Abstract
Purpose.
Targeted inhibition of Müller cell (MC)–produced VEGF or broad inhibition of VEGF with an intravitreal anti-VEGF antibody reduces intravitreal neovascularization in a rat model of retinopathy of prematurity (ROP). In this study, we compared the effects of these two approaches on retinal vascular development and capillary density in the inner and deep plexi in the rat ROP model.
Methods.
In the rat model of ROP, pups received 1 μL of (1) subretinal lentivector-driven short hairpin RNA (shRNA) to knockdown MC-VEGFA (VEGFA.shRNA) or control luciferase shRNA, or (2) intravitreal anti-VEGF antibody (anti-VEGF) or control isotype goat immunoglobulin G (IgG). Analyses of lectin-stained flat mounts at postnatal day 18 (p18) included: vascular/total retinal areas (retinal vascular coverage) and pixels of fluorescence/total retinal area (capillary density) of the inner and deep plexi determined with the Syncroscan microscope, and angles between cleavage planes of mitotic vascular figures labeled with anti-phosphohistone H3 and vessel length.
Results.
Retinal vascular coverage and density increased in both plexi between p8 and p18 in room air (RA) pups. Compared with RA, p18 ROP pups had reduced vascular coverage and density of both plexi. Compared with respective controls, VEGFA.shRNA treatment significantly increased vascular density in the deep plexus, whereas anti-VEGF reduced vascular density in the inner and deep plexi. Vascular endothelial growth factor-A.shRNA caused more cleavage angles predicting vessel elongation and fewer mitotic figures, whereas anti-VEGF treatment led to patterns of pathologic angiogenesis.
Conclusions.
Targeted treatment with lentivector-driven VEGFA.shRNA permitted physiologic vascularization of the vascular plexi and restored normal orientation of dividing vascular cells, suggesting that regulation of VEGF signaling by targeted treatment may be beneficial.
Keywords: VEGF, vascular extent, vascular density, intrvitreal neovscularization, rat model of retinopathy of prematurity
Targeted inhibition of Müller cell (MC)-produced VEGF or broad inhibition of VEGF with anti-VEGF antibody reduces intravitreal neovascularization (IVNV) in the rat retinopathy of prematurity (ROP) model. We compared the effects of these two approaches on retinal vascular area and capillary density of the inner and deep plexi.
Introduction
Lack of retinal capillary support is a pathologic consequence that precedes intravitreal neovascularization (IVNV) in a number of diseases, including retinopathy of prematurity (ROP) and diabetic retinopathy.1,2 Loss of vascular support to the retina causes hypoxia3,4 and triggers a cascade of events5–7 that enables transcription of angiogenic factors, including vascular endothelial growth factor (VEGF),5 erythropoietin,8 angiopoietin,9 and others.10–12 The goals of previous clinical treatments were to destroy the avascular retina, which was believed responsible for stimulating angiogenic factor production and to reduce oxygen debt created by the avascular retina. Later approaches interfered with angiogenic growth factor-receptor binding and activation (e.g., antibodies to ligands or receptors for VEGF, platelet-derived growth factor,13,14 or tyrosine kinase inhibitors to inhibit signaling pathways) or inhibited inflammatory pathways that also increase angiogenesis (e.g., steroids and angiotensin II type I receptor).15,16 Currently, efforts seek to promote physiologic retinal vascularization through the use of growth factors17 or omega-3 fatty acids in growth-restricted preterm infants for ROP,18 to prevent metabolic damage to physiologic vascularization of the retina in diabetic retinopathy,19,20 or to regulate aberrant angiogenic signaling in endothelial cells to minimize disoriented retinal vascular growth21 and intravitreal angiogenesis in ROP.22
Recent clinical studies reported a persistent avascular retina and recurrent IVNV following treatment with nonspecific intravitreal anti-VEGF agents for severe ROP.23 Using the rat ROP model, we found that a neutralizing antibody to VEGF significantly inhibited IVNV but also reduced pup weight gain and serum VEGF.24 These studies suggest that targeted inhibition of overproduced VEGF may be a safer strategy to treat ROP. We located the mRNA signal of VEGFA-splice variants in the regions corresponding to cellular retinaldehyde-binding protein (CRALBP)-labeled Müller cells, and therefore developed lentivector-driven VEGFA short hairpin RNA (shRNA) to target Müller cell VEGFA using a CD44 promoter.21 Using the rat ROP model, we found that the lentivector