 2, SD = 0.21) and 32% (n = 2, SD = 0.12) increase, respectively, at 3 days, and a decrease to baseline rates at 7 days, similar to LC3-II levels (Fig. 1a).
Figure 1.
Autophagy-related gene 5 activity following retinal detachment and Fas activation of 661W cells. (a) Protein from attached retinas and those detached for 1, 3, and 7 days were analyzed by Western blot. Actin was used as a protein-loading control. There was an increase in LC3-II, Atg5, and Atg5-12, peaking at the 1- and 3-day time points. (b) The attached retina, 3- and 7-day detached samples were again compared. Bafilomycin-A or equal volume of DMSO was injected into the subretinal space at the time of detachment. There was no significant difference in Atg5 or Atg5-12 at 7 days with Baf-A added. (c) 661W cells were treated with Fas activating antibody. Whole-cell lysates were collected at various time points, listed by number of hours following Fas activation. Both LC3-II and Atg5-12 peaked at 12 hours. Actin served as a loading control.
We showed previously that autophagy is still present at 7 days, but that LC3-II levels decrease in part because LC3-II itself is degraded in the autophagosomes, a concept known as “autophagy flux.”11 When the degradation of the autophagosome is inhibited by Baf-A, LC3-II does still remain elevated compared with untreated detached retinas at 7 days.11 Our current data shows that when Baf-A was injected at the time of detachment, there was no increase in Atg5 or Atg5-12 levels as compared with detachments alone, suggesting that Atg5 levels truly decreased by day 7 in a manner unrelated to autophagosome turnover (Fig. 1b).
After determining that Atg5 levels increased following retinal detachment in vivo, we wanted to confirm the results in our in vitro system where 661W cells, a mouse photoreceptor cell line, are activated by a Fas-receptor activating antibody. We have previously shown that following activation, the 661W cells undergo peak autophagy at approximately 12 hours and apoptosis at approximately 48 hours.11 Similar to our prior published results,11 LC3-II peaked at approximately 12 hours, decreasing to basal levels by approximately 48 hours (Fig. 1c). Like LC3-II, the Atg5-12 complex also peaked at approximately 12 hours, confirming peak autophagy activation at approximately 12 hours after Fas activation (Fig. 1c).
Calpain 1 Activity Following Detachment
We next looked to confirm that calpains are activated following retinal detachment and assess the temporal relationship of calpain activation to autophagy. Calpain activity was measured by looking at α-spectrin, which when cleaved by calpain 1 or a downstream member of the caspase cascade, forms a breakdown product of around 150 kd.14 Compared to eyes with attached retinas, there was a significant increase in cleaved α-spectrin that peaked at 7 days following detachment (Fig. 2a). In addition, there was a significant rise in cleaved α-spectrin following Fas activation of 661W cells that peaked at 24 hours (Fig. 2b). Both of these time periods correlated with decreased Atg5 levels.
Figure 2.
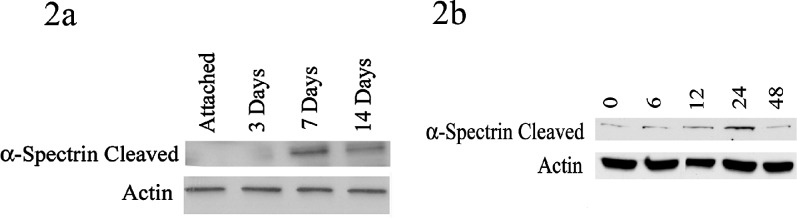
Calpain 1 activity following retinal detachment and Fas activation. (a) Whole retinas were harvested 3, 7, and 14 days after detachments were created. Attached retina served as controls. Cleaved α-spectrin levels were up sharply at 7 days compared with attached retinas and down by 14 days. Actin was used a loading control for all experiments. (b) 661W cells were treated with Fas activating antibody for 6 to 48 hours. There was an increase in total cleaved α-spectrin compared with untreated cells, which peaked at 24 hours.
