Abstract
Emerging findings suggest that brain-derived neurotrophic factor (BDNF) serves widespread roles in regulating energy homeostasis by controlling patterns of feeding and physical activity, and by modulating glucose metabolism in peripheral tissues. BDNF mediates beneficial effects of energetic challenges such as vigorous exercise and fasting on cognition, mood, cardiovascular function and peripheral metabolism. By stimulating glucose transport and mitochondrial biogenesis BDNF bolsters cellular bioenergetics and protects neurons against injury and disease. By acting in the brain and periphery, BDNF increases insulin sensitivity and parasympathetic tone. Genetic factors, a ‘couch potato’ lifestyle and chronic stress impair BDNF signaling, which may contribute to the pathogenesis of metabolic syndrome. Novel BDNF-focused interventions are being developed for obesity, diabetes and neurological disorders.
Keywords: Alzheimer’s disease, BDNF, diabetes, exercise, glucocorticoid, insulin resistance, learning and memory, obesity
BDNF is a central regulator of energy homeostasis
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of proteins, plays crucial roles in the development, maintenance and plasticity of the central and peripheral nervous systems [1]. BDNF promotes the differentiation of neurons from stem cells, enhances neurite outgrowth and synaptogenesis, and can prevent programmed cell death/apoptosis. BDNF is expressed in neurons throughout the developing and adult mammalian nervous system, wherein it is produced in relatively low amounts but is highly potent, eliciting biological responses at picomolar concentrations. Neurons in energy homeostasis centers within the hypothalamus also produce BDNF, as do neurons in other appetite-regulating areas including the dorsal vagal complex, hindbrain and ventral tegmental area of the midbrain [2]. But the expression of BDNF and its high affinity receptor TrkB are now known to be much broader, being present in neurons throughout the CNS and periphery, as well as in skeletal muscle, cardiac, liver and adipose cells. Thus, the anorexogenic action of BDNF in hypothalamic regulatory centers provided the first clue to what has recently been found to be a widespread role for BDNF as a mediator of adaptive responses of the brain and body to fluctuations in energy intake and expenditure. Here we highlight recent evidence suggesting key roles for BDNF in the effects of exercise and food intake on neuroplasticity, and the vulnerability of the brain and peripheral organs to metabolic morbidity and obesity. Moreover, emerging findings suggest that BDNF is a master regulator of energy homeostasis with sites and mechanisms of action that extend well beyond those hormones such as insulin and leptin.
BDNF synthesis, processing and signaling
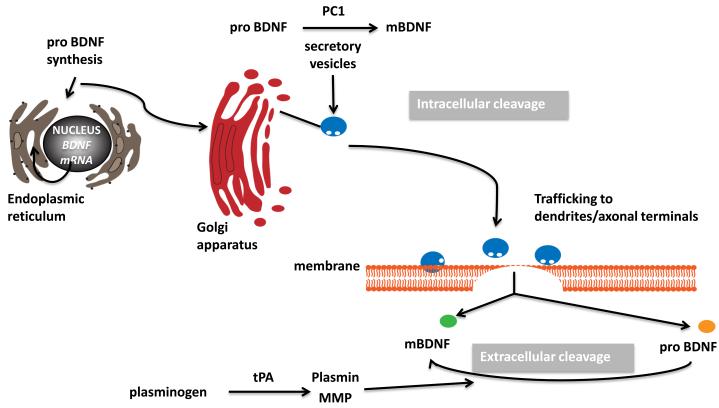
BDNF is synthesized as a pre-pro neurotrophin that is cleaved into pro-BDNFand further processed to mature mBDNF (Figure 1). Pro-BDNF is converted to biologically active mBDNF by furin and proconvertases within secretory vesicles before release [1]. Neurons also release pro-BDNF, which is converted by the tissue plasminogen activator tPA/plasmin system to mBDNF [1]. The expression and release of BDNF are stimulated by excitatory synaptic activity, and certain neuropeptides and hormones [75]. Glutamate released from excitatory synapses binds to receptors on the synaptic membrane resulting in the influx of Na+ and Ca2 via α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, N-methyl-D-aspartate (NMDA) receptors and voltage-dependent Ca2+ channels (Figure 2). Ca2+ activates Ca2+-calmodulin-dependent protein kinases (CaMK), protein kinase C (PKC) and mitogen-activated protein kinases (MAPKs) which, in turn, activate the transcription factors cyclic AMP response element-binding protein (CREB) and nuclear factor κB (NF-κB) to induce Bdnf gene transcription [76]. BDNF is concentrated in vesicles that are transported into axons/presynaptic terminals and dendrites from which it is released in response to glutamate receptor activation [77]. BDNF mRNA is also located in dendrites where protein translation can be stimulated by synaptic activity. Local BDNF production and release activates its high-affinity receptor tropomyosin-related kinase B (TrkB) or the low affinity p75 neurotrophin receptor, on synaptic partner neurons and other cells in the immediate vicinity. TrkB is a receptor tyrosine kinase that upon activation engages phospholipase C gamma (PLC-y), phosphatidylinositol-3 kinase (PI3-K) and MAPK intracellular signaling pathways leading to activation of transcription factors that regulate expression of proteins involved in neuronal survival, plasticity, cellular energy balance and mitochondrial biogenesis [1, 26]. BDNF can prevent neuronal apoptosis by inducing expression of anti-apoptotic Bcl-2 family members and caspase inhibitors, and by inhibiting pro-apoptotic proteins such as Bax and Bad. BDNF also up-regulates antioxidant enzymes and enhances repair of damaged DNA in neurons [1, 78]. BDNF stimulates neurite outgrowth and synaptogenesis in the brain and periphery by mechanisms involving activation of p21 ras, enhancement of cytoskeletal dynamics, modulation of cell adhesion, and stimulation of mitochondrial biogenesis. By promoting neuronal survival, neurite outgrowth and synaptogenesis, BDNF plays critical roles in the formation of neuronal circuits throughout the brain including those that regulate energy homeostasis [79] and is also involved in the control of multiple aspects of circadian patterns of behaviors and neuroendocrine processes related to energy homeostasis (Box 1).
