Abstract
Background
Clinical trials have demonstrated that second-generation cobalt-chromium everolimus-eluting stent (CoCr-EES) is superior to first-generation paclitaxel-eluting stent (PES) and is non-inferior or superior to sirolimus-eluting stent (SES) in terms of safety and efficacy. It remains unclear whether vascular responses to CoCr-EES are different from SES and PES, since the pathology of CoCr-EES has not been described in humans.
Methods and Results
A total of 204 lesions (SES=73, PES=85, CoCr-EES=46) from 149 autopsy cases with duration of implant >30 days and ≤3 years were pathologically analyzed, where comparison of vascular responses was corrected for duration of implant. The observed frequency of late and very late stent thrombosis (LST/VLST) was less in CoCr-EES (4%) versus SES (21%, p=0.029) and PES (26%, p=0.008). Neointimal thickness was comparable among the groups, while the percent uncovered struts was strikingly lower in CoCr-EES (median=2.6%) versus SES (18.0%, p≤0.0005) and PES (18.7%, p<0.0005). CoCr-EES showed less inflammation score (with no hypersensitivity) and less fibrin deposition versus SES and PES. The observed frequency of neoatherosclerosis, however, did not differ significantly among the groups (CoCr-EES=29%, SES=35%, PES=19%). CoCr-EES had the least frequency of stent fracture (CoCr-EES=13%, SES=40%, PES=19%; p=0.007 for CoCr-EES versus SES), whereas fracture-related restenosis or thrombosis was comparable among the groups (CoCr-EES=6.5%, SES=5.5%, PES=1.2%).
Conclusions
CoCr-EES demonstrated greater strut coverage with less inflammation, less fibrin deposition, and less LST/VLST as compared to SES and PES in human autopsy analysis. Nevertheless, the observed frequencies of neoatherosclerosis and fracture-related adverse pathologic events were comparable in these devices, indicating that careful long-term follow-up remains important even after CoCr-EES placement.
Keywords: coronary disease, pathology, restenosis, stents, thrombosis
Introduction
Delayed arterial healing with poor strut coverage has been identified as the major substrate responsible for late and very late stent thrombosis (LST/VLST) following 1st-generation stainless steel sirolimus-eluting stent (SES) and paclitaxel-eluting stent (PES) placement.1, 2 Human autopsy studies have demonstrated that 1st-generation drug-eluting stents (DES) placed for “off-label” indications exhibit further delayed healing as compared to those implanted for “on-label” indications.3, 4 SES and PES show divergent mechanisms of LST/VLST: hypersensitivity reaction with diffuse extensive inflammation in the former versus malapposition with excessive fibrin deposition in the latter.4 In addition, in-stent neoatherosclerosis and stent fracture have emerged as other important contributing factors for late adverse events including LST/VLST and late target-lesion revascularization (TLR) following SES and PES placement. Neoatherosclerosis develops rapidly and more frequently within 1st-generation DES as compared to bare metal stent (BMS).5 The incidence of stent fracture in 1st-generation DES has been reported to vary from 1.3 to 8.4% in clinical studies.6, 7 However, in autopsy study where high-contrast film-based radiography was used, the prevalence of fracture was 29% in the 1st-generation DES where grade V fracture was identified in 5% of the lesions and was associated with increased risk of restenosis and thrombosis.8
Cobalt-chromium everolimus-eluting stent (CoCr-EES), a second-generation DES, consists of a thin (81 μm) strut platform, coated with 7.8-μm-thick durable fluorinated copolymer and 1.0 μg/mm2 everolimus.9 Pivotal randomized clinical trials have consistently demonstrated superiority of CoCr-EES over PES in reducing stent thrombosis, myocardial infarction, and TLR up to 2 years of follow-up.10, 11 On the other hand, randomized comparisons of CoCr-EES and SES have shown similar TLR rates between the devices, with comparable or lower incidence of stent thrombosis in CoCr-EES versus SES.12, 13 While better safety profile of CoCr-EES versus SES has not been consistently reported in head-to-head randomized trials, recent large-scale registry data14 and meta-analysis of randomized trials15, 16 have revealed that CoCr-EES shows substantially less stent thrombosis as compared to SES and PES.
Nevertheless, vascular responses to CoCr-EES versus SES and PES need further clarification since pathology of CoCr-EES has not been reported in humans. Although clinical studies utilizing optical coherence tomography have reported better strut coverage in CoCr-EES versus SES and PES at 6 to 9 months following stent placement,17 detailed assessment of vascular response to CoCr-EES including the degree of inflammation, fibrin deposition, and strut coverage in relation to underlying plaque morphology, along with the mechanism(s) of stent thrombosis, can only be determined by histopathologic studies. Moreover, the prevalence and characteristics of neoatherosclerosis as well as the impact of stent fracture on adverse pathologic outcomes in CoCr-EES remain to be elucidated. In the current study, we investigated pathologic response to CoCr-EES as compared to SES and PES in human coronary arteries using a registry database of autopsy cases.