Calpain Inhibition and Prolongation of Autophagy
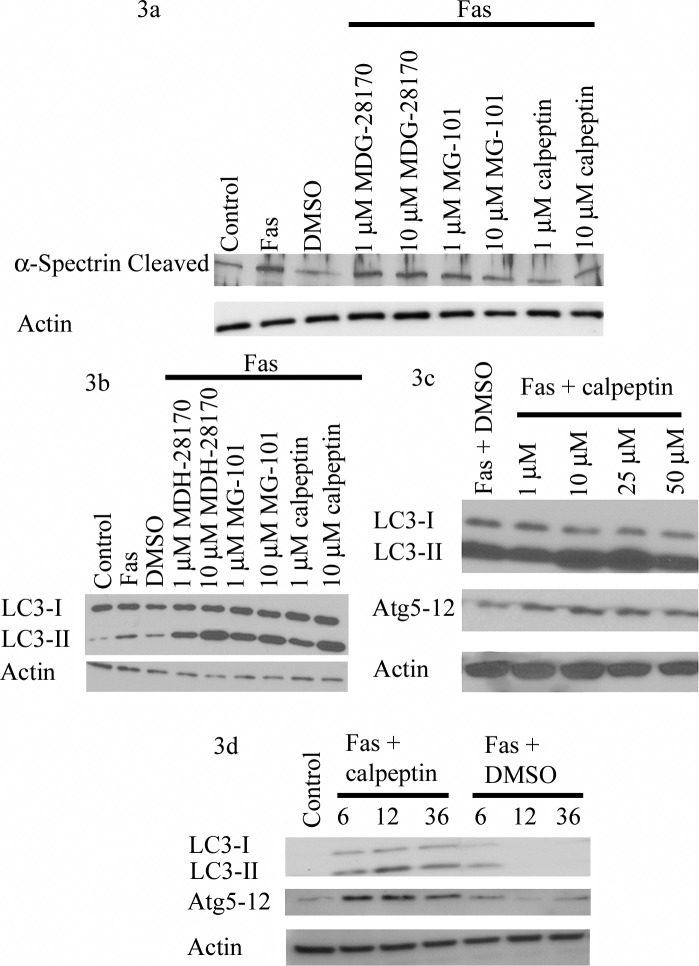
Given that Atg5 levels increased with retinal detachment and that calpain 1 activity peaked as autophagy was decreasing, we next wanted to see if we could prolong autophagy with calpain inhibitors, which prevent Atg5 cleavage. We tried a number of pan-calpain inhibitors in our in vitro model, including MDG-28170, MG-101, and calpeptin. All three led to decreased cleaved α-spectrin levels at 24 hours in the 661W cells as compared with Fas activation alone. The largest effect, however, was found with calpeptin (Fig. 3a). We then looked to see if decreased calpain 1 activity was correlated with increased autophagy. As predicted, there was a significant rise in LC3-II with all the calpain inhibitors at the 24-hour time point, compared with Fas activation alone (Fig. 3b). After determining that calpeptin inhibited calpain 1 the best, we did a dose-response curve to determine the optimal concentration for increasing autophagy. Both LC3-II and Atg5-12 peaked around the 25-μM dose of calpeptin (Fig. 3c). We then looked to see if calpeptin would prolong the two markers of autophagy over time. When compared with cells with Fas activation alone, those cells treated with calpeptin had elongated presence of LC3-II and Atg5-12, indicating prolonged autophagy (Fig. 3d).
Figure 3.
Calpain inhibition in 661W cells with Fas activation. (a) Cells were treated for 24 hours with Fas activating antibody and various calpain inhibitors at two concentrations. Calpain inhibitors were added 1-hour prior to Fas activating antibody. All caused a drop in cleaved α-spectrin, with calpeptin causing the largest effect. Actin was used as a loading control for all experiments. (b) Similar samples were assayed for LC3-II. LC3-II was up at both concentrations at 24 hours after Fas activation. (c) Cells were treated at various concentrations of calpeptin for 24 hours. Both LC3-II and Atg5-12 complex peaked at the 25-μM levels. (d) 661W cells were treated with 25 μM of calpeptin for various lengths of time (in hours). There was an increase in both Atg5-12 and LC3-II following Fas activation. This signal lasted for an extended period of time with calpeptin when compared with vehicle (DMSO) alone.