Figure 1. Mechanisms for the production and release of BDNF.
BDNF mRNA is translated into proBDNF protein in the endoplasmic reticulum. ProBDNF is transported into the Golgi and processed to the mature form of BDNF (mBDNF) by extracellular protein convertase 1 (PC1) within the vesicles. The secretory granules are trafficked to the sites of release in the axonal or dendritic terminals. Neurons secrete both proBDNF and mBDNF in an activity-dependent manner. The tissue-type plasminogen activator (tPA) form mBDNF by activating a plasminogen, which then cleaves the precursor molecule. Alternatively, extracellular metalloproteinases process proBDNF to generate mBDNF.
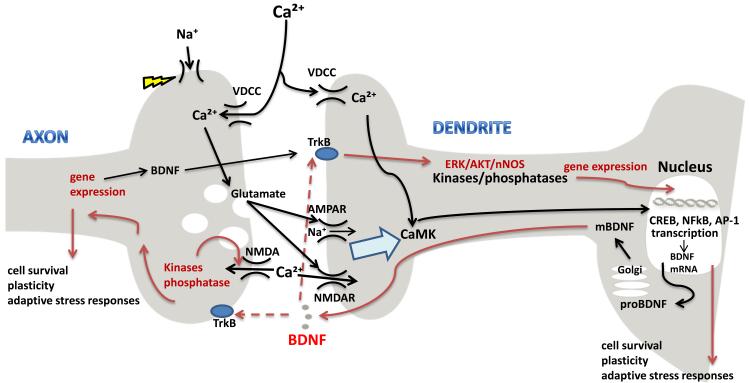
Figure 2. Biological actions of BDNF.
When an axon potential reaches the presynaptic terminal of an axon, Na+ influx depolarizes the plasma membrane, which triggers Ca2+ influx and release of the excitatory neurotransmitter glutamate into the synaptic cleft. Glutamate binds to AMPA and NMDA receptors at the postsynaptic membrane. Activation of the AMPA receptors results in membrane depolarization and Ca2+ influx via NMDA and VDCC. Ca2+ engages CaMKs that activates CREB and NF-κB which in turn induce the transcription of the Bdnf gene. mBDNF is released at synpases and activates TrkB receptors resulting in activation of downstream signaling cascades including PLCγ, PI3K and MAPKs and subsequent expression of genes critical for the survival and plasticity of neurons. BDNF signaling also elicits rapid effects on membrane excitability and synaptic transmission via altering the activation kinetics of NMDA receptors and increasing the number of docked synaptic vesicles in the presynaptic terminal.
Abbreviations: alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic 689 acid (AMPA); N-methyl-D-aspartate (NMDA) receptors; voltage dependent Ca2+ channels (VDCC); Ca2+/calmodulin-dependent kinases (CaMKs); phospholipase Cγ (PLCγ); phosphatidylinositol 3-kinase (PI3K); mitogen-activated protein kinases (MAPKs).
Box 1. BDNF and Circadian Rhythms.
Energy homeostasis is modulated in a circadian rhythm-dependent manner by neural circuits in the hypothalamus and higher brain centers. Disruptions of circadian control of energy metabolism are associated with the metabolic syndrome and obesity [80]. Emerging evidence suggests roles for BDNF in regulating circadian rhythms and implicates impaired BDNF signaling in disturbed circadian control of energy metabolism in metabolic disorders. BDNF expression oscillates in a circadian manner in rodents, with expression being greater during the dark phase in the hippocampus and cerebellum and greater during the light phase in the retina and visual cortex [81]. TrkB expression levels are also greater in hippocampal neurons during the dark phase in rodents, possibly as a response to increased physical activity [82].
BDNF signaling plays important roles in the regulation of circadian rhythms. Infusion of BDNF into the suprachiasmatic nucleus (SCN) of rats results in large phase advances when the rats are exposed to light during a period (subjective day) when the circadian 609 pacemaker is normally exposed to light during a period (subjective day) when the circadian pacemaker is normally insensitive to light; in contrast, BDNF+/− mice exhibit decreased amplitude of light-induced phase-shifts during subjective night [83]. Inhibition of TrkB signaling abolishes circadian changes in astrocyte interactions with dendrites of vasoactive intestinal polypeptide (VIP)-expressing neurons in the SCN, indicating that BDNF-mediated circadian changes of SCN cytoarchitecture are involved in the light synchronization process [84]. The involvement of BDNF in the control of multiple aspects of circadian patterns of behaviors and neuroendocrine processes related to energy homeostasis (e.g., feeding behavior and insulin sensitivity) suggest the possibility that perturbed circadian control of energy metabolism contributes to the obesity and diabetes that occurs when BDNF signaling is selectively impaired.