Methods
Study population
Between July 2002 and October 2012, CVPath registry had received a total of 347 DES lesions with duration of implant >30 days, which include 294 lesions with 1st-generation DES (SES [Cypher, Cordis Corp., Miami Lakes, FL] and PES [TAXUS Express or TAXUS Liberté, Boston Scientific, Natick, MA]) and 53 lesions with 2nd-generation DES (zotarolimus-eluting stent [Endeavor or Resolute, Medtronic, Santa Rosa, CA] and CoCr-EES [XIENCE V, Abbott Vascular, Santa Clara, CA; or PROMUS, Boston Scientific]), from 220 autopsy cases. Of these, all available CoCr-EES (n=46 lesions) were included in the current study and the maximum duration of implant was 3 years (median=200 days, 25th to 75th percentiles [121 – 360]). For 1st-generation DES, 126 lesions in the last 3 years were excluded from the current analysis due to the longer duration of implant (721 days, [361 – 1204]) as compared to CoCr-EES. Of the remaining 168 lesions in the 1st-generation DES, 10 lesions with duration of implant >3 years were also excluded, and the remaining 158 lesions were included into the study (total SES=73 and PES=85 [63 SES and 79 PES have been included in previous reports4]). Consequently, a total of 204 DES lesions from 149 cases with similar duration of implant (SES, PES, and CoCr-EES [>30 days, ≤3 years]) were evaluated in the current study. The current study did not include lesions with platinum-chromium (PtCr) paclitaxel-eluting TAXUS Element stent (Boston Scientific) or PtCr everolimus-eluting PROMUS Element stent (Boston Scientific). Overlapping or consecutively implanted stents were treated as 1 lesion, while stents including a gap of >5 mm were considered to be separate lesions.1 All available clinical records were reviewed for patient history, duration of implant, risk factors, and medications. “Off-label” indication was defined as stents deployed for acute myocardial infarction (AMI), bifurcation lesion, left main coronary artery, bypass graft, restenosis, chronic total occlusion, or lesion length >30 mm.18 Cause of death was reported as stent-related death, nonstent-related cardiac death, and noncardiac death, as described previously.1
Histologic preparation
Epicardial coronary arteries were dissected from the heart and radiographed using high-contrast film-based radiography. Stented arteries were submitted for plastic embedding in methylmethacrylate. The entire stent was then sawed serially at 2 to 3 mm intervals. Histologic sections were cut at 6 μm and stained with hematoxylin and eosin (H&E) along with Movat pentachrome, as previously described.4
Pathologic assessment and morphometric analysis
The severity of calcification in the stented lesion was assessed based on the radiographs as previously described: none, mild, moderate, and severe.8 Stent fracture was identified by assessment of high-contrast film-based radiographs and classified as grade I to V, where grade V fracture was defined as multiple strut fractures with acquired transection with gap in the stent body, as previously described.8 Acute stent thrombosis was defined as a platelet-rich thrombus occupying >30% of the cross-sectional area of the lumen while stent restenosis was defined as >75% cross sectional area narrowing by neointimal formation within the stented area, with or without atherosclerosis.1, 4 Timing of the stent thrombosis was classified based on the Academic Research Consortium definition as LST (31 days to 1 year) or VLST (>1 year). The underlying plaque morphology (outside stent struts) was classified using traditional definitions of pathologic intimal thickening, fibroatheroma, thin-cap fibroatheroma, plaque rupture, and fibrocalcific plaque.19 Newly formed atherosclerotic changes within the stented segment (neoatherosclerosis) were classified into peri-strut foamy macrophage clusters, fibroatheroma, thin-cap fibroatheroma, and in-stent plaque rupture, as defined previously.5 Diffuse neoatherosclerosis was characterized by involvement of >50% of stent length by either foamy macrophages, fibroatheroma, thin-cap fibroatheroma, or rupture, and ≤50% involvement was defined as focal neoatherosclerosis. Morphometric analysis and histologic assessment of coronary sections were performed as previously described.4 Morphometric measurements (IPLab, Scanalytics, Rockville, MD) included external elastic lamina, stent, and lumen areas, as well as the thickness above each strut. Uncovered struts were identified by the presence of platelet and/or fibrin thrombus or bare struts with absence of neointima, and reported as ratio of uncovered-to-total stent struts per section.1 Strut coverage was also evaluated based on the presence or absence of >30% uncovered struts in at least one cross section, which has been shown to be a strong predictor of LST/VLST.1 The degree of fibrin deposition was evaluated as the percentage of struts with fibrin, while the severity of inflammation was assessed based on a grading scale of 0 to 4 (score 0 = <25% struts with fewer than 10 inflammatory cells, score 1 = up to 25% struts with greater than 10 inflammatory cells, score 2 = 25–50% struts with greater than 10 inflammatory cells, score 3 = >50% struts with greater than 10 inflammatory cells, and score 4 = two or more strut-associated granulomatous inflammatory reactions). Hypersensitivity reaction was defined as diffuse circumferential inflammation predominantly consisting of T-lymphocytes and eosinophils. The percentage of stent struts with giant cells and maximum number of eosinophils around each strut were also evaluated.4 In select cases, Luna’s staining method was used to confirm the infiltration of eosinophils. Immunohistochemical staining was performed in select cases using standard avidin-biotin techniques as previously described.5 Immunohistochemistry was carried out for the identification of macrophages using an anti-CD68 antibody (dilution 1:400, Dako, Carpinteria, CA), T-lymphocytes by an anti-CD45RO antibody (dilution 1:100, Dako), and B-lymphocytes using an anti-CD20 antibody (dilution 1:50, Dako).
Statistical analysis
Results for continuous variables with normal distribution were expressed as mean ± SD. Normality of distribution was tested with the Shapiro-Wilk test. Variables with non-normal distribution were expressed as median and 25th to 75th percentiles. For per patient analysis, comparisons of continuous variables with normal distribution were tested by one-way analysis of variance (ANOVA) with Dunnett’s multiple-comparison correction, and categorical variables were analyzed by chi-square test with the Bonferroni adjustment. These analyses were performed with the use of SPSS software version 19 (IBM Corporation, Armonk, NY). For per lesion analysis, both linear regression and logistic regression were used as appropriate where corrections for intra-class correlations were applied and robust variance estimates were employed. The Bonferroni adjustment was used to account for multiple comparisons in these regression models. Dependent variates that did not pass the Shapiro-Wilk test for normality were transformed with generalized log-normal transformations before the regression analyses to de-skew the distribution of errors in the variables. Ordered dependent variables, such as number of stents per lesion, were treated with ordered logistic regression analysis. Lesion location, underlying plaque morphology, and distribution of neoatherosclerosis involved multiple categorical variables where a multinomial logistic regression was employed for the analysis. Fisher’s exact test or Poisson regression substituted for logistic regression analysis when regression failed due to a low observed frequency. Even though there were no significant difference in duration of implant among the three groups, to insure that the impact of duration of implant was accounted for, all regression analyses of dependent variates with regard to vascular responses (i.e., stent outcome, morphometric analysis, and prevalence of neoatherosclerosis and stent fractures) included duration of implant as an independent covariate, whether this correction was statistically significant or not. The tests at the level of the lesions were performed with STATA 9.2 (StataCorp LP, College Station, TX). All tests were two-tailed and the analyses with Bonferroni adjustment required p<0.025 (0.05 divided by 2) for statistical significance except when involving multiple categorical variables, where the appropriate divisor of the nominal alpha value (threshold of significance for a p-value) of 0.05 was noted in the footnotes. A value of p<0.05 was considered to be significant for ANOVA with Dunnett’s correction.