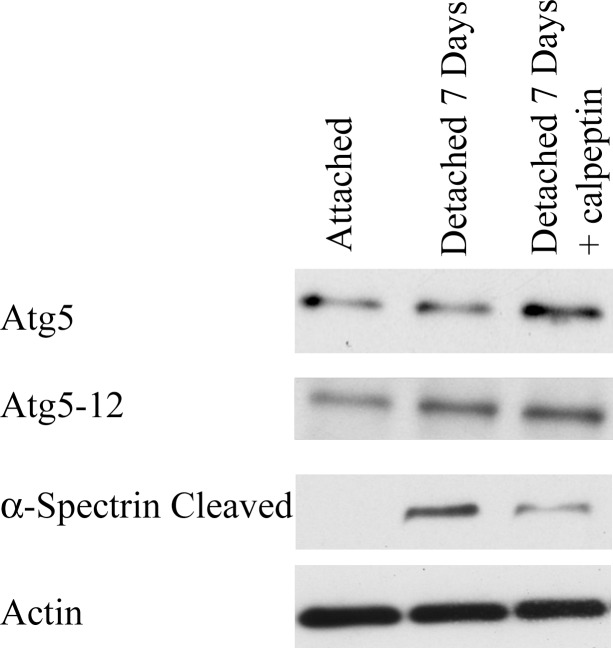
When assessing the effect of calpain inhibition in vivo we found similar results. There was significantly less cleaved α-spectrin with calpeptin at the 7-day time point as compared to without calpeptin, confirming less calpain activity (Fig. 4). When looking at the autophagy markers, there was more Atg5 and Atg5-12, 33% (n = 2, SD = 0.03) and 21% (n = 2, SD= 0.27), respectively, at the 7-day time period with calpain inhibition (Fig. 4). This confirmed the results from the cell culture model that calpeptin is capable of inhibiting calpain in the retina and prolonging autophagy.
Figure 4.
Calpeptin inhibits calpain activity after retinal detachment. Retinas were harvested 7 days following detachment. Attached retinas served as controls. Either 0.2 μg of calpeptin or vehicle (DMSO) was injected into the subretinal space at the time of detachment. Protein was assayed by Western blot. Actin served as a loading control. There was significantly less cleaved α-spectrin with calpeptin. There was an increase in Atg5 and Atg5-12 with calpain inhibition when compared with detached retinas alone. Similar results were found at 3 days with and without calpeptin (data not shown).
Calpain Inhibition Leads to Decreased Apoptosis and Prolonged Cell Survival
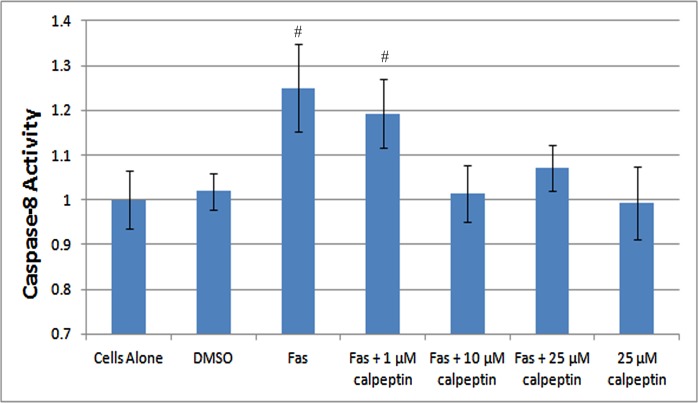
Finally, we tested to see if inhibiting calpain activity, thereby inhibiting Atg5 cleavage and extending autophagy, led to decreased apoptosis. We tested this on the 661W cells using a caspase-8 activity assay. Fas activation caused increased caspase-8 activity compared with control cells at 48 hours, similar to prior data.7 Both 10 and 25 μM of calpeptin significantly reduced its activity back to baseline, therefore rescuing the cells from apoptosis (Fig. 5).