targeted knockdown of Müller cell VEGFA significantly inhibited IVNV without affecting pup weight or serum VEGF. In this study, we evaluated the safety effects on the retinal vasculature and capillary density from the two methods of anti-VEGF treatment. We developed the hypothesis that targeted knockdown of VEGF in Müller cells that overproduced VEGF21 would preserve normal-ordered developing intraretinal vascularization and capillary density, whereas broad anti-VEGF treatment would interfere with endothelial cell receptor activation necessary for physiologic vascularization and cause a persistent avascular retina with reduced capillary density. We used the rat model of ROP25 to recreate pathologic features of severe ROP and measured capillary support in the inner and deep vascular plexi of the retina by two different methods. We determined the vascular coverage as areas of vascularized retinal area and capillary density as pixels fluorescence from lectin-stained flat mounts showing a vascularized retina in both inner and deep plexi. In both measurements, outcomes were normalized to total retinal area. We also determined the number and orientation of dividing vascular cells within the retina at the junction of vascularized and avascular retinas. We compared targeted VEGFA knockdown of Müller cell–VEGFA to neutralizing VEGF with an antibody to rat VEGF. Both treatments were delivered at doses and time points that significantly inhibit IVNV.21,24,26 Here, we report that targeted VEGFA knockdown permitted physiologic vascularization of the retinal vascular plexi and restored normal orientation of dividing vascular cells at the junction of vascular and avascular retinas. These data support additional studies to develop methods to target overproduced VEGF in order to treat IVNV.
Methods
Rat Model of Oxygen-Induced Retinopathy (Rat ROP Model)
All animal studies were performed in compliance with the University of Utah (Guide for the Care and Use of Laboratory Animals) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The rat ROP model has been previously described.25 Entire litters of newborn Sprague-Dawley rat pups (Charles River, Wilmington, MA) and dams were placed into an oxygen environment that cycled oxygen concentration between 50% and 10% every 24 hours for 14 days, and then placed into room air (RA). Pup number was maintained at 12 to 14 pups/litter. At postnatal day 18 (p18), pups were euthanized by intraperitoneal injection (IP) of ketamine (60 mg/kg) and xylazine (18 mg/kg) followed by IP pentobarbital (80 mg/kg).
Ocular Injections
Intravitreal Injections of Neutralizing Antibody to VEGF.
As described previously,24 following anesthesia, 1 μL of 50 ng neutralizing antibody to rat VEGF164 (anti-VEGF; R&D Systems, Minneapolis, MN) or isotype goat immunoglobulin G (IgG; R&D Systems) was delivered into the vitreous with a 33-gauge needle attached to a Hamilton syringe (Hamilton, Reno, NV) at the beginning of the 50% oxygen cycle on p12 in order to inhibit retinal secreted VEGF at its highest concentration in the model at p1426–28 and subsequent IVNV. As shown in our previous study, 50 ng of neutralizing antibody to rat VEGF164 significantly reduced IVNV by 3.5-fold over IgG control.24
Subretinal Injections of Lentivector-Driven VEGFA.shRNA.
Lentivector-driven VEGFA shRNA was constructed and tested as previously described.21 Briefly, shRNAs were designed as microRNAs against rat VEGFA (VEGFA.shRNA) or luciferase (luc.sRNA) and cloned into the lentiviral transfer vector (pFmCD44.1GW) with the CD44 promoter, which targets Müller cells and not astrocytes,21,29 and a green fluorescence protein (GFP) reporter gene. Micron III (Phoenix Research Laboratories, Inc., Pleasanton, CA) live imaging showed that 30% of retina was transduced by subretinal injection of lentivector and achieved 80% knockdown of retinal VEGFA by VEGFA.shRNA compared with luc.shRNA determined by ELISA in retinal lysates from the rat model of ROP. However, an intravitreal injection of lentivirus yielded a poor retinal virus transduction, which was consistent with the report from Greenberg et al.29 Vascular endothelial growth factor A.shRNA effectively reduced IVNV by 4-fold over luc.shRNA at p18 in the rat model of ROP.21
In this study, at the beginning of the 50% oxygen cycle of the 50/10 ROP model on p8,21 pups received 1 μL (1 × 109 viral particles/mL) of lentivectors containing VEGFA.shRNA or luc.shRNA as subretinal injections that created a transient retinal detachment, which resolved within 24 hours. Both eyes of each pup were injected with the same lentivector preparation.