Results
Patient and lesion characteristics and outcome of DES
There were no differences in clinical characteristics between CoCr-EES and 1st-generation DES (Table 1). Risk factors were similar between different stents except that CoCr-EES had greater prevalence of diabetes than SES while hyperlipidemia was less frequent in CoCr-EES as compared to SES and PES. The duration of implant in all groups were similar, and lesion characteristics were comparable among the groups (Table 1). Representative histologic images of SES, PES, and CoCr-EES implanted for stable coronary artery disease (CAD) and for acute coronary syndromes (ACS) are shown in Figure 1.
Table 1.
Patient and Lesion Characteristics and Outcomes of DES
| SES | PES | CoCr-EES | p value CoCr-EES vs. SES |
p value CoCr-EES vs. PES |
|
|---|---|---|---|---|---|
| Patient characteristics | n=56 | n=61 | n=32 | ||
|
| |||||
| Age (years) | 59 ± 12 | 61 ± 12 | 64 ± 13 | 0.14 | 0.35 |
| Male gender | 41 (73%) | 46 (75%) | 20 (63%) | 0.29 | 0.19 |
| Hypertension | 28/39 (72%) | 39/48 (81%) | 19/30 (63%) | 0.45 | 0.078 |
| Diabetes mellitus | 8/40 (20%) | 15/48 (31%) | 14/30 (47%) | 0.017 | 0.17 |
| Hyperlipidemia | 26/39 (67%) | 29/48 (60%) | 10/30 (33%) | 0.006 | 0.020 |
| Previous MI | 27/47 (57%) | 30/53 (57%) | 18/30 (60%) | 0.82 | 0.76 |
| Previous CABG | 10/53 (19%) | 12/57 (21%) | 10 (31%) | 0.19 | 0.28 |
| Aspirin | 21/28 (75%) | 30/40 (75%) | 4/6 (67%) | 0.67 | 0.66 |
| Thienopyridines | 17/28 (61%) | 25/39 (64%) | 4/6 (67%) | 0.79 | 0.90 |
| Cause of death | |||||
| Stent-related | 14 (25%) | 20 (33%) | 5 (16%) | 0.30 | 0.076 |
| Nonstent-related cardiac | 20 (36%) | 24 (39%) | 15 (47%) | 0.30 | 0.48 |
| Noncardiac | 18 (32%) | 15 (25%) | 11 (34%) | 0.83 | 0.32 |
| Explant | 4 (7%) | 2 (3%) | 1 (3%) | 0.43 | 0.97 |
|
| |||||
| Lesion characteristics | n=73 | n=85 | n=46 | ||
|
| |||||
| Duration of implant (days) | 270 (116 – 510) | 210 (120 – 361) | 200 (121 – 360) | 0.75 | 0.71 |
| Indications for stenting | |||||
| Stable CAD/ACS | 42 (58%)/31 (42%) | 56 (66%)/29 (34%) | 27 (59%)/19 (41%) | 0.92 | 0.51 |
| Lesion location* | |||||
| LM/LAD/LCX/RCA/Graft | 1/27/20/24/1 | 3/37/17/21/7 | 2/17/11/11/5 | 0.36/Ref/0.78/0.51/0.078 | 0.69/Ref/0.46/0.79/0.57 |
| Stent length (mm) | 22.0 (15.5 – 29.0) | 21.0 (16.0 – 30.0) | 22.0 (15.0 – 36.3) | 0.71 | 0.62 |
| Overlapping stents | 23 (32%) | 26 (31%) | 17 (37%) | 0.59 | 0.50 |
| with BMS | 6 (8%) | 6 (7%) | 1 (2%) | 0.22 | 0.27 |
| with same type of DES | 14 (19%) | 17 (20%) | 10 (22%) | 0.74 | 0.82 |
| with different type of DES | 4 (5%) | 4 (5%) | 9 (20%) | 0.057 | 0.032 |
| Bifurcation multistenting | 7 (10%) | 15 (18%) | 2 (4%) | 0.31 | 0.049 |
| Number of stents per lesion | 1.4 ± 0.8 | 1.4 ± 0.7 | 1.6 ± 1.0 | 0.37 | 0.57 |
| Underlying plaque morphology* | |||||
| Rupture·TCFA/FA/FC/PIT/Others† | 25/28/8/7/5 | 20/42/11/8/4 | 11/19/5/4/7 | 0.37/Ref/0.91/0.83/0.37 | 0.70/Ref/0.99/0.88/0.060 |
| Lesion calcification | |||||
| None/Mild/Moderate/Severe | 16/28/15/14 | 20/38/19/8 | 6/22/7/11 | 0.57 | 0.15 |
| Off-label indication | 41 (56%) | 48 (56%) | 24 (52%) | 0.71 | 0.67 |
| Stent outcome‡ | |||||
| Thrombosis (late and very late) | 15 (21%) | 22 (26%) | 2 (4%) | 0.029 | 0.008 |
| Late stent thrombosis | 7/40 (18%) | 17/64 (27%) | 2/36 (6%) | 0.39 | 0.031 |
| Very late stent thrombosis§ | 8/33 (24%) | 5/21 (24%) | 0/10 (0%) | <0.0005 | <0.0005 |
| Restenosis | 10 (14%) | 10 (12%) | 8 (17%) | 0.63 | 0.43 |
Values are expressed as mean ± SD, median (25th – 75th percentiles), or n (%), unless otherwise specified. All tests at the level of the lesions use either logistic regression or linear regression with correction for intra-class correlations among lesions and robust variance estimates, after appropriate de-skewing transformations in the case of interval dependent variables. Ordered dependent variables, such as number of stents per lesion, are treated with ordered logistic regression.
Multiple p values refer to each category in the order given. The second category serves as the reference category in a multinomial logistic regression for each of the five categories in lesion location and underlying plaque morphology. Since each of these two dependent variables involves 8 tests each, multiple comparisons protection via the Bonferroni method requires that no p value be considered significant unless p< 0.05/8 = 0.00625. In all other cases, the Bonferroni criterion for significance is p< 0.05/2 = 0.025.
Others include nodular calcification and restenotic lesions.
Only these dependent variates were corrected with the covariate duration of implant, because the other lesion variates in this table do not reflect vascular healing response and therefore they are not associated with duration of implant.
For very late stent thrombosis, Poisson regression (with the same conditions, e.g. duration of implant as covariate and robust variance estimate) was used instead of logistic regression, because the use of the CoCr-EES perfectly predicts no very late stent thrombosis, and logistic regression yields no confidence estimate under this condition.