Figure 5.
Caspase-8 activity following Fas activation. 661W cells were treated with Fas activating antibody for 48 hours. Calpeptin was added 1-hour prior to treatment. Cells were then assayed for caspase-8 activity. Fas activation caused a significant elevation in caspase-8 activity. While 1 μM of calpeptin had little effect, both the 10- and 25-μM concentrations returned caspase-8 activity to baseline levels. Calpeptin alone had no effect on cells that were not treated with Fas activating antibody (P < 0.05; # compared with cells alone). Error bars indicate SD.
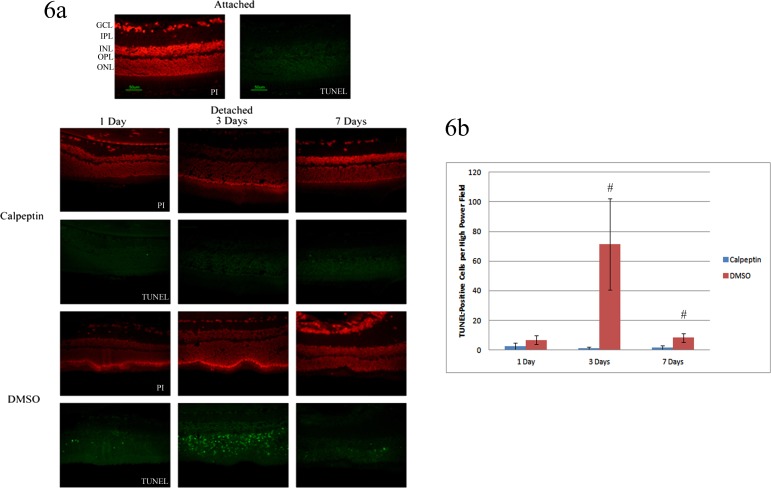
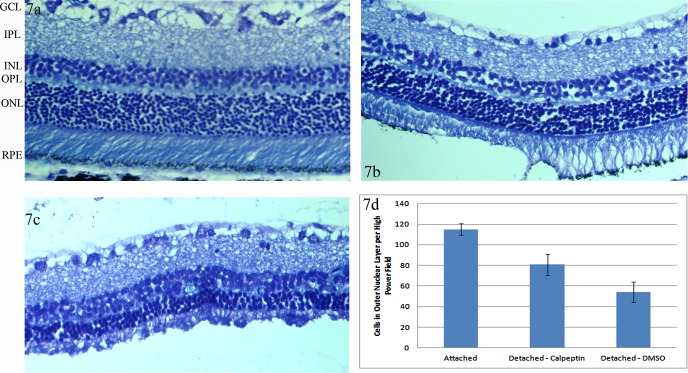
We then wanted to test if decreased apoptosis in vitro correlated with decreased apoptosis and increased photoreceptor cell survival in vivo. We first looked at TUNEL staining. It is known from prior reports that the TUNEL marker peaks at 3 days following a detachment,7 as was true here (Fig. 6). However, in the presence of calpeptin, there was a marked reduction in TUNEL-positive photoreceptors. The persistently reduced level of TUNEL-positive cells at 7-days postdetachment suggest that calpain inhibition is reducing the number of cells entering the apoptotic cascade, not just delaying the entry of cells into apoptosis. This reduced number of apoptotic cells resulted in 49% more photoreceptors in the calpeptin-treated eyes as compared with detachments treated with DMSO following prolonged (2 month) detachments (Fig. 7).
Figure 6.
Photoreceptor apoptosis following retinal detachment with calpain inhibition. (a) Whole retinas were detached for 1 to 7 days. Calpeptin or DMSO alone was injected into the subretinal space at the time of detachment. Slides were analyzed via TUNEL staining at 1, 3, and 7 days following detachment. Propidium iodine (PI) was used to stain the nuclei of retinal cells. Attached retinas served as controls. (b) Three representative high power fields of three separate eyes were counted for number of TUNEL-positive cells in the ONL. The average of each eye was calculated and then the average of the three eyes for each condition was reported. There were significantly fewer TUNEL-positive cells at 3 and 7 days in retinas treated with calpeptin compared to vehicle alone (#, P < 0.05). Error bars indicate SD. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Figure 7.