Linking Energy Availability and Physical Activity to Cognitive Function
BDNF signaling may have evolved to play a role in cognition-enhancing effects of running, and intermittent food deprivation [3]. Indeed, rodent and human studies show that cognitive function is enhanced by running and intermittent fasting, and BDNF may play a role in this [4]. Rats and mice will voluntarily run as much as 10-15 kilometers in a 24 hour period on a running wheel, and compared to more sedentary rodents, runners exhibit improved performance in tests of spatial learning and memory [5, 6]. BDNF signaling-mediated synaptogenesis and neurogenesis contribute to such cognition-enhancing effects of exercise [7-9]. Running and other types of aerobic exercise can also enhance cognitive performance [10] and increase serum BDNF levels [11] in human subjects. Running also lessens anxiety and depression in animal models by BDNF-mediated mechanisms, consistent with a role for BDNF in alleviating anxiety and depression in humans [12-14]. Dietary energy restriction can also exert anxiolytic effects, which may involve BDNF and ketone bodies [15-17].
The cellular and molecular mechanisms by which running and fasting up-regulate BDNF signaling, resulting in enhanced cognitive function, are emerging. Running induces BDNF expression in neurons by stimulating CREB via a Ca2+ influx- and CaMK-mediated mechanism [18]. In addition, an exercise-induced muscle protein called FNDC5 is induced by exercise in neurons where it mediates up-regulation of BDNF [19]. Interestingly, FNDC5 in muscle and liver cells is cleaved and a protein fragment called irisin is released into the blood and may enter the brain and induce BDNF expression in neurons [19]. Thus, the effects of exercise on neuroplasticity are mediated, in part, by BDNF induced by local changes in the brain and by signals from peripheral tissues including muscle and liver. Fasting/food deprivation can also induce BDNF expression in neuronal circuits involved in cognition by increasing their activity, and by shifting cellular energy substrate utilization from glucose to ketones. From an evolutionary standpoint, this activity-dependent production of BDNF likely contributed to optimization of brain function during fasting and running [3]. Activation of TrkB by BDNF enhances synaptic plasticity, and learning and memory, by multiple transcriptional and post-transcriptional mechanisms that involve the PI3-kinase – Akt pathway and extracellular signal regulate kinases (ERKs 1 and 2). Two weeks of running wheel exercise results in activation of the PI3-kinase and Akt in the hippocampus of rats [20]. Shorter bouts of exercise that enhance spatial learning and memory also increase hippocampal Akt and CREB activities [21]. Genes up-regulated by BDNF that likely contribute to the beneficial effects of running and fasting on neuronal plasticity and cognition include those encoding proteins involved in cytoskeletal and synaptic plasticity, cell survival, cellular energy metabolism and mitochondrial biogenesis [22]. BDNF signaling increases the levels of the NMDA glutamate receptor subunits NR1 by a CREB and early growth response factor 3 (Egr3)-mediated mechanism [23]. Enhanced NMDA receptor function would be expected to bolster cognition because Ca2+ influx through the NMDA receptor channel is fundamental to the long-lasting changes at synapses that are critical for learning and memory.
Cellular Bioenergetics and Neuroplasticity
The activation of synapses and ‘firing’ of action potentials to propagate signals along axons are fundamental mechanisms by which neurons perform their functions; the amount of energy required to support this ongoing activity of neurons is considerably greater than other cell types [24]. The major energy substrates utilized by neurons to generate the ATP and NAD+ required to support their diverse biochemical processes, are glucose and ketones. BDNF can enhance neuronal ATP production in several ways (Figure 3). BDNF increases 150 glucose transport by inducing the expression of GLUT3, and also increases Na+-dependent amino acid transport and protein synthesis [25]. BDNF also up-regulates the master regulator of mitochondrial biogenesis peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and the increased number of mitochondria sustains cellular energy substrates (ATP and NAD+) and the formation and maintenance of synapses [26]. BDNF enhances the respiratory coupling efficiency of synaptic mitochondria by a mechanism involving MEK and Bcl-2 [27]. BDNF signaling via the forkhead transcription factor FOXO3a can further enhance mitochondrial health by up-regulating the expression of manganese superoxide dismutase [28],and by enhancing utilization of ketones by neurons by up-regulating MCT2 [29]. Whether BDNF mediates the effects of exercise and fasting on bioenergetic pathways involving mammalian target of rapamycin (mTOR) and AMP kinase (AMPK) remains to be determined. However, in cultured neurons, hippocampal slices and isolated synaptosomes BDNF increases mTOR activity and dendritic protein synthesis [30, 31,90, 91]. In addition, the mTOR inhibitor rapamycin can prevent late-phase synaptic long-term potentiation (LTP) in the hippocampus and can block the ability of BDNF to enhance LTP [32]. On the other hand, while energy restriction can enhance neurogenesis, it typically reduces mTOR and suppresses overall cellular protein synthesis, while preserving synthesis of proteins critical for the ongoing function and survival of the cells. It will therefore be important to understand the molecular mechanisms by which BDNF regulates adaptive responses of neurons to activity-dependent changes in neuronal energy metabolism and protein synthesis.
Figure 3. Mechanisms by which BDNF regulates neuronal bioenergetics.
Binding of BDNF to trkB results in the activation of phosphatidylinositol-3 kinase (PI3K) and Akt kinase. Akt can activate the mammalian target of rapamycin (mTOR) to stimulate the translation of mRNAs encoding the neuronal glucose transporter GLUT3 and the monocarboxylic acid transporter 2 (MCT2) to enhance cellular uptake of the fuels glucose and lactate. BDNF can also induce Ca2+ influx through transient receptor potential C (TRPC) channels. Ca2+ then activates a Ca2+/calmodulin-dependent protein kinase (CaMK) resulting in the activation of the transcription factor cyclic AMP response element-binding protein (CREB) which, in turn, induces the expression of peroxisome proliferator receptor γ coactivator 1α (PGC-1α). PGC-1α is a master regulator of mitochondrial biogenesis that increases the number of mitochondria in neurons to provide more energy substrates (ATP and NAD+) to support the function and adaptive plasticity of the neurons. One energy status-sensitive enzyme that is supported by NAD+ is sirtuin-1 (Sirt1), a deacetylase that can activate the transcription factor FOXO3a resulting in the production of the mitochondrial antioxidant enzyme manganese superoxide dismutase (MnSOD). BDNF production is increased in response to bioenergetic challenges such as exercise, intermittent fasting (IF) and cognitive stimulation. By enhancing the uptake of energy substrates, mitochondrial biogenesis and protein synthesis capabilities of neurons, BDNF signaling plays pivotal roles in the adaptive changes in neuronal circuits including synapse formation/modification and neurogenesis (see text for further information).