ACS=acute coronary syndromes; CABG=coronary artery bypass grafting; CAD=coronary artery disease; CoCr-EES=cobalt-chromium everolimus-eluting stent; DES=drug-eluting stent; FA=fibroatheroma; FC=fibrocalcific plaque; LAD=left anterior descending artery; LCX=left circumflex artery; LM=left main coronary artery; MI=myocardial infarction; RCA=right coronary artery; PES=paclitaxel-eluting stent; PIT=pathologic intimal thickening; Ref=reference category; SES=sirolimus-eluting stent; TCFA=thin-cap fibroatheroma
Figure 1.
Representative images of SES, PES, and CoCr-EES implanted for stable CAD (A: a to f) and for ACS (B: g to l). (a, b) Histologic sections from a 53 year-old-man with SES implanted in the proximal LAD for 13 months. A low-power image (a) shows mild neointimal growth and underlying fibrocalcific plaque. Focal uncovered struts are highlighted in a high-power image in b. (* indicates stent strut.) (c, d) Histologic sections from a 71-year-old man with PES implanted in the RCA 11 months antemortem. A low-power image (c) shows mild to moderate neointimal proliferation and underlying fibroatheroma. Note uncovered struts with persistent peri-strut fibrin deposition shown at high power image in d. (e, f) Histologic sections from a 60-year-old man who received CoCr-EES in the mid LCX 6 months antemortem. A low-power image (e) shows mild neointimal proliferation and underlying fibrocalcific plaque. All struts are covered with proteoglycan-rich neointima with absence of fibrin, which is highlighted in a high-power image in f. (g, h) Histologic sections from a 74-year-old woman who received SES in the proximal LAD for AMI 18 months antemortem, who died of diffuse severe CAD. A low-power image (g) shows mild neointimal proliferation. Note focal uncovered struts and strut penetration into the necrotic core (NC) (h). (i, j) Histologic sections from a 64-year-old woman with PES implanted in the RCA for AMI 9 months antemortem, who died of congestive heart failure. A low-power image (i) shows patent lumen with stent struts surrounded by fibrin and an underlying NC. Note uncovered struts with fibrin deposition which overlie the NC (j). (k, l) Histologic sections from a 67-year-old man who received CoCr-EES in the proximal LAD for non-ST elevation AMI 5 months antemortem, who died of non-cardiac causes. A low-power image (k) shows mild neointimal proliferation and an underlying large NC. All struts are covered with a thin neointima overlying the NC, which is highlighted in the high-power image in l. All histologic sections are stained with Movat pentachrome. AMI=acute myocardial infarction. Other abbreviations as in Table 1.
The observed frequency of LST and VLST within the study population was less in CoCr-EES (4%) as compared to SES (21%, p=0.029) and PES (26%, p=0.008) (after adjustment for duration of implant) (Table 1). There were two lesions with CoCr-EES showing LST, and no VLST was observed in CoCr-EES. Both of the lesions with LST in the CoCr-EES group had CoCr-EES implanted over an underlying PES implant. Pathologic etiologies for the two LST in CoCr-EES were identified as uncovered struts associated with overlapping stents and neointimal erosion with restenosis, respectively (Figure 2). The prevalence of restenosis observed for CoCr-EES (17%) did not differ significantly from SES (14%) and PES (12%).
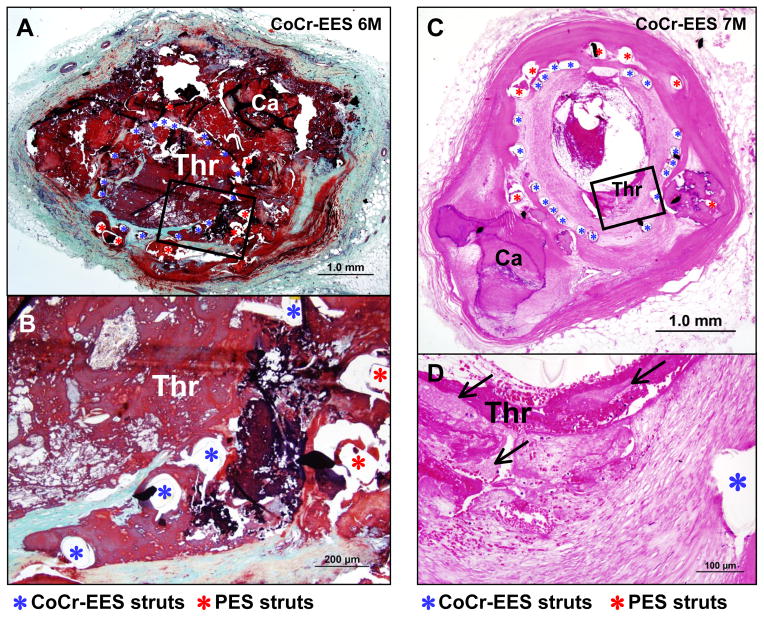
Figure 2.
Late stent thrombosis in two cases with CoCr-EES. (A and B) Histologic sections from a 55-year-old man with CoCr-EES implanted over an underlying PES in the proximal RCA 6 months antemortem, who died suddenly of stent thrombosis. A low-power image (A) shows occlusive luminal thrombus (Thr) within the stents with underlying calcified plaque (Ca=calcification). A few struts of CoCr-EES are covered with thin neointima but majority of the struts are uncovered, which is highlighted in a high-power image in B. (C and D) Histologic sections from a 72-year-old woman with CoCr-EES implanted over an underlying PES in the proximal LAD 7 months antemortem. The patient presented with AMI from stent thrombosis and underwent balloon angioplasty which resulted in rupture of LAD. A low-power image (C) shows in-stent restenosis with luminal thrombus (Thr) where neointima is focally dissected due to the balloon angioplasty with overlying non-occlusive thrombus. A high-power image (D) shows erosive neointima with overlying fibrin and platelet thrombus. (A) and (B) are Movat pentachrome staining, and (C) and (D) are stained with hematoxylin and eosin (H&E).