Cell survival with calpain inhibition. Whole-rat retinas were harvested 2 months following detachments. (a) Attached retinas were compared with retinas detached in the presence of (b) 0.2 μg calpeptin and (c) DMSO alone. (d) Three representative high power fields of three separate eyes were counted for number of cells in the ONL. The average of each eye was calculated and then the average of the three eyes for each condition was reported. The number of cells in the ONL was significantly greater in retinas detached in the presence of calpeptin compared with DMSO. Both detachment conditions had significantly fewer cells in the ONL compared with attached retinas. Error bars represent SD. All three conditions were significantly different with P < 0.05.
Discussion
Photoreceptor–RPE separation is a common feature of many retinal diseases, and the resultant photoreceptor cell death contributes greatly to the visual morbidity in these patients. Previously, we have shown how both cell survival and cell death pathways are activated soon after photoreceptor–RPE separation, leading to a molecular “tug-of-war” pulling the cell toward survival or death, respectively.10,11 In other words, the photoreceptors are triggered to undergo apoptosis as their supply of nutrition is taken away. However, the cells evolved ways of stopping that terminal program for at least a short period of time, in order to recover if the stress is only temporary. Our data show that inhibition of calpain activation can shift the molecular cascades toward the prosurvival autophagy pathways, with fewer photoreceptors entering the apoptotic cascade.
Strategies being investigated to prolong survival pathways have enormous clinical implications. We know that after 7 days of detachment, visual acuity outcomes decrease as compared with patients where detachments are repaired before 7 days.8–9 When patients present early with rhegmatogenous retinal detachments, they can be fixed surgically with good visual outcomes. However, in diseases such as AMD and exudative detachments, where the period of retina–RPE separation is chronic and rapid reattachment is not easily achieved, prolonging photoreceptor survival allows hope of improving visual outcomes.
In this study, we looked at calpain inhibition as a way of prolonging autophagy, a known cell survival pathway upregulated in many cell types following stress. We did this by following the activity of Atg5, a protein critical to autophagy activation and whose cleavage by calpain 1 resulted in cells aborting autophagy and entering apoptosis in other cell lines.12,13 We first showed that Atg5 does rise early after detachment, similar to other markers of autophagy. We also showed that its levels fall considerably at 7 days, which was confirmed by inhibiting autophagosome turnover with Baf-A. In addition, we showed that calpain 1 activity peaks at 7 days, which causes the cleavage and inactivation of Atg5.12,13
This 7-day time period of decreased Atg5 correlates well with the clinical knowledge that retinal detachments affecting the central macula need to be fixed by 7 days to preserve quality central vision. Prior to this study, there has been no explanation as to why vision starts decreasing at this point. This study suggests a possible explanation as to why photoreceptors start decreasing at 1 week.
We have also shown that the inhibition of the calpain family, including calpain 1, prolongs autophagy. Both in our in vitro and in vivo system we saw extended presence of Atg5 with calpain inhibitors. This extension of autophagy correlated with reduction of apoptosis. The number of cells surviving out to 2 months was significantly increased in retinas treated with the calpain inhibitor, but perhaps not as much as would be expected by the more dramatic reduction in TUNEL staining seen at the 3-day time point. This might suggest that alternative prodeath pathways become activated at intermediate time points. One such pathway is necroptosis, which has been shown to be activated as a shunt pathway leading to cell death during retinal detachment.22
This data correlates well with what is already known about calpain inhibition. Nakajima et al.14 showed calpain inhibition led to significant reduction of apoptosis in a retinal hypoxia model using an orally available calpain inhibitor. Koumuro et al.23 showed similar results in acute cerebral ischemia. Russo et al.24 demonstrated that with an intravitreal injection of various calpain inhibitors following transient ocular ischemia, there was upregulation of autophagy and decreased apoptosis of retinal cells. Smith et al.16 demonstrated that intraperitoneal injection of calpeptin, the calpain inhibitor used in our study, significantly reduced apoptosis of retinal ganglion cells in a model of optic neuritis. Finally, Kuro et al.25 demonstrated that an intraperitoneal injection of SNJ-1945 in a retinitis pigmentosa model restored retinal cellular autophagy and reduced photoreceptor cell death. The importance of our study is that it expands our knowledge about calpain inhibition and retinal survival to retina–RPE separation, a very common pathology found in a number of diseases.