Each litter typically had an equal distribution of either lentivector preparation. After the injection, topical antibiotic (0.5% erythromycin) was applied to each eye, and pups were allowed to recover on a warming pad before being returned to the Oxycycler.
For both intravitreal and subretinal injections, litters were typically out of the oxygen cycler for 3 hours. At p18, the time point of maximum IVNV in this model,26 pups were euthanized for analysis.
Retinal Flat-Mount Preparation, Imaging, and Analysis
Lectin-stained retinal flat mounts were prepared using Alexa Fluor 568–conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (5 μg/mL; Invitrogen, Molecular Probes, Eugene, OR), as previously described, and imaged30 using an inverted fluorescence microscope (Olympus, Tokyo, Japan). Flat mounts were created using the scan-slide stitching function of Metamorph imaging software (Molecular Devices, Inc., Sunnyvale, CA). Measurements were made by two masked reviewers using ImageJ (National Institutes of Health, Bethesda, MD). High resolution multi-Z plane images of retinal flat mounts were created by autostitching individual 20× fluorescence images of lectin-stained vasculature using the Syncroscan fluorescence microscope (Olympus). Fluorescence was converted to grayscale prior to stitching of each Z plane. The number of Z-planes needed to capture both primary and tertiary plexi was determined during imaging. The inner (primary plexus) and deep (tertiary plexus) layers were separated using filters in Adobe Photoshop CS5 extended (Version 12.1; Adobe Systems, Inc., San Jose, CA). In this study, only data from the inner and deep capillary layers were analyzed. Images corresponding to inner and deep layers had different color channels in Photoshop (Adobe Systems, Inc.) to differentiate the inner and deep layers. Total pixels covered by inner and deep layers were measured using histograms in Photoshop. The flat-mount vascular and avascular areas were measured by ImageJ 1.45S (National Institutes of Health). Retinal vascular coverage was defined as area of vascular extent to total retina area. Retinal vascular density was the pixels of lectin fluorescence to total retinal area.
Phosphohistone Labeling and Measurement of Mitoses and Cleavage Angles
Quantitative image analysis was performed using the freeware ImageTool, version 3 (University of Texas, Austin, TX). As described previously,31 cell division cleavage planes were identified in Alexa Fluor 568–conjugated isolectin-stained vessels by bisection of the separating chromosomes labeled with Alexa Fluor 488–conjugated anti-phosphohistone H3 (10 μg/mL; EMD Millipore, Billerica, MA) during late metaphase to anaphase. Mitotic figures were identified as cells labeled with isolectin and phosphohistone H3. Lines were drawn using image management software (Photoshop 7.0; Adobe Systems, Inc.) along the cleavage plane and along the long axis of the blood vessel for each mitotic division. The angle between these two lines was calculated. Angles of 0° predicted widening and 90° elongation of vessels.31 Angles between these values predict disordered divisions.
Statistical Analysis
Significant differences between treatment groups were determined by ANOVA. A minimum value of P less than 0.05 was considered statistically significant. At least two different litters were used for each experiment to account for potential effects within individual litters. For each condition, three to five flat mounts were analyzed. For each analysis, one data point was one eye from an individual pup.
Results
Retinal Vascular Coverage and Density in the Inner and Deep Plexi at Different Time Points During Development
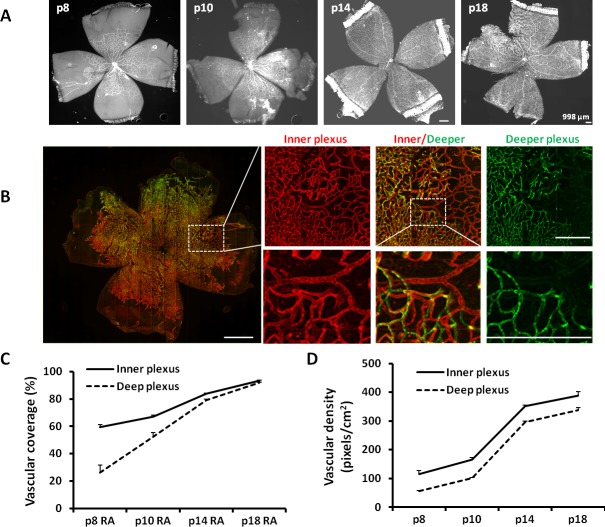
To analyze retinal vascular development in inner and deep plexi at various time points in development, we used two different approaches. In the first, we measured the extent of the retina covered by blood vessels as vascular/total retinal area for each plexus (termed vascular coverage) in retinal flat mounts (Fig. 1A). In the second, we measured the number of pixels of lectin-stained fluorescence/total retinal area in the inner and deep vascular plexi (termed vascular density), separated by assigning different colors to the plexi (Fig. 1B). The second approach measures not only retinal vascular/total retinal area, but incorporates capillary density/total retinal area. At time points from p8 to p18 in RA-raised rats, we found vascular coverage increased on average 1.5-fold in the inner plexus and 3.5-fold in the deep plexus (Fig. 1C). Vascular density increased 3.5-fold in the inner plexus and 6-fold in the deep plexus from p8 to p18 (Fig. 1D). Even though the slope of vascular coverage of the deep plexus was steeper than that of the inner plexus at the time points measured, the vascular density of the deep plexus increased with a similar slope as the inner plexus, suggesting that maintaining capillary density may be important to overall retinal development.
