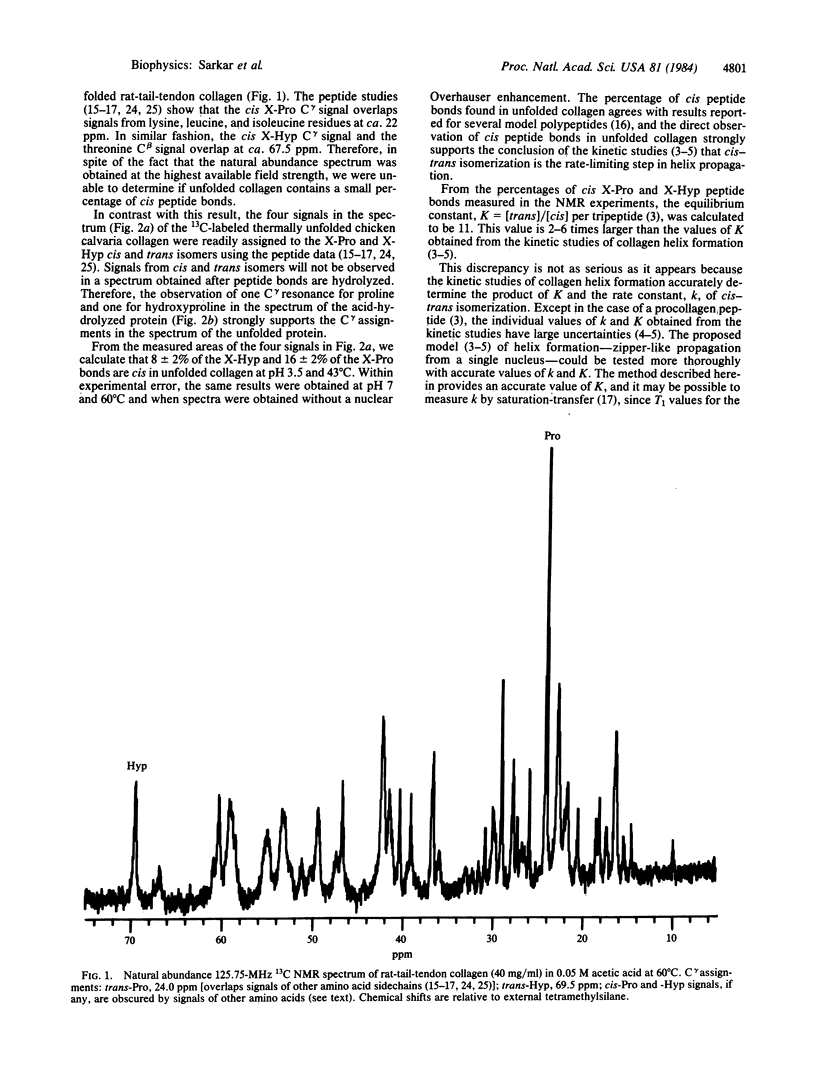
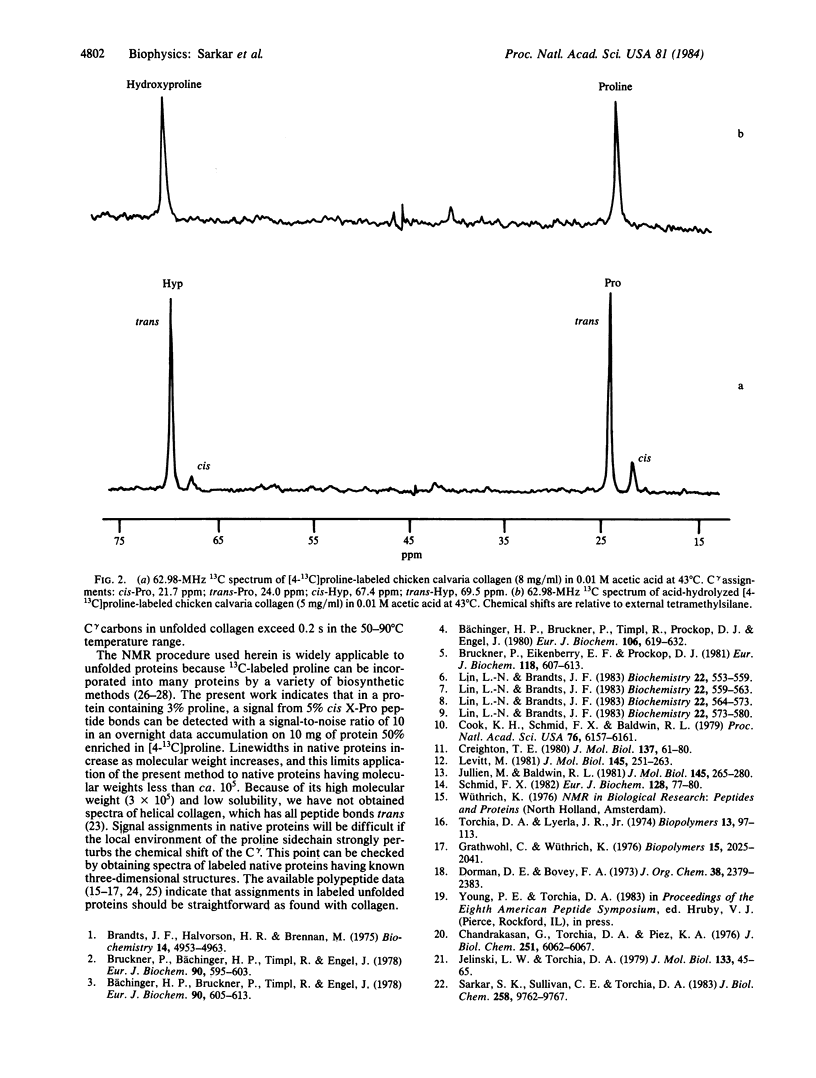
Abstract
Heretofore the complexity of natural abundance spectra has precluded the use of 13C NMR to detect cis peptide bonds in proteins. We have incorporated [4-13C]proline into chicken calvaria collagen and report here well-resolved C gamma signals, arising from cis and trans X-Pro and X-Hyp peptide bonds (where X is any amino acid residue) in the 13C NMR spectrum of the thermally unfolded protein. Measurement of 13C signal areas shows that 16% of the X-Pro and 8% of X-Hyp bonds are cis in the unfolded collagen. These results strongly support the conclusion drawn from kinetic studies that cis-trans isomerization of peptide bonds is the rate-limiting step in helix propagation after nucleation. Our method can be applied to other proteins as well and should aid in testing the generality of the hypothesis of Brandts, Halvorson, and Brennan that cis-trans isomerization is the rate-limiting step in protein folding when proline is present.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandts J. F., Halvorson H. R., Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry. 1975 Nov 4;14(22):4953–4963. doi: 10.1021/bi00693a026. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Bächinger H. P., Timpl R., Engel J. Three conformationally distinct domains in the amino-terminal segment of type III procollagen and its rapid triple helix leads to and comes from coil transition. Eur J Biochem. 1978 Oct 16;90(3):595–603. doi: 10.1111/j.1432-1033.1978.tb12640.x. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Eikenberry E. F., Prockop D. J. Formation of the triple helix of type I procollagen in cellulo. A kinetic model based on cis-trans isomerization of peptide bonds. Eur J Biochem. 1981 Sep 1;118(3):607–613. doi: 10.1111/j.1432-1033.1981.tb05562.x. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Bruckner P., Timpl R., Engel J. The role of cis-trans isomerization of peptide bonds in the coil leads to and comes from triple helix conversion of collagen. Eur J Biochem. 