However, there are several limitations of our study. The main limitation of this study is that we are assuming that it is the prevention of Atg5 cleavage by calpain inhibition that is responsible for reduced apoptosis. We do know that knocking out Atg5, and thus autophagy, causes the cells to immediately enter apoptosis.11 We also know that autophagy is upregulated and apoptosis is decreased with calpain inhibition. However, the calpain inhibitors are not specific. It may be that calpain cleavage of another target, and not Atg5, is the true reason the cells enter apoptosis and that Atg5 activity simply correlates with the real factor. This will remain unknown until Atg5 cleavage can be specifically targeted.
Another major limitation is the lack of a good antibody to detect the cleaved form of Atg5 and cleaved α-spectrin. It has been shown that cleaved Atg5 correlates well with decreased Atg5 and Atg5-12. In fact, most authors choose to follow the full-length form due to the difficulty in detecting cleaved Atg5 in different models.12,13 However, it would still be beneficial to prove that decreased Atg5 is associated with an increase in the cleaved form in our system. With respect to α-spectrin levels, the particular antibody we used does not appear to be able to differentiate calpain versus caspase-mediated cleavage, and there may be some contribution of both. However, in Figures 3 and 4 we used calpain specific inhibitors, which clearly reduced the level of α-spectrin, supporting the conclusion that the changes shown in Figure 2 are largely calpain driven.
An additional limitation of the study is the possibility of an off-target effect of the calpain inhibitors. As seen in Figure 3a, there did not appear to be a significant difference in α-spectrin levels at 1 and 10 μM. However, when looking at Figure 3b, there appeared to be a significant rise in LC3 at the higher concentrations. This could be interpreted as an off-target effect of the higher concentration of the drug. However, we do not expect a 1:1 concordance in the dose-response curves because we are measuring two different outcomes, α-spectrin cleavage and LC3 lipidation. Alpha-spectrin cleavage is a direct effect of activated calpains, and thus very sensitive to calpain inhibition. LC3 lipidation, on the other hand, is the result of a complex set of transduction events, and thus may require a higher level of calpain inhibition in order to achieve the downstream effect. Therefore, the difference may not be an off-target effect, but rather just demonstrating a difference in sensitivities of the assays.
At this time, there are no clinical trials looking at calpain inhibition on retinal or other systemic diseases. However, in all the data to date, it appears as if calpain inhibitors increase cell survival following stress, are well tolerated by animals and can be delivered in multiple ways, either systemically or, potentially, intravitreally. Based on our data, it would be feasible to use calpain inhibitors in a number of clinical settings. For example, it could be an adjunct to retinal detachment surgery and anti-VEGF therapy for AMD, with the goal of increasing final visual outcomes in these debilitating diseases.
Acknowledgments
Supported by a grants from the National Eye Institute (R01-EY020823 [DNZ]), the Foundation Fighting Blindness (DNZ), the Research to Prevent Blindness, Inc. Sybil B. Harrington Special Scholar Award for Macular Degeneration (DNZ), and the Core Center for Vision Research funded by EY007003 from the National Eye Institute.