Figure 1.
Retinal vascular coverage and density in the inner and deeper plexi are increased during development at p8, p10, p14, and p18 in RA. (A) Syncroscan images of lectin-stained retinal flat mounts. (B) A portion of p18 VEGFA.shRNA-treated flat mount in the ROP model showing inner plexus, deep plexus, and combined image of both plexi assigned different colors (inner-red, deep-green; inner and deep plexi offset to permit visualization; lines indicate magnification size at the same unit). (C) Vascular coverage determined by vascular area normalized to total retina area. (D) Vascular density determined by the number of pixels of lectin fluorescence normalized to total retinal area (overall one-way ANOVA ***P < 0.001; results are means ± SD).
Retinal Vascular Coverage and Density in the Inner and Deep Plexi is Reduced in the Rat ROP Model
Delayed physiological retinal vascular development accounts for much of the avascular retina in phase I ROP in places in which oxygen is regulated.32 When rat pups are exposed to the ROP model, retinal vascular coverage in the inner and deep plexi was decreased compared with RA-raised pups at p18 (Figs. 2A, 2B).33 At p18, RA vascular coverage in both inner and deep plexi was 96% on average; however, for pups exposed to the ROP model, both vascular coverage (Fig. 2B) and vascular density (Fig. 2C) were significantly reduced in both inner and deep plexi compared with RA-raised pups.
Figure 2.
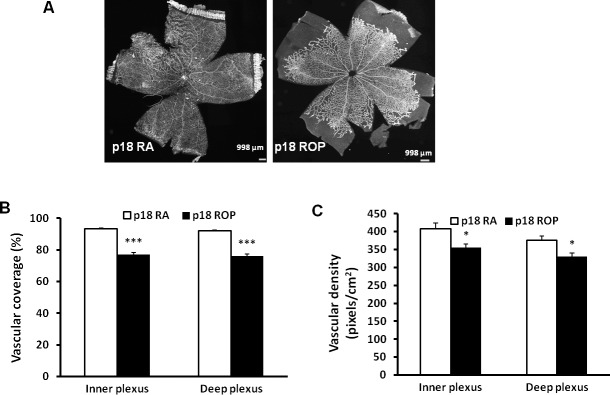
Retinal vascular coverage and density are reduced in both inner and deep plexi of pups raised in the ROP model at p18. (A) Images of retinal flat mounts of pups raised in RA or exposed to the ROP model. (B) Retinal vascular coverage and (C) retinal vascular density in the inner and deep plexi (*P < 0.05, ***P < 0.001 versus RA, two-way ANOVA; results are means ± SD).
Effects of Methods to Inhibit VEGF on Vascular Density and the Extent of Vascular Coverage in Inner and Deep Plexi
We tested two different methods to reduce VEGF at doses previously found to successfully inhibit VEGF-induced IVNV.21,24,26 In one, intravitreal anti-VEGF antibody (anti-VEGF) was compared with its control nonimmune IgG and in the other a subretinal injection of lentivectors carrying VEGFA shRNA (VEGFA.shRNA) that knocked down Müller cell VEGF was compared with control shRNA to the nonmammalian gene, luciferase (luc.shRNA). We measured vascular coverage and vascular density of the inner and deep plexi in lectin stained flat mounts at p18 ROP (Fig. 3). Compared with respective controls, luc.shRNA or IgG-treated ROP pups, VEGFA.shRNA or anti-VEGF did not significantly affect vascular coverage in the inner and deep plexi (Fig. 4A). However, compared with respective controls, anti-VEGF significantly reduced vascular density of the inner and deep plexus, and VEGFA.shRNA treatment significantly increased vascular density of the deep plexus (Fig. 4B). Therefore, compared with respective controls, anti-VEGF treatment reduced capillary density in the inner and deep plexi of the retina in the ROP model, and targeted knockdown of VEGFA increased vascular density in the deep plexus, even though both treatments had no effect on the vascular coverage.