Morphometric analysis
Morphometric analysis was performed on a total of 1357 histologic sections (SES=479, PES=578, CoCr-EES=300) with 14,456 struts (SES=4546, PES=6037, CoCr-EES=3873) (Table 2). Areas of external elastic lamina, stents, and underlying plaques were observed to be smaller in CoCr-EES as compared to SES and PES, where the difference in external elastic lamina area between the CoCr-EES and the SES groups was statistically significant (p=0.010). The mean and maximum neointimal thickness in CoCr-EES did not differ significantly from those in SES and PES (Table 2). On the contrary, the observed frequency of uncovered struts was strikingly lower in CoCr-EES (median=2.6%, 25th to 75th percentiles [0 – 7.1]) as compared to SES (18.0%, [0 – 51.4]; p≤0.0005) and PES (18.7%, [7.1 – 44.4]; p<0.0005). The prevalence of DES lesions with >30% uncovered struts was also significantly lower in CoCr-EES (20%) as compared to SES (60%, p<0.0005) and PES (67%, p<0.0005).
Table 2.
Morphometric Analysis and Neoatherosclerosis Assessment
| SES (n=73 lesions) | PES (n=85 lesions) | CoCr-EES (n=46 lesions) | p value CoCr-EES vs. SES |
p value CoCr-EES vs. PES |
|
|---|---|---|---|---|---|
| Number of histologic sections evaluated | 479 | 578 | 300 | ||
| Number of stent struts evaluated | 4546 | 6037 | 3873 | ||
| External elastic lamina area (mm2) | 14.2 ± 5.1 | 14.1 ± 5.6 | 12.5 ± 5.0 | 0.010 | 0.089 |
| Stent area (mm2) | 6.7 ± 2.2 | 7.2 ± 3.2 | 6.2 ± 2.4 | 0.15 | 0.034 |
| Underlying plaque area (mm2) | 7.5 ± 3.9 | 6.9 ± 3.3 | 6.3 ± 3.1 | 0.16 | 0.34 |
| Mean neointimal thickness (mm) | 0.09 (0.04 – 0.18) | 0.11 (0.06 – 0.19) | 0.09 (0.03 – 0.18) | 0.89 | 0.32 |
| Maximum neointimal thickness (mm) | 0.19 (0.08 – 0.32) | 0.26 (0.17 – 0.35) | 0.20 (0.10 – 0.39) | 0.93 | 0.13 |
| Uncovered struts (%) | 18.0 (0 – 51.4) | 18.7 (7.1 – 44.4) | 2.6 (0– 7.1) | <0.0005 | <0.0005 |
| Prevalence of >30% uncovered struts* | 44 (60%) | 57 (67%) | 9 (20%) | <0.0005 | <0.0005 |
| Struts with fibrin (%) | 29.9 (12.1 – 59.9) | 51.1 (36.9 – 72.9) | 8.5 (0 – 28.2) | 0.001 | <0.0005 |
| Inflammation score† | 1.00 (0.33 – 2.00) | 1.00 (0.13 – 1.44) | 0.26 (0 – 0.60) | <0.0005 | 0.006 |
| Maximum number of eosinophils per strut‡ | 0 (0 – 9.5) | 0 (0 – 2.0) | 0 (0 – 1.3) | 0.009 | 0.59 |
| Struts with giant cells (%) | 16.7 (9.5 – 41.4) | 3.0 (0 – 9.0) | 7.5 (3.3 – 18.2) | 0.002 | 0.001 |
| Prevalence of malapposition | 12 (16%) | 15 (18%) | 2 (4%) | 0.065 | 0.043 |
| Prevalence of hypersensitivity reaction§ | 6 (8%) | 0 (0%) | 0 (0%) | 0.081 | - |
| Prevalence of neoatherosclerosis|| | 25/72 (35%) | 15/78 (19%) | 12/41 (29%) | 0.91 | 0.19 |
| Foamy macrophage clusters | 8/72 (11%) | 13/78 (17%) | 8/41 (20%) | 0.15 | 0.69 |
| Fibroatheroma | 15/72 (21%) | 2/78 (3%) | 4/41 (10%) | 0.32 | 0.13 |
| TCFA/in-stent plaque rupture§ | 2/72 (2%) | 0/78 (0%) | 0/41 (0%) | 0.52 | - |
| Distribution of neoatherosclerosis||¶ | |||||
| None/Focal/Diffuse | 47/10/15 | 63/11/4 | 29/7/5 | Ref/0.62/0.48 | Ref/0.47/0.13 |
| Prevalence of calcification within the neointima|| | 14/72 (19%) | 4/78 (5%) | 3/41 (7%) | 0.048 | 0.88 |
All regression analyses were corrected with the covariate duration of implant. Multiple-comparison thresholds to be used as in Table 1.
Prevalence of DES lesion with >30% uncovered struts in at least one cross section.1
Using ordered logistic regression on 16 cuts in counts dominated by 0, because no transformation justified linear regression. This retained robust variance estimates and correction for intra-class correlations among lesions.
Using Poisson regression for these counts data.
Tabular Fisher’s exact test substituted for regression, because regression fails where CoCr-EES perfectly predicts absence of hypersensitivity reaction and TCFA/in-stent plaque rupture vs. SES.
Evaluated on DES implanted in native coronary arteries.
Using multinomial logistic regression with None as the reference.
Abbreviations as in Table 1.
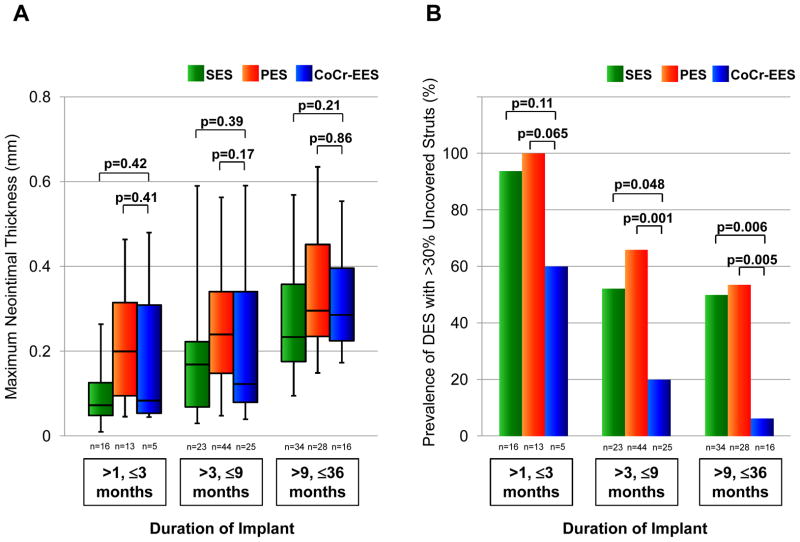
The maximum neointimal thickness and the prevalence of DES with >30% uncovered struts in CoCr-EES versus SES or PES were further compared based on stratified duration of implant (Figure 3). All DES showed progressive increase in the maximum neointimal thickness and gradual decrease in the prevalence of DES lesions with >30% uncovered struts as the duration of implant increased. The maximum neointimal thickness did not differ significantly between the groups within each period of the duration of implant. In contrast, the prevalence of lesions with >30% uncovered struts in CoCr-EES was lower than that in SES and PES at each duration of implant where the differences between the groups were statistically significant for 3 to 9 months (for CoCr-EES versus PES) and 9 to 36 months (for CoCr-EES versus both SES and PES) (Figure 3).