The abilities of exercise and energy restriction to enhance synaptic plasticity and stimulate neurogenesis [4, 33] may depend, at least in part, on BDNF-mediated enhancement of neuronal bioenergetics (Figure 3). Selective knockdown of PGC-1α abolishes the ability of BDNF to enhance synaptogenesis in cultured hippocampal neurons, and reduces synapse numbers in dentate granule neurons in adult mice, suggesting that mitochondrial biogenesis is necessary for BDNF to stimulate the formation of new synapses and to maintain existing synapses [26]. BDNF also plays a key role in the process of hippocampal neurogenesis induced by intermittent fasting and exercise, by promoting the differentiation of neurons from stem cells, and the survival and synaptic integration of newly generated neurons [33]. Adult hippocampal neurogenesis is associated with some forms of hippocampal-dependent learning including spatial pattern separation [33]. BDNF induces robust mitochondrial biogenesis in newly generated neurons [26], and regulates the expression of the glutamate receptor GluR1 to enhance synaptic plasticity and memory by a transient receptor potential channel (TRPC)- Ca2+- and mTOR-mediated mechanism [34, 35].
BDNF Signaling Regulates Energy Metabolism
While mice lacking BDNF die shortly after birth with severe abnormalities in their nervous system, BDNF haploinsufficient mice are hyperphagic, obese and diabetic [36], and mice lacking BDNF in hippocampal and forebrain neurons develop obesity and an anxiety disorder [37]. Similarly, targeted deletion of BDNF in the dorso- and ventro-medial hypothalamus results in increased food intake and obesity, in mice [38]. On the other hand, fasting induces BDNF expression in the brain [39] and BDNF haploinsufficient mice maintained on an intermittent fasting diet exhibit improvement in their behavioral abnormalities, as well as reduced insulin resistance and obesity [40]. Infusion of BDNF into the lateral ventricle of rats during a 2 week period results in reduction in body weight, increases levels of corticotropin-releasing hormone (CRH) and vasopressin in the paraventricular, supraoptic and suprachiasmatic nuclei, and increases locomotor activity and body temperature [41]. Intracerebroventricular (ICV) infusion of BDNF in rats induces dose-dependent appetite suppression and weight loss [42], and conditional deletion of Bdnf gene in mice causes hyperphagia and obesity associated with elevated serum levels of leptin, insulin, glucose and cholesterol [43].
Although suppression of appetite is one mechanism by which CNS BDNF signaling influences energy metabolism throughout the body, recent findings suggest additional and more direct energy-regulating actions of BDNF on neuroendocrine pathways and organs involved in energy metabolism. Independent of an effect on food intake, CNS administration of BDNF lowers blood glucose levels and increases energy expenditure in leptin receptor mutant (db/db) mice [44]. The mechanisms by which CNS BDNF improves glucose metabolism in the periphery include regulation of the insulin signaling pathway because ICV BDNF treatment enhances the tyrosine phosphorylation of the insulin receptor accompanied by increased PI3K signaling in the liver of diabetic mice [45].
BDNF influences multiple cell types in the body that are involved in glucose metabolism including pancreatic β-cells (increased insulin production), hepatocytes (decreased glucose production) and skeletal muscle (increased insulin sensitivity). In db/db mice, BDNF administration significantly lowers blood glucose levels compared to pair-fed vehicle-treated mice, and this effect of BDNF is associated with increased numbers and total area of pancreatic islets, and increased secretory granules in β-cells [46]. Infusion of BDNF into the brain of rats results in a decrease in levels of glucagon in the portal vein, an effect eliminated by denervation of pancreatic efferent nerves [47]. The latter study further showed that intraportal administration of GLP-1 increases BDNF levels in the pancreas and reduces glucagon secretion. GLP-1 receptors are expressed in pancreatic cells, muscle and liver cells, and in neurons throughout the brain. Activation of GLP-1 receptors results in cyclic AMP production and activation of CREB which is known to induce BDNF expression. Similar to GLP-1, BDNF signaling increases glucose uptake by liver, skeletal and cardiac muscle cells [48]. Both BDNF and trkB are expressed in skeletal muscle cells, and exercise can induce the expression of BDNF in muscles. Ex vivo and in vivo studies showed that BDNF treatment increases phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase (ACCβ) resulting in enhanced fat oxidation in the skeletal muscle [49].
Some of the metabolic effects of BDNF are mediated via changes in energy expenditure of peripheral tissues. A major site for regulation of body temperature in rodents is brown adipose tissue (BAT) where energy is dissipated as heat through uncoupling fatty acid oxidation from ATP generation by uncoupling protein 1 (UCP-1). Hypothalamic injection of BDNF up-regulates UCP-1 expression and increases energy expenditure by enhancing metabolic rate and thermogenesis in BAT [50]. Moreover, hypothalamic overexpression of BDNF can induce the switching of white adipose tissue (WAT) to the BAT phenotype through sympathetic neuron activation, and endogenous BDNF is critical for the WAT to BAT transformation in response to environmental (sensory, cognitive, motor and social) stimuli [51]. In addition to its effects on the activity of peripheral neurons, BDNF may regulate peripheral energy metabolism by direct actions on non-neuronal cells. Thus, selective knockout of BDNF in liver cells in mice results in a reduction in hyperglycemia and hyperinsulinemia caused by a high fat diet [52].