Figure 3.
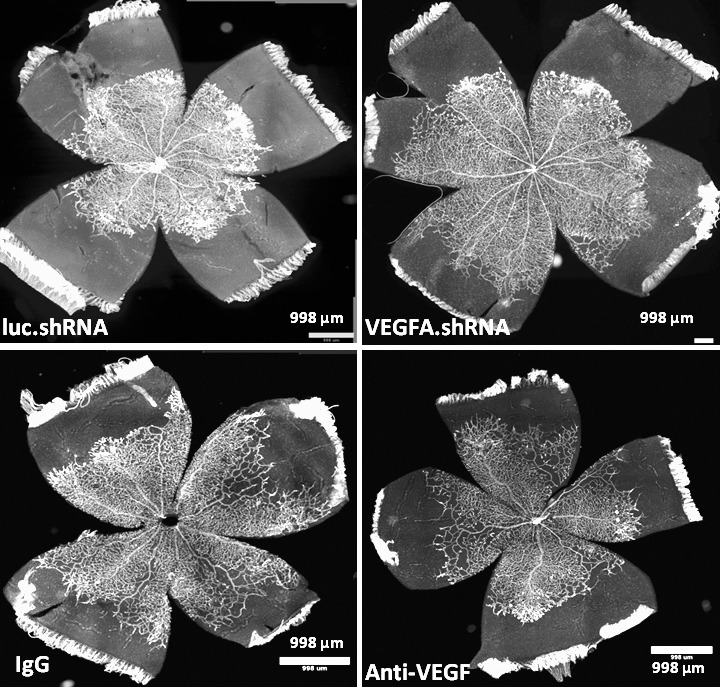
Vascular endothelial growth factor-A.shRNA versus anti-VEGF treatments on retinal vascular morphology. Images of lectin-stained retinal flat mounts from pups treated with luc.shRNA, VEGFA.shRNA, IgG, and anti-VEGF at p18 in the ROP model.
Figure 4.
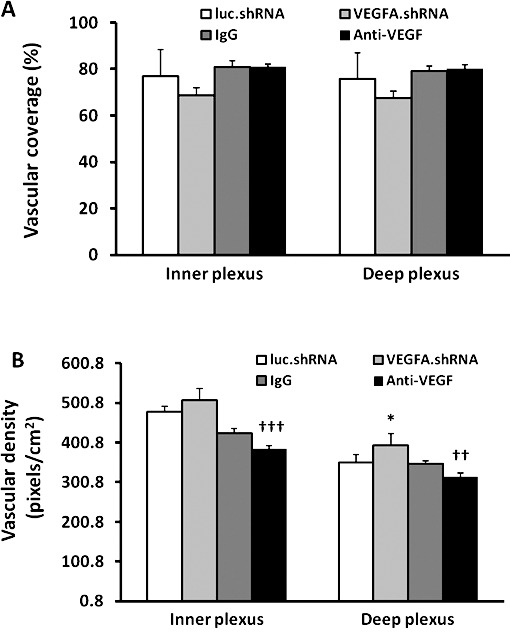
Retinal vascular coverage and density in the inner and deep plexi of pups treated with VEGFA.shRNA or anti-VEGF in the ROP model at p18 compared with respective controls. (A) Retinal vascular coverage and (B) vascular density (*P < 0.05 versus luc.shRNA; ††P < 0.01 and †††P < 0.001 versus IgG, two-way ANOVA; results are means ± SD).