Figure 3.
Box-and-whisker plots showing maximum neointimal thickness (A) and bar graphs showing prevalence of DES lesions with >30% uncovered struts (B) stratified by duration of implant in SES, PES, and CoCr-EES. In box-and-whisker plots, lines within boxes represent median values; the upper and lower lines of the boxes represent the 75th and 25th percentiles, respectively; and the upper and lower bars outside the boxes represent the 90th and 10th percentiles, respectively. P values for CoCr-EES versus SES and for CoCr-EES versus PES are presented. Multiple-comparison threshold is used as in Table 1.
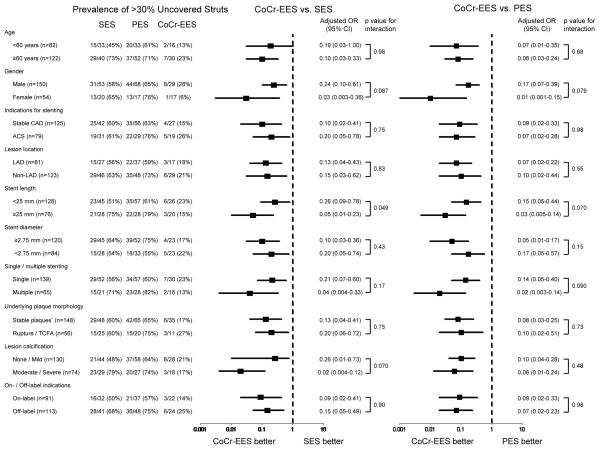
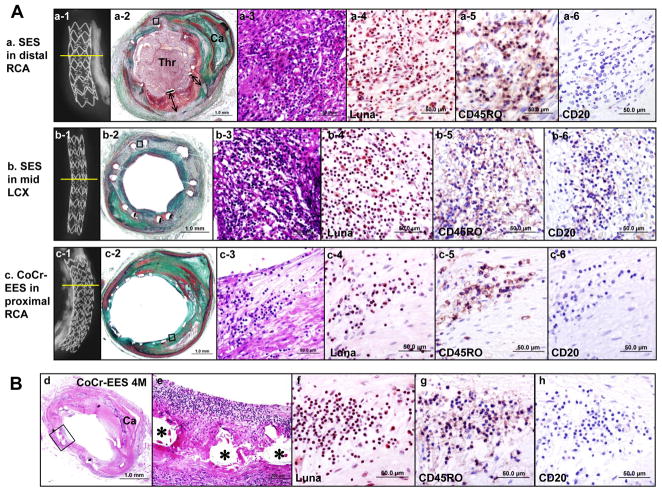
Further subgroup analysis demonstrated that the superiority of CoCr-EES over SES and PES in terms of lower prevalence of >30% uncovered struts was consistently observed irrespective of age, gender, indications for stenting (stable CAD versus ACS, and “on- versus off-label”), and lesion characteristics including lesion location, stent length, stent diameter, single or multiple stenting, underlying plaque morphology (stable versus unstable plaques), and the degree of lesion calcification (Figure 4). Strut coverage in devices implanted for “off-label” indications was less as compared to that for “on-label” indications in all DES. However, CoCr-EES as compared to SES and PES showed greater strut coverage for both “on- and off-label” indications. Stent struts with fibrin deposition were significantly less in CoCr-EES (8.5%, [0 – 28.2]) as compared to SES (29.9%, [12.1 – 59.9]; p=0.001) and PES (51.1%, [36.9 – 72.9]; p<0.0005) (Table 2). CoCr-EES showed significantly less inflammation score (0.26, [0 – 0.60]) as compared to SES (1.00, [0.33 – 2.00]; p<0.0005) and PES (1.00, [0.13 – 1.44]; p=0.006). Eosinophil infiltration and giant cell reaction were also significantly less in CoCr-EES as compared to SES; however, CoCr-EES showed greater giant cell reaction as compared to PES. The observed frequency of malapposition was lower in CoCr-EES (4%) as compared to SES (16%) and PES (18%), although the differences were not statistically significant. No hypersensitivity reaction was observed in CoCr-EES, whereas 8% of SES (6 of 73) showed hypersensitivity. One patient had received two SES and one CoCr-EES implants: both of the SES showed diffuse hypersensitivity reaction and one of them had occlusive thrombus, while the CoCr-EES showed only focal inflammation with eosinophils and T-lymphocytes without hypersensitivity reaction (Figure 5A). A total of 4 lesions (8.7%) had focal eosinophil infiltration (>10 per strut) with T-lymphocytes in CoCr-EES (Figure 5B).
Figure 4.
Sub-group analysis for the presence of >30% uncovered struts in CoCr-EES versus SES and PES. All regression analyses include duration of implant as a covariate. Multiple-comparison threshold is used as in Table 1. *Underlying stable plaques include fibroatheroma, fibrocalcific plaque, nodular calcification, pathologic intimal thickening, and restenotic lesions. OR=odds ratio; CI=confidence interval. Other abbreviations as in Table 1.
Figure 5.