1978 Oct 16;90(3):605–613. doi: 10.1111/j.1432-1033.1978.tb12641.x. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Bruckner P., Timpl R., Prockop D. J., Engel J. Folding mechanism of the triple helix in type-III collagen and type-III pN-collagen. Role of disulfide bridges and peptide bond isomerization. Eur J Biochem. 1980 May;106(2):619–632. doi: 10.1111/j.1432-1033.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Chandrakasan G., Torchia D. A., Piez K. A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the nonhelical ends in solution. J Biol Chem. 1976 Oct 10;251(19):6062–6067. [PubMed] [Google Scholar]
- Cook K. H., Schmid F. X., Baldwin R. L. Role of proline isomerization in folding of ribonuclease A at low temperatures. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6157–6161. doi: 10.1073/pnas.76.12.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Kinetic study of protein unfolding and refolding using urea gradient electrophoresis. J Mol Biol. 1980 Feb 15;137(1):61–80. doi: 10.1016/0022-2836(80)90157-6. [DOI] [PubMed] [Google Scholar]
- Grathwohl C., Wüthrich K. The X-Pro peptide bond as an nmr probe for conformational studies of flexible linear peptides. Biopolymers. 1976 Oct;15(10):2025–2041. doi: 10.1002/bip.1976.360151012. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Torchia D. A. 13C/1H high power double magnetic resonance investigation of collagen backbone motion in fibrils and in solution. J Mol Biol. 1979 Sep 5;133(1):45–65. doi: 10.1016/0022-2836(79)90250-x. [DOI] [PubMed] [Google Scholar]
- Jullien M., Baldwin R. L. The role of proline residues in the folding kinetics of the bovine pancreatic trypsin inhibitor derivative RCAM(14-38). J Mol Biol. 1981 Jan 5;145(1):265–280. doi: 10.1016/0022-2836(81)90343-0. [DOI] [PubMed] [Google Scholar]