Effects of Targeted VEGF Knockdown and Anti-VEGF on Number and Angle of Proliferating Vascular Endothelial Cells
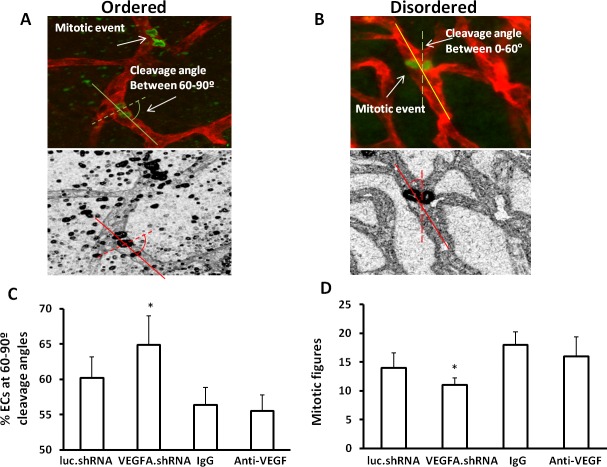
The orientation of dividing endothelial cells predicts whether the subsequent vessel is elongated, widened, or disordered.31 Previously, we found that excessive signaling through VEGFR2 disordered the orientation of dividing daughter endothelial cells in an embryonic stem cell model31 and vascular cells leading to increased vessel tortuosity in the ROP model.34 Knockdown of Müller cell VEGFA with shRNA appeared to reduce retinal vascular tortuosity (Fig. 3) that occurred in the ROP model compared with anti-VEGF antibody post treatment. We, therefore, postulated that targeted VEGFA knockdown in Müller cells would orient dividing endothelial cells into a more ordered physiologic pattern. To test this, we labeled retinal flat mounts from pups in the ROP model after treatment with VEGFA.shRNA, luc.shRNA, anti-VEGF, or IgG with lectin, and an antibody to anti-phosphohistone H3 that manifests mitotic figures in anaphase. We counted the number of mitotic figures and also determined the cleavage angles between the long axis of lectin-labeled vessels and the cleavage plane drawn between phosphohistone H3–labeled figures (Figs. 5A, 5B). We predicted that cleavage angles between the cleavage planes of dividing cells and respective long axes of the vessels would align closer to 90° (predicting elongation or physiologic angiogenesis31; Fig. 5A) with targeted VEGFA knockdown, whereas angles away from 90° (predictive of widening or disordered angiogenesis; Fig. 5B) would occur after nonspecific anti-VEGF treatment.
Figure 5.
Vascular endothelial growth factor-A.shRNA treatment reduces disordered angiogenesis in the ROP model. Diagram of cleavage angles produced from lectin-stained flat mounts colabeled for phosphohistone H3 showing cells in anaphase: (A) cleavage angles between 60° and 90° predict ordered angiogenesis; (B) cleavage angles between 0° and 60° predict widened or disordered angiogenesis; (C) percentage of vascular cells with cleavage angles between 60° and 90°; and (D) total number of mitotic figures determined as phosphohistone H3–labeled vascular cells in retinal flat mounts from pups treated with luc.shRNA, VEGFA.shRNA, IgG, or anti-VEGF (*P < 0.05 versus luc.shRNA, two-way ANOVA; results are means ± SD).
We clustered angles into groups every 15° between 0° and 90°, and determined the absolute number of antiphosphohistone H3–labeled cells at the plane of the inner and deep plexi counted throughout retinal flat mounts. Most mitotic figures were found at the junctions between vascularized and avascular retina where IVNV occurs. Compared with luc.shRNA, we found that VEGFA.shRNA treatment caused more mitotic figures clustered between 60° and 90°, predicting elongation and ordered angiogenesis (Fig. 5C). We also found that the absolute number of dividing vascular cells was decreased following VEGFA.shRNA treatment compared with luc.shRNA (Fig. 5D). There was no difference in the percent of mitotic figures clustered between 60° and 90° and total mitotic figures between anti-VEGF and IgG (Figs. 5C, 5D). In agreement with this, retinal flat mounts of inner plexi after each treatment and respective controls provided evidence that targeted overproduction of VEGF restored more normal retinal vascular morphology than did broad anti-VEGF inhibition (Fig. 3). Altogether, these data provide evidence that targeting overproduced VEGFA with the VEGFA.shRNA treatment restored ordered angiogenesis, whereas anti-VEGF treatment did not improve ordered angiogenesis.