Hypersensitivity reaction in SES versus focal inflammation in CoCr-EES. (A) Histologic sections from a 58-year-old man with two SES and one CoCr-EES, who died suddenly one day after nasal polyp surgery. Dual antiplatelet therapy was stopped 5 days prior to the surgery. (a and b) SES implanted in distal RCA (a) and in the mid LCX (b) for 3 years. Radiographs show SES with (a-1) and without (b-1) underlying severe calcification, with no stent fracture. A low-power histology image in a-2 (Movat) shows occlusive platelet rich thrombus (Thr) with transmural inflammation and extensive malapposition of stent struts with fibrin deposition (double arrows). A low-power image in b-2 shows mild neointimal proliferation with transmural inflammation but no luminal thrombus. High-power images (a-3 to a-6 and b-3 to b-6) show extensive inflammation predominantly consisting of eosinophils (Luna stain [a-4 and b-4]) and T-lymphocytes (CD45RO [a-5 and b-5]) but rare B-lymphocytes (CD20 [a-6 and b-6]). (c) A CoCr-EES implanted in the proximal RCA of 7 months duration. A radiograph (c-1) shows a stent with underlying severe calcification and no fracture. A low-power histology image (c-2) shows a patent lumen with thin neointima. High power images (c-3 to c-6) show focal mild inflammation consisting of eosinophils (c-4) and T-lymphocytes (c-5) but no B-lymphocytes (c-6). (B) Histologic sections from a 51-year-old man who received CoCr-EES in the distal LCX 4 months antemortem. A low-power image (d) (H&E) shows a patent lumen with mild neointimal proliferation and underlying calcified plaque. High power images (e to h) show focal inflammation within the neointima consisting of eosinophils (f) and T-lymphocytes (g), but no B-lymphocytes were observed (h). (* indicates stent strut.)
Prevalence and characteristics of neoatherosclerosis
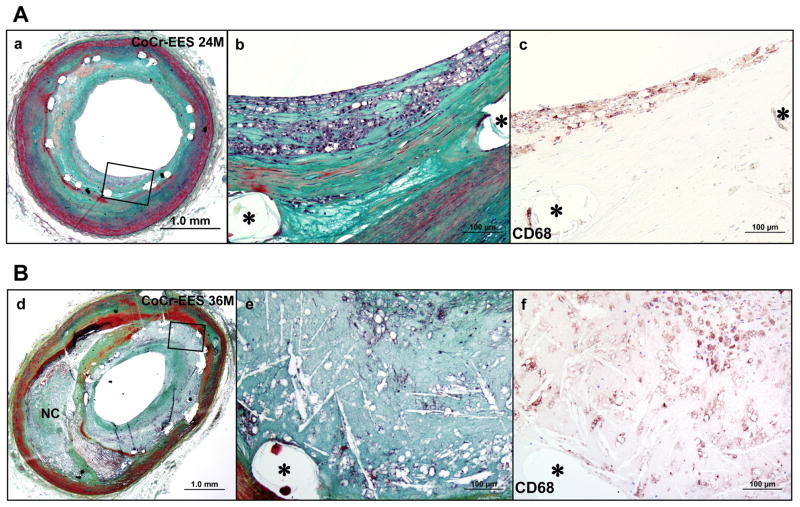
The overall prevalence of neoatherosclerosis following CoCr-EES implantation in native coronary arteries was 29%, which did not differ significantly from SES (35%) and PES (19%) (Table 2). There was no significant difference in the observed frequency of each characteristic of neoatherosclerosis (foamy macrophage clusters, fibroatheroma, and thin-cap fibroatheroma or in-stent plaque rupture) between the groups; however, a dominant morphology in CoCr-EES and PES was foamy macrophage clusters (CoCr-EES=67% [8 of 12]; PES=87% [13 of 15]) which was less frequent in SES (32% [8 of 25]). No unstable features of neoatherosclerosis (thin-cap fibroatheroma or plaque rupture) were observed in CoCr-EES. Diffuse nature of neoatherosclerotic change was observed in 42% (5 of 12) of CoCr-EES with neoatherosclerosis, which was not significantly different from SES (60% [15 of 25]) and PES (27% [4 of 15]). The earliest duration of implant showing neoatherosclerosis in CoCr-EES within the native coronary arteries was 270 days, which was relatively longer than that in SES (120 days) and PES (70 days). Representative images of neoatherosclerosis in CoCr-EES implanted in native coronary arteries are shown in Figure 6.
Figure 6.
Neoatherosclerosis in CoCr-EES. (A) Histologic sections from a 49-year-old man with CoCr-EES implanted in the mid LAD 2 years antemortem. A low-power image (a) (Movat) shows a patent lumen with moderate neointimal growth (50% cross-sectional area narrowing). (b) A high-power image of the boxed area in (a) shows foamy macrophage accumulation within the neointima close to the luminal surface, which is confirmed by imuunostaining for CD68-positive macrophages (c). (B) Histologic sections from a 73-year-old man with CoCr-EES implanted in the mid LAD for 3 years. A low-power image (d) (Movat) shows moderate luminal narrowing with moderate neointimal growth (69% stenosis) and underlying fibroatheroma. A high-power image (e) of the boxed area in (d) shows necrotic core formation within the neointima where CD68-positive macrophages are identified (f). (* indicates stent strut.)
Prevalence of stent fracture and fracture-related complications
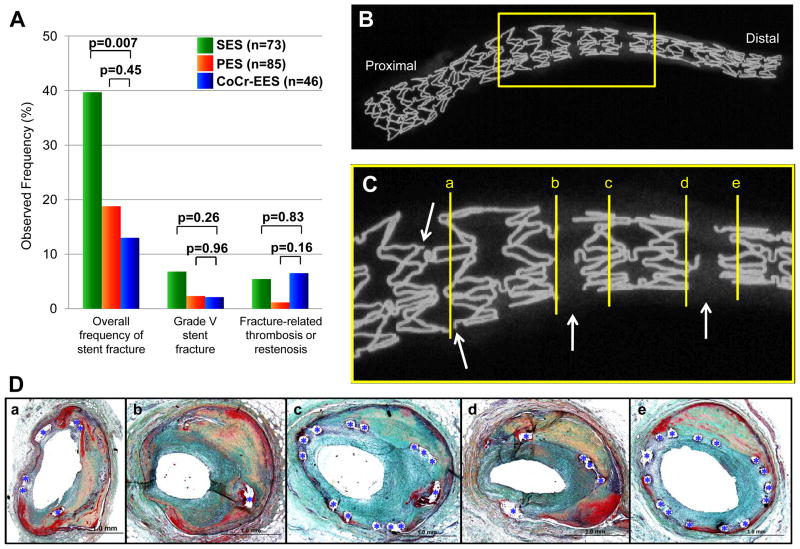
The overall observed frequency of stent fracture in CoCr-EES was 13%, which was significantly lower than SES (40%, p=0.007), but was comparable to PES (19%, p=0.45) (Figure 7A). The observed frequency of grade V fracture of CoCr-EES (2.2%) did not differ from SES (6.9%, p=0.26) and PES (2.4%, p=0.96). Moreover, there was no significant difference in fracture-related adverse pathologic events (restenosis or thrombosis) of CoCr-EES (6.5% [n=3 restenosis]) versus SES (5.5% [n=4; 2 restenosis and 2 thrombosis], p=0.83) and PES (1.2% [n=1 thrombosis], p=0.16). The six lesions (five patients) showing CoCr-EES fracture are listed in Table 3. Majority of the CoCr-EES fractures were identified in the middle, adjacent to the hairpin bend in the non-linear link. Several fractures were also identified along the crown at the base of the non-linear link (Table 3) (Figure 7 B to D).