In line with the observations in animals, inherited BDNF deficiency causes severe obesity in humans [53], and a recent genome-wide association study revealed a link between a polymorphism of BDNF and obesity [54]. One poignant example is the case of an 8 year-old girl with a spontaneous chromosomal inversion with the breakpoint affecting the BDNF gene resulting in BDNF haploinsufficiency that presented with prominent hyperphagia, obesity and cognitive impairment [55]. More subtle reductions in BDNF have also been associated with impaired glucose metabolism. In a study of 233 subjects, plasma BDNF levels were inversely associated with fasting plasma glucose levels and, interestingly, measurements of brain ‘BDNF output’ suggest that elevated glucose levels reduce the amount of BDNF produced and released from the brain into the blood [56]. Compromised BDNF signaling may also contribute to the suboptimal/impaired ANS function associated with obesity and the metabolic syndrome [57].
Potential role for BDNF signaling in the regulation of heart rate
Heart rate regulation is often linked strongly to metabolic status, such that exercise and energy restriction reduce resting heart rate and blood pressure by increasing parasympathetic tone, whereas obesity promotes elevated heart rate and blood pressure by increasing sympathetic tone. BDNF has emerged as a factor playing a role in the adaptive plasticity of the heart and regulation of heart rate, via the autonomic nervous system (ANS). Yang et al. [85] reported that when ANS neurons are cultured with cardiac myocytes, the neurons form synapses on the myocytes and bath application of BDNF increases the release of acetylcholine from ANS neurons and lowers cardiac myocyte beat frequency. BDNF haploinsufficiency in mice results in elevated resting heart rate, and infusion of BDNF into the cerebral ventricles reduces heart rate [86]. BDNF signaling in brainstem cholinergic neurons enhances production and release of acetylcholine resulting in reduced resting heart rate and increased heart rate variability (Figure 4). In addition, mice expressing a mutant form of the huntingtin protein that causes Huntington’s disease that results in reduced levels of BDNF in their brainstem, have an elevated resting heart rate, and infusion of BDNF into the cerebral ventricles reduced resting heart rate to a normal level [87]. Both dietary energy restriction and running, which induce BDNF expression in multiple brain regions, lower resting heart rate and increase heart rate variability by a mechanism involving increased parasympathetic tone [3, 88]. Rats on calorie restricted or intermittent fasting diets have lower resting heart rate, blood pressure and improved cardiovascular adaptation to stress, compared with rats fed ad libitum [88]. In humans, an energy-restricted diet ameliorates age-associated changes in ANS function, and markedly improves the heart rate variability profile, compared to individuals eating a standard Western diet [89]. Roles for BDNF in ANS responses to energy intake and exercise in humans remain to be established.
Figure 4. BDNF is involved in responses of the autonomic nervous system to energy intake and exercise.
By increasing the activity of the parasympathetic outflow from brainstem cholinergic neurons in the dorsal motor nucleus of the vagus (DMNV), regular exercise and intermittent fasting (IF) reduce heart rate and blood pressure, increase heart rate variability, and reduce inflammation in the cardiovascular system. Similarly, activation of the parasympathetic input to the gut can enhance motility and reduce local inflammation. BDNF signaling can enhance the activity of brainstem cholinergic neurons, suggesting possible roles for BDNF in the responses of the cardiovascular and gastrointestinal systems to exercise and IF. On the other hand, a sedentary gluttonous lifestyle can increase activity of sympathetic neurons (SN) and reduce BDNF signaling resulting in a relative in reduced parasympathetic tone. As a consequence heart rate and blood pressure are elevated and gut motility is reduced.
Implications for the Prevention and Treatment of Metabolic Syndrome and Associated Diseases
The findings from studies of animal models and human subjects described above suggest roles for BDNF in some of the beneficial effects of exercise and energy restriction on brain and body. Interventions that target BDNF signaling are therefore being developed for a range of metabolic and neurological disorders (Table 1). BDNF or TrkB agonists can be administered peripherally, but this approach risks many adverse side effects because of the widespread pattern of TrkB expression. One example of such adverse effects is lowered sensory thresholds and increased pain [58]. BDNF can be infused directly into brain regions of interest, expressed from viral vectors delivered into brain cells, or produced by genetically engineered cells transplanted into the brain. These approaches have all been validated in animal models of metabolic and/or neurodegenerative disorders (Box 2), and clinical trials using some of these approaches are in progress in human patients with obesity, depression, AD and PD [59, 60]. Up-regulation of endogenous BDNF by targeted stimulation of specific brain areas is another emerging therapeutic strategy for the treatment of obesity and diabetes [60]. In a recent proof-of-principal study, 3 obese patients were implanted with an electrode to stimulate the lateral hypothalamus, and this treatment reduced their food intake and resulted in weight loss [61]. Vagus nerve stimulation tested in rats showed potential for treating metabolic disorders.; during a 4-week period vagus nerve stimulation resulted in significant reductions in body weight and adipose tissue in rats, and these effects were accompanied by increased expression of BDNF in the hypothalamus [62]. However, this has not been tested in humans. Deep brain stimulation of the thalamus and subthalamic nucleus is increasingly used to treat PD, with dramatic relief of motor symptoms in many patients; BDNF is believed to play a role in the therapeutic effects of such deep brain stimulation [63]. While promising, clearly, the invasive nature of BDNF infusion, gene therapy and electrical stimulation, render these approaches not ideal for widespread application.