Discussion
In countries lacking resources to treat severe ROP, the use of intravitreal anti-VEGF antibodies has increased. However, there have been some reports of persistent avascular retina, recurrent IVNV, retinal detachment,23,35,36 and reduction in serum VEGF leading to concern about potential effects of intravitreal anti-VEGF on growth and development of preterm infants. The current guidelines for ROP treatment recommend longer follow-up after anti-VEGF treatment than for standard of care laser and only use of anti-VEGF agents for zone I stage 3+ severe ROP until more information on proper dose and safety is available.37–39
Many studies report inner plexus coverage determined by area of vascular extent as an outcome, whereas fewer have measured capillary density or the vascular extent of both inner and deep plexi.40 In this study, we sought to determine the effects of different strategies to inhibit VEGF on physiologic retinal vascularization by measuring the intensity of pixel fluorescence of the inner and deep plexi in lectin-stained retinal flat mounts, which provided data on capillary density and extent of these plexi. Compared with RA-raised pups of the same developmental age, we found that the ROP model reduced vascular extent and density compared with normal development. This finding goes along with a previous study in which retinal hypoxia measured with Hypoxyprobe (HPI, Burlington, MA), which detects tissue where oxygen levels are approximately 1%, was increased in retinal flat mounts from p18 rat pups in the ROP model compared with RA-raised pups.41 Although vascular extent after treatment with either strategy to reduce VEGF was no different compared to untreated ROP, broad inhibition using an intravitreal antibody against rat VEGF at a dose shown to inhibit IVNV in the rat ROP model24,26 significantly reduced capillary density in both inner and deep retinal plexi compared with control IgG. In contrast, targeted VEGFA knockdown in Müller cells that overproduce VEGF increased capillary density in the deep plexus and did not inhibit capillary density in the inner plexus compared with luc.shRNA control. Vascular endothelial growth factor is a survival factor for endothelial cells and broad anti-VEGF needed to neutralize excess VEGF may adversely reduce VEGF essential to the survival of newly developed intraretinal capillaries.42 Vascular endothelial growth factor also has survival effects on retinal neurons and Müller glia,43 and it is increasingly clear that neurovascular interactions are important in retinovascular development and angiogenesis.40,44–46
Finally, we found that the number of dividing vascular cells was increased following treatment with anti-VEGF and the orientation of dividing cells predicted vessel widening or disordered angiogenesis instead of more physiologic elongation. If vascular cell divisions are disordered, they may divide outside the plane of the retina and into the vitreous as IVNV. Even though the change in area of IVNV was comparable after treatment with intravitreal anti-VEGF24 or subretinal VEGFA.shRNA21 compared with respective controls at p18 in the ROP model, it is possible that hypoxia from reduced capillary density was greater after anti-VEGF antibody and may have stimulated later recurrent intravitreal angiogenesis reported at p25.24 These findings may also lend insight into what occurs in some cases of human ROP when recurrent intravitreal angiogenesis occurs after treatment with intravitreal anti-VEGF antibody.
In summary, we show that broad inhibition of VEGF was associated with reduced capillary density and may have led to disordered angiogenesis from retina that had too little capillary support and oxygenation. The data from these studies support the need for both greater knowledge of the effect of dose on developing angiogenesis as it relates to ROP as well as to future studies into targeted treatment of disordered angiogenesis causing IVNV.
Acknowledgments
The authors thank Wes Stuker for assistance preparing experiments and Justin Miller for help cloning lentivector-shRNA.
Supported by National Institutes of Health (NIH)/National Eye Institute (NEI) Grants R01 R01EY015130 (MEH), R01EY017011 (MEH), and R01-DK058702-10 (TK); and in part by an unrestricted grant from Research to Prevent Blindness, Inc., to the Department of Ophthalmology and Visual Sciences, University of Utah.
Disclosure: H. Wang, None; Z. Yang, None; Y. Jiang, None; J. Flannery, None; S. Hammond, None; T. Kafri, None; S.K. Vemuri, None; B. Jones, None; M.E. Hartnett, None
References
- 1. Chan-Ling T, Gock B, Stone J. Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1995; 36: 1215–1230 [PubMed] [Google Scholar]
- 2. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487 [DOI] [PubMed] [Google Scholar]
- 3. Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-{alpha} improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol. 2005; 166: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007; 13: 840–853 [PMC free article] [PubMed] [Google Scholar]
- 5. Ema M, Taya S, Yokotani N, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia inducible factor 1 alpha regulates VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997; 94: 4273–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006; 83: 473–483 [DOI] [PubMed] [Google Scholar]
- 7. Ema M, Hirota K, Mimura J, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999; 18: 1905–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Progr Retin Eye Res. 2012; 31: 89–119 [DOI] [PubMed] [Google Scholar]