Disclosure: N.D. Chinskey, P; Q.-D. Zheng, None; D.N. Zacks, ONL Therapeutics, LLC (I), P
References
- 1. Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139: 87–99 [DOI] [PubMed] [Google Scholar]
- 2. Burton TC. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982; 80: 475–497 [PMC free article] [PubMed] [Google Scholar]
- 3. Zacks DN, Hänninen V, Pantcheva M, Ezra E, Grosskreutz C, Miller JW. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2003; 44: 1262–1267 [DOI] [PubMed] [Google Scholar]
- 4. Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2004; 45: 4563–4569 [DOI] [PubMed] [Google Scholar]
- 5. Zacks DN, Boehlke C, Richards AL, Zheng QD. Role of the Fas signaling pathway in photoreceptor neuroprotection. Arch Ophthalmol. 2007; 125: 1389–1395 [DOI] [PubMed] [Google Scholar]
- 6. Dunaief JL, Dentchev T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002; 120: 1435–1442 [DOI] [PubMed] [Google Scholar]
- 7. Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Inhibition of retinal detachment-induced apoptosis in photoreceptors by a small peptide inhibitor of the Fas receptor. Invest Ophthalmol Vis Sci. 2010; 51: 2177–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ross WH, Stockl FA. Visual recovery after retinal detachment. Curr Opin Ophthalmol. 2000; 11: 191–194 [DOI] [PubMed] [Google Scholar]
- 9. Ross WH. Visual recovery after macula-off retinal detachment. Eye. 2002; 16: 440–446 [DOI] [PubMed] [Google Scholar]
- 10. Zacks DN, Han Y, Zeng Y, Swaroop A. Activation of signaling pathways and stress-response gene in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2006; 47: 1691–1695 [DOI] [PubMed] [Google Scholar]
- 11. Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Autophagy activation in the injured photoreceptor inhibits Fas-mediated apoptosis. Invest Ophthalmol Vis Sci. 2011; 52: 4193–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature Cell Bio. 2006; 8: 1124–1132 [DOI] [PubMed] [Google Scholar]
- 13. Xia H, Zhang L, Chen G, et al. Control of basal autophagy by calpain1 mediated cleavage of Atg5. Autophagy. 2010; 6: 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakajima E, Hammond KB, Rosales JL, Shearer TR, Azuma M. Calpain, not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells. Invest Ophthalmol Vis Sci. 2011; 52: 7059–7067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma AK, Rohrer B. Sustained elevation of intracellular cGMP causes oxidative stress triggering calpain-mediated apoptosis in photoreceptor degeneration. Curr Eye Res. 2007; 32: 259–269 [DOI] [PubMed] [Google Scholar]
- 16. Smith AW, Das A, Guyton MK, Ray SK, Rohrer B, Banik NL. Calpain inhibition attenuates apoptosis of retinal ganglion cells in acute optic neuritis. Invest Ophthalmol Vis Sci. 2011; 52: 4935–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Das A, Garner DP, Del Re AM, et al. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res. 2006; 1084: 146–157 [DOI] [PubMed] [Google Scholar]
- 18. McKernan DP, Guerin MB, O'Brien CJ, Cotter TG. A key role for calpains in retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2007; 48: 5420–5430 [DOI] [PubMed] [Google Scholar]
- 19. Tamada Y, Fukiage C, Mizutani K, et al. Calpain inhibitor, SJA6017, reduces the rate of formation of selenite cataract in rats. Curr Eye Res. 2001; 22: 280–285 [DOI] [PubMed] [Google Scholar]
- 20. Kupina NC, Nath R, Bernath EE, et al. The novel calpain inhibitor SJA6017 improves functional outcome after delayed administration in a mouse model of diffuse brain injury. J Neurotrauma. 2001; 18: 1229–1240 [DOI] [PubMed] [Google Scholar]
- 21. Das A, Sribnick EA, Wingrave JM, et al. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motor neurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005; 81: 551–562 [DOI] [PubMed] [Google Scholar]
- 22. Murakami Y, Notomi S, Hisatomi T, et al. Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retina Eye Res. 2013; 37: 114–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koumuro A, Nonaka Y, Hyakkoku K, et al. A novel calpain inhibitor, SNJ-1945, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience. 2008; 157: 309–318 [DOI] [PubMed] [Google Scholar]
- 24. Russo R, Berliocchi L, Adornetto A, et al. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011; 2: e144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuro M, Katsuhiko Y, Norihisa U, et al. Calpain inhibition restores basal autophagy and suppresses MNU-induced photoreceptor cell death in mice. In Vivo. 2011; 25: 617–624 [PubMed] [Google Scholar]