Table 1.
BDNF centric approaches for preventing and treating metabolic and neurological disorders
| Intervention | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Peripheral BDNF administration |
non-invasive | BBB penetrance; reduced pain threshold |
45, 46, 58 |
| CNS infiision of BDNF | on site delivery | delivery invasive | 44, 47 |
| Transplantation of BDNF- producing cells |
continuous delivery/one surgery | possible aberrant growth of implanted cells immune reaction; not reversible |
92 |
| BDNF-producing iPS cells | tailored cell type; no immune response |
requires transplantation; long-term safety? | NR |
| BDNF gene delivery using AAV |
Focal, long-term delivery/one surgery |
possible side effects related to excessive BDNF |
93 |
| Low molecular weight TrkB agonists |
easy administration, reversible | potential systemic side effects | 94 |
| Antidepressants | low cost, relatively safe | variable effectiveness | 64 |
| GLP-1 analogs | dual effects on brain and periphery |
long-term safety profde uncertain | 95, 96 |
| Electroconvulsive shock | non-invasive, long-lasting effects |
potential CNS side-effects, seizures | 97 |
| Deep brain stimulation | focal, activity-induced action | invasive | 60 |
| Exercise | safe, activity-dependent BDNF production |
requires motivation and dedication | 4, 5, 7 |
| Intermittent fasting | safe, activity-dependent BDNF production; improves overall health |
requires motivation and discipline | 4, 98, 99 |
| Intellectual stimulation | safe, activity-dependent BDNF production |
requires effort | 51, 100 |
BOX 2. Impaired BDNF Signaling and the Pathogenesis of Neurodegenerative Disorders.
Analysis of postmortem brain tissue samples from patients and control subjects have shown that BDNF levels are associated with dysfunction and degeneration of neurons in several major neurological disorders including Alzheimer’s disease (AD), Huntington’s disease (HD) and major depression [89]. Measurements of BDNF in cerebrospinal fluid samples from living patients and controls have revealed reductions in BDNF levels in patients with AD, age-related cognitive decline and PD. Studies of animal models support the involvement of diminished BDNF signaling in neurodegenerative disorders and, indeed, interventions that increase BDNF levels or activate TrkB have been shown to ameliorate clinical symptoms and underlying cellular and molecular neuropathologies in mouse models of AD, PD, HD, stroke and depression [4, 90]. There are mechanistic links between perturbed systemic and brain cell energy metabolism, impaired BDNF signaling, and the pathogenesis of neurodegenerative and psychiatric disorders. Abnormalities of systemic and brain energy metabolism have been described in studies of AD, PD and depression, and midlife obesity and diabetes/insulin resistance are risk factors for these disorders [91] . Altogether, the data suggest that impaired BDNF signaling may be a consequence of a combination of a chronically perturbed energy balance, the aging process, and disease-specific molecular alterations that occur in AD and PD.
Antidepressants that act by inhibiting serotonin and/or norepinephrine reuptake induce the expression of BDNF in animal models, and data from clinical studies support a BDNF-mediated mechanism of action of antidepressants in humans [64]. Some antidepressants may even activate TrkB independent of any effect on BDNF levels [65]. However, the impact of long-term antidepressant use on the risk for, and treatment of, metabolic and neurodegenerative disorders remains to be determined.
Several anti-diabetic drugs that are based on targets involved in glucose regulation may act, in part, by inducing BDNF expression. The GLP-1 analogs exenatide and liraglutide suppress appetite, and improve neuroplasticity and cognitive function, by activating receptors coupled to The GLP-1 analogs are highly effective in improving glucose regulation in diabetes patients, and because of their effectiveness in animal models [66-68], are currently being evaluated for PD and AD (clinicaltrials.gov NCT01255163, NCT01469351, and NCT01843075) with promising initial results in PD patients [69].
Natural products and synthetic drugs that stimulate BDNF production or activate TrkB hold potential for the prevention and treatment of metabolic and brain disorders. Extracts or purified phytochemicals from a range of plants have been shown to induce the expression of BDNF in rat neurons [70, 71]. A general mechanism of action of such phytochemicals is that they activate adaptive cellular stress response pathways resulting in the up-regulation of defenses against oxidative stress, energy deficits and inflammation [72]. Another approach is the development of TrkB agonists. Putative TrkB agonists were reported effective in reversing obesity and hyperglycemia in mice [73]. Molecular modeling and evaluation of more than 1 million chemicals, indentified top ‘hits’ with neuroprotective actions in cultured neurons, and resulted in the identification of several low molecular weight BDNF mimetics [74].
Concluding remarks and future perspectives
Emerging findings suggest that BDNF is a critical player in adaptive responses of the brain and body to metabolic challenges such as intermittent fasting and exercise, as well as to intellectual challenges (see box 3). Produced in a neuronal activity-dependent manner, BDNF signaling enhances synaptic plasticity and promotes neurogenesis, and may thereby mediate beneficial effects of intermittent energetic challenges on cognition and mood. Activation of the BDNF receptor TrkB bolsters neuronal bioenergetics by stimulating glucose transport and mitochondrial biogenesis, while simultaneously increasing neuronal resistance to metabolic, oxidative and excitotoxic stress. A chronic positive energy balance resulting from lack of exercise and overeating can result in reduced BDNF signaling which may contribute to the development of the metabolic syndrome and obesity, and to susceptibility to AD and PD, the most common age-related neurodegenerative disorders.
Box 3. Emerging Questions and Trends.
Questions
What are the gene targets that mediate the actions of BDNF on neuronal bioenergetics and adaptive plasticity?
What are the specific contributions of BDNF to the beneficial effects of exercise and energy restriction on the brain and body?