- Levitt M. Effect of proline residues on protein folding. J Mol Biol. 1981 Jan 5;145(1):251–263. doi: 10.1016/0022-2836(81)90342-9. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Determination of cis-trans proline isomerization by trypsin proteolysis. Application to a model pentapeptide and to oxidized ribonuclease A. Biochemistry. 1983 Feb 1;22(3):553–559. doi: 10.1021/bi00272a005. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Isomerization of proline-93 during the unfolding and refolding of ribonuclease A. Biochemistry. 1983 Feb 1;22(3):559–563. doi: 10.1021/bi00272a006. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Mechanism for the unfolding and refolding of ribonuclease A. Kinetic studies utilizing spectroscopic methods. Biochemistry. 1983 Feb 1;22(3):564–573. doi: 10.1021/bi00272a007. [DOI] [PubMed] [Google Scholar]
- Lin L. N., Brandts J. F. Mechanism for the unfolding and refolding of ribonuclease A. Simulations using a simple model with no structural intermediates. Biochemistry. 1983 Feb 1;22(3):573–580. doi: 10.1021/bi00272a008. [DOI] [PubMed] [Google Scholar]
- Sarkar S. K., Sullivan C. E., Torchia D. A. Solid state 13C NMR study of collagen molecular dynamics in hard and soft tissues. J Biol Chem. 1983 Aug 25;258(16):9762–9767. [PubMed] [Google Scholar]
- Schmid F. X. Proline isomerization in unfolded ribonuclease A. The equilibrium between fast-folding and slow-folding species is independent of temperature. Eur J Biochem. 1982 Nov;128(1):77–80. [PubMed] [Google Scholar]
- Torchia D. A., Lyerla J. R., Jr Molecular mobility of polypeptides containing proline as determined by 13C magnetic resonance. Biopolymers. 1974 Jan;13(1):97–114. doi: 10.1002/bip.1974.360130106. [DOI] [PubMed] [Google Scholar]
- Torchia D. A., Lyerla J. R., Jr, Quattrone A. J. Molecular dynamics and structure of the random coil and helical states of the collagen peptide, alpha 1-CB2, as determined by 13C magnetic resonance. Biochemistry. 1975 Mar 11;14(5):887–900. doi: 10.1021/bi00676a004. [DOI] [PubMed] [Google Scholar]
- Torchia D. A. Solid state NMR studies of protein internal dynamics. Annu Rev Biophys Bioeng. 1984;13:125–144. doi: 10.1146/annurev.bb.13.060184.001013. [DOI] [PubMed] [Google Scholar]