- 9. Xin X, Rodrigues M, Umapathi M, et al. Hypoxic retinal Müller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A. 2013; 110: E3425–E3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamakawa M, Liu LX, Date T, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003; 93: 664–673 [DOI] [PubMed] [Google Scholar]
- 11. Brafman A, Mett I, Shafir M, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004; 45: 3796–3805 [DOI] [PubMed] [Google Scholar]
- 12. Shoshani T, Faerman A, Mett I, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol. 2002; 22: 2283–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartmann JS, Thompson H, Wang H, et al. Expression of vascular endothelial growth factor and pigment epithelial-derived factor in a rat model of retinopathy of prematurity. Mol Vis. 2011; 17: 1577–1587 [PMC free article] [PubMed] [Google Scholar]
- 14. Mori K, Gehlbach P, Ando A, McVey D, Wei L, Campochiaro PA. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002; 43: 2428–2434 [PubMed] [Google Scholar]
- 15. Sobel DB, Philip AGS. Prolonged dexamethasone therapy reduces the incidence of cryotherapy for retinopathy of prematurity in infants of less than one kilogram birth weight with bronchopulmonary dysplasia. Pediatrics. 1992; 90: 529–533 [PubMed] [Google Scholar]
- 16. Nagai N, Noda K, Urano T, et al. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005; 46: 1078–1084 [DOI] [PubMed] [Google Scholar]
- 17. Engstrom E, Niklasson A, Albertsson-Wickland K, Ewald U, Hellstrom A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005; 57: 605–610 [DOI] [PubMed] [Google Scholar]
- 18. Hard AL, Smith LE, Hellstrom A. Nutrition, insulin-like growth factor-1 and retinopathy of prematurity [ published online ahead of print February 18, 2013]. Semin Fetal Neonatal Medicine. doi:10.1016/j.siny.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sapieha P, Chen J, Stahl A, et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr Diabetes. 2012; 2: e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mustad VA, Demichele S, Huang YS, et al. Differential effects of n-3 polyunsaturated fatty acids on metabolic control and vascular reactivity in the type 2 diabetic ob/ob mouse. Metabolism. 2006; 55: 1365–1374 [DOI] [PubMed] [Google Scholar]
- 21. Wang H, Smith GW, Yang Z, et al. Short hairpin RNA-mediated knockdown of VEGFA in Müller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity. Am J Pathol. 2013; 183: 964–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal low-dosage bevacizumab for retinopathy of prematurity [ published online ahead of print September 11, 2013]. Acta Ophthalmol. doi:10.1111/aos.12266 [DOI] [PubMed] [Google Scholar]
- 23. Hu J. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012; 130: 1000–1006 [DOI] [PubMed] [Google Scholar]
- 24. McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013; 54: 2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994; 36: 724–731 [DOI] [PubMed] [Google Scholar]
- 26. Geisen P, Peterson L, Martiniuk D, Uppal A, Saito Y, Hartnett M. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008; 14: 345–357 [PMC free article] [PubMed] [Google Scholar]
- 27. Budd SJ, Hartnett ME. Increased angiogenic factors associated with peripheral avascular retina and intravitreous neovascularization: a model of retinopathy of prematurity. Arch Ophthalmol. 2010; 128: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2010; 108: 96–119 [PMC free article] [PubMed] [Google Scholar]
- 29. Greenberg KP, Geller SF, Schaffer DV, Flannery JG. Targeted transgene expression in Müller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci. 2007; 48: 1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am J Pathol. 2012; 180: 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007; 109: 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012; 367: 2515–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004; 10: 512–520 [PMC free article] [PubMed] [Google Scholar]
- 34. Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008; 49: 3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006; 26: 699–700 [DOI] [PubMed] [Google Scholar]
- 36. Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004; 79: 623–630 [DOI] [PubMed] [Google Scholar]
- 37. Mintz-Hittner HA. Treatment of retinopathy of prematurity with vascular endothelial growth factor inhibitors. Early Hum Dev. 2012; 88: 937–941 [DOI] [PubMed] [Google Scholar]
- 38. Mititelu M, Chaudhary KM, Lieberman RM. An evidence-based meta-analysis of vascular endothelial growth factor inhibition in pediatric retinal diseases: part 1. Retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012; 49: 332–340 [DOI] [PubMed] [Google Scholar]
- 39. Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013; 131: 189–195 [DOI] [PubMed] [Google Scholar]
- 40. Weidemann A, Krohne TU, Aguilar E, et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010; 58: 1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008; 49: 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruberte J, Ayuso E, Navarro M, et al. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest. 2004; 113: 1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS One. 2008; 3: e3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dorrell MI, Aguilar E, Jacobson R, et al. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010; 58: 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004; 103: 4527–4535 [DOI] [PubMed] [Google Scholar]
- 46. Joyal JS, Sitaras N, Binet F, et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011; 117: 6024–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]