How does BDNF mediate behavioral responses to fluctuations in energy intake and expenditure?
What are the cellular sources and triggers of BDNF production in peripheral tissues?
Does impaired BDNF signaling play major roles in chronic diseases promoted by a ‘couch potato’ lifestyle?
Can BDNF signaling be targeted for the development of effective therapeutic interventions for metabolic disorders?
Trends
Molecular pathways that trigger BDNF production and release are being discovered.
Biological actions of BDNF on peripheral tissues such as muscle, liver and pancreas are being described.
Genetic variations in genes involved in BDNF signaling are being linked to neurological and metabolic disorders.
Methods for targeted delivery of BDNF to specific sites of cellular dysfunction and pathology are being developed.
BDNF is not only a prominent mediator of neuronal adaptations to energetic challenges, but it has far-reaching roles in controlling peripheral energy metabolism via actions in the brain, peripheral neurons and target organs including the pancreas, liver, skeletal muscle and heart. Data from studies of animal models and, in some cases human subjects, suggest that BDNF can protect against metabolic syndrome and obesity by suppressing appetite, increasing insulin sensitivity, and enhancing parasympathetic cardiovascular tone.
However, it is unlikely that the beneficial effects of exercise and intermittent fasting can be fully reproduced by artificially delivering BDNF or inducing a global increased in BDNF production. One reason is that BDNF is normally produced and released from neurons in an activity-dependent manner at the level of individual synapses, and this cannot be reproduced by crude stimulation methods or drugs. It is therefore important to continue to pursue novel approaches for the implementation of public policies and medical practices that incentivize and facilitate healthy diets and exercise programs. Educating doctors and the general public concerning why exercise and intermittent fasting are good for the brain and body will be fundamental to effecting society-wide changes to prevent and treat metabolic and brain disorders that involve impaired BDNF signaling.
Highlights.
Energetic challenges (e.g., exercise and energy restriction) induce BDNF signaling
BDNF enhances neuronal bioenergetics and promotes optimal brain health
BDNF signaling improves peripheral energy metabolism and cardiovascular function
Deficits in BDNF may contribute to metabolic morbidity and associated diseases
Acknowledgement
This work was supported by the intramural research program of the National Institute on Aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chao MV. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 2.Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci. 2006:126–127. doi: 10.1016/j.autneu.2006.02.027. 30-38. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Evolutionary aspects of human exercise--born to run purposefully. Ageing Res Rev. 2012;11:347–352. doi: 10.1016/j.arr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metabolism. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins ME, et al. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molteni R, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Vaynman S, et al. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 8.Stranahan AM, et al. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobilo T, et al. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter B, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Griffin ÉW, et al. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Duman CH, et al. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marais L, et al. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis. 2009;24:587–597. doi: 10.1007/s11011-009-9157-2. [DOI] [PubMed] [Google Scholar]
- 14.Sartori CR, et al. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 15.Levay EA, et al. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav. 2007;92:889–896. doi: 10.1016/j.physbeh.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwaya Y, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddle MC, et al. Caloric restriction enhances fear extinction learning in mice. Neuropsychopharmacology. 2013;38:930–937. doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaynman S, et al. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrann CD, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18 doi: 10.1016/j.cmet.2013.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Aguiar AS, Jr., et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, et al. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J Neurochem. 2012;120:210–219. doi: 10.1111/j.1471-4159.2011.07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris JJ, et al. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Burkhalter J, et al. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng A, et al. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markham A, et al. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35:366–374. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- 28.Kops GJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 29.Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takei N, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briz V, et al. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J Neurosci. 2013;33:4317–4328. doi: 10.1523/JNEUROSCI.4907-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang SJ, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarov O, et al. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33:569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slipczuk L, et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4(6):e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortin DA, et al. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci. 2012;32:8127–8137. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kernie SG, et al. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 38.Unger TJ, et al. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, et al. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 40.Duan W, et al. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 41.Naert G, et al. Continuous i.c.v. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal axis activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Pelleymounter MA, et al. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 43.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 44.Nonomura T, et al. Brain-derived neurotrophic factor regulates energy expenditure through the central nervous system in obese diabetic mice. Int J Exp Diabetes Res. 2001;2:201–209. doi: 10.1155/EDR.2001.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchida A, et al. The effects of brain-derived neurotrophic factor on insulin signal transduction in the liver of diabetic mice. Diabetologia. 2001;44:555–566. doi: 10.1007/s001250051661. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka M, et al. Protective effect of brain-derived neurotrophic factor on pancreatic islets in obese diabetic mice. Metabolism. 2006;55:1286–1292. doi: 10.1016/j.metabol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 47.Gotoh K, et al. Hypothalamic brain-derived neurotrophic factor regulates glucagon secretion mediated by pancreatic efferent nerves. J Neuroendocrinol. 2013;25:302–311. doi: 10.1111/jne.12003. [DOI] [PubMed] [Google Scholar]
- 48.Yamanaka M, et al. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab. 2007;9:59–64. doi: 10.1111/j.1463-1326.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- 49.Matthews VB, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchida A, et al. Acute effects of brain-derived neurotrophic factor on energy expenditure in obese diabetic mice. Int J Obes Relat Metab Disord. 2001;25:1286–1293. doi: 10.1038/sj.ijo.0801678. [DOI] [PubMed] [Google Scholar]
- 51.Cao L, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teillon S, et al. Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARalpha and FGF21 in mice with hepatic ablation of brain-derived neurotropic factor. J Endocrinol. 2010;205:37–47. doi: 10.1677/JOE-09-0405. [DOI] [PubMed] [Google Scholar]
- 53.Gray J, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 55.Gray J, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krabbe KS, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 57.Yang AC, et al. BDNF Val66Met polymorphism alters sympathovagal balance in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1024–1030. doi: 10.1002/ajmg.b.31069. [DOI] [PubMed] [Google Scholar]
- 58.Bretzner F, Liu J, Currie E, Roskams AJ, Tetzlaff W. Undesired effects of a combinatorial treatment for spinal cord injury--transplantation of olfactory ensheathing cells and BDNF infusion to the red nucleus. Eur J Neurosci. 2008;28:1795–1807. doi: 10.1111/j.1460-9568.2008.06462.x. [DOI] [PubMed] [Google Scholar]
- 59.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 60.Halpern CH, et al. Deep brain stimulation in the treatment of obesity. J Neurosurg. 2008;109:625–634. doi: 10.3171/JNS/2008/109/10/0625. [DOI] [PubMed] [Google Scholar]
- 61.Whiting DM, et al. Lateral hypothalamic area deep brain stimulation for refractory obesity: a pilot study with preliminary data on safety, body weight, and energy metabolism. J Neurosurg. 2013;119:56–63. doi: 10.3171/2013.2.JNS12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banni S, et al. Vagus nerve stimulation reduces body weight and fat mass in rats. PLoS One. 2012;7(9):e44813. doi: 10.1371/journal.pone.0044813. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spieles-Engemann AL, et al. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis. 2011;1:123–136. [PMC free article] [PubMed] [Google Scholar]
- 64.Neto FL, et al. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol. 2011;9:530–552. doi: 10.2174/157015911798376262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rantamäki T, et al. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One. 2011;6(6):e20567. doi: 10.1371/journal.pone.0020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin B, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aviles-Olmos I, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang R, et al. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008;1210:84–91. doi: 10.1016/j.brainres.2008.01.104. [DOI] [PubMed] [Google Scholar]
- 71.Rendeiro C, et al. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One. 2013;8(5):e63535. doi: 10.1371/journal.pone.0063535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Son TG, et al. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsao D, et al. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology. 2008;149:1038–1048. doi: 10.1210/en.2007-1166. [DOI] [PubMed] [Google Scholar]
- 74.Massa SM, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koppel I, et al. Tissue-specific and neural activity-regulated expression of human BDNF gene in BAC transgenic mice. BMC Neurosci. 2009;10:68. doi: 10.1186/1471-2202-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marini AM, et al. Preconditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32:299–304. doi: 10.1007/s00726-006-0414-y. [DOI] [PubMed] [Google Scholar]
- 77.Dean C, et al. Distinct subsets of Syt-IV/BDNF vesicles are sorted to axons versus dendrites and recruited to synapses by activity. J Neurosci. 2012;32:5398–5413. doi: 10.1523/JNEUROSCI.4515-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. NeuroMolecular Med. 2013 doi: 10.1007/s12017-013-8270-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fargali S, et al. Role of neurotrophins in the development and function of neural circuits that regulate energy homeostasis. J Mol Neurosci. 2012;48:654–659. doi: 10.1007/s12031-012-9790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Pollock GS, et al. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21:3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dolci C, et al. Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain Res. 2003;994:67–72. doi: 10.1016/j.brainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Liang FQ, et al. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20:2978–2987. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Girardet C, et al. Brain-derived neurotrophic factor/TrkB signaling regulates daily astroglial plasticity in the suprachiasmatic nucleus: Electron-microscopic evidence in mouse. Glia. 2013 doi: 10.1002/glia.22509. doi: 10.1002/glia.22509. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85.Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]
- 86.Griffioen KJ, et al. Evidence for the involvement of brain-derived neurotrophic factor in the autonomic control of heart rate. Soc Neurosci Abst. 2011 283.10/QQ5. [Google Scholar]
- 87.Griffioen KJ, et al. Aberrant heart rate and brainstem brain-derived neurotrophic factor (BDNF) signaling in a mouse model of Huntington's disease. Neurobiol Aging. 2012;33:f1481.e1–5. doi: 10.1016/j.neurobiolaging.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wan R, et al. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 89.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 90.Levivier M, et al. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci. 1995;15:7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riederer P, Bartl J, Laux G, Grünblatt E. Diabetes type II: a risk factor for depression-Parkinson-Alzheimer? Neurotox Res. 2011;19:253–265. doi: 10.1007/s12640-010-9203-1. [DOI] [PubMed] [Google Scholar]
- 92.Rodrigues Hell RC, Silva Costa MM, Goes AM, Oliveira AL. Local injection of BDNF producing mesenchymal stem cells increases neuronal survival and synaptic stability following ventral root avulsion. Neurobiol Dis. 2009;33:290–300. doi: 10.1016/j.nbd.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 93.Ramaswamy S, Kordower JH. Gene therapy for Huntington's disease. Neurobiol Dis. 2012;48:243–254. doi: 10.1016/j.nbd.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 94.Massa SM, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197. doi: 10.1016/S0140-6736(11)60207-9. [DOI] [PubMed] [Google Scholar]
- 96.Schéle E, et al. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (gcg) and brain-derived neurotrophic factor (BDNF) in the central nervous system. Endocrinology. 2013;154:3643–3651. doi: 10.1210/en.2012-2151. [DOI] [PubMed] [Google Scholar]
- 97.Mughal MR, et al. Electroconvulsive shock ameliorates disease processes and extends survival in huntingtin mutant mice. Hum Mol Genet. 2011;20:659–669. doi: 10.1093/hmg/ddq512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harvie MN, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson JB, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goshen I, et al. Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J Neurosci. 2009;29:3395–3403. doi: 10.1523/JNEUROSCI.5352-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]