Figure 7.
Stent fracture in CoCr-EES. (A) Bar graph showing observed frequency of overall fracture, grade V fracture (acquired transection with gap in the stent body), and fracture-related thrombosis or restenosis, in CoCr-EES versus SES and PES. The analyses include duration of implant as a covariate. Multiple-comparison threshold is used as in Table 1. (B to D) A case of grade V CoCr-EES fracture showing restenosis at fracture site (Case #3 in Table 3). Radiograph of left obtuse marginal branch (B) shows a single CoCr-EES with multiple grade V fractures, which are highlighted in (C) (arrows=fracture sites). (D) Histologic sections of the fracture site (corresponding with [a] to [e] in panel C) showing focal restenosis. Note single stent struts (*) in the most severely narrowed section in b.
Table 3.
Six Lesions (Five Patients) with Cobalt-Chromium Everolimus-Eluting Stent Fracture
| Case | Age (yrs) | Sex | Vessel | Stent type | Stent length (mm) | Indication of stenting | Lesion calcification | Duration of implant (days) | Cause of death | Stent outcome | Number of fracture site (non-linear link/crown) | Fracture grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | RCA (prox) | Xience V x2 + Taxus Ex x1 | 30 | Stable CAD | Severe | 180 | SRD (LST) | LST at non-FS | 2 (2/0) | II |
| 2 | 56 | M | LAD (prox) | Xience V x1 + ML Vision x2 | 45 | Stable CAD | None | 80 | NCD (ARDS, vasculitis) | Restenosis at non-FS | 1 (1/0) | I |
| 3 | 51 | M | LOM (dist) | Xience V x1 | 28 | Stable CAD | Mild | 101 | SRD (restenosis with diffuse CAD) | Restenosis at FS | 9 (9/0) | V |
| 4 | 82 | M | SVG (prox) | Xience V x1 | 18 | ACS | Mild | 360 | NSRCD (diffuse CAD) | Patent | 1 (1/0) | I |
| 5* | 70 | F | LM-LCX (prox) | Xience V x2 | 28 | ACS | Severe | 167 | SRD (restenosis) | Restenosis at FS | 4 (1/3) | III |
| 70 | F | RCA (dist) | Xience V x1 | 12 | ACS | Severe | 167 | SRD (restenosis) | Restenosis at FS | 3 (2/1) | III |
Previously published case (Foerst JR, et al. JACC. Cardiovascular Interventions 2012;5: 239–242).
ARDS=acute respiratory distress syndrome; Ex=Express; FS=fracture site; LOM=left obtuse marginal branch; LST=late stent thrombosis; ML=Multi-Link, NCD=noncardiac death; NSRCD=nonstent-related cardiac death; SRD=stent-related death; SVG=saphenous vein graft. Other abbreviations as in Table 1.
Discussion
The principal findings of the current autopsy study are: (1) CoCr-EES showed a lower frequency of LST/VLST with less uncovered struts, less inflammation (with no hypersensitivity), and less fibrin deposition as compared to SES and PES in humans; (2) greater strut coverage in CoCr-EES versus SES and PES was consistently identified irrespective of lesion characteristics and indications for stenting; (3) neointimal thickness in CoCr-EES was similar to SES and PES, and progressively increased with time; (4) CoCr-EES showed presence of neoatherosclerosis, the frequency of which was comparable to SES and PES; and (5) overall stent fracture was less frequent in CoCr-EES versus SES, whereas the prevalence of fracture-related adverse events (restenosis and thrombosis) in CoCr-EES did not differ from SES and PES. To our knowledge, the current study represents the first report of the pathology of CoCr-EES as compared to SES and PES in human coronary arteries.
Poor strut coverage has been shown to be the best predictor of LST/VLST following 1st-generation DES placement.1 In this regard, fewer uncovered struts in CoCr-EES likely contributed to a lower frequency of LST/VLST, which could be associated with improved DES components. In vivo and ex vivo experimental studies have shown that thin stent struts are associated with less flow disturbance, greater endothelial cell coverage, and less thrombogenicity, as compared to thick stent strut.20, 21 Fluorinated copolymer of CoCr-EES consists of vinylidene fluoride and hexafluoropropylene, which is clinically used for permanent surgical sutures and thus proven to be biocompatible.9 In addition, fluorinated copolymer coating has been shown in ex vivo studies to be thromboresistant as compared to CoCr BMS (Multi-Link Vision).21 Complex lesion characteristics and underlying unstable plaque morphologies are associated with greater delayed healing following 1st-generation DES placement as compared to simple and stable CAD lesions.3, 4 The current study demonstrated greater strut coverage in CoCr-EES versus SES and PES regardless of lesion characteristics, which is in line with several registry-based studies and pooled analysis from randomized clinical trials showing the safety and efficacy of CoCr-EES versus 1st-generation DES in patients with ACS, long and/or small vessel lesions, and unprotected left main coronary disease.22–25 A recent randomized comparison of CoCr-EES and BMS in patients with ST-elevation AMI showed a significantly lower incidence of TLR and stent thrombosis in CoCr-EES versus BMS at 1 year following stent implantation.26 Although the long-term follow-up for safety and efficacy of CoCr-EES in these settings are still needed, our pathologic findings support greater clinical safety of CoCr-EES versus 1st-generation DES for “off-label” indications.
Hypersensitivity vasculitis with eosinophils and T-lymphocytes has been shown to be an important pathologic etiology of LST/VLST in SES, which is likely a response to the polymer rather than the drug.4 Reduced inflammation without hypersensitivity vasculitis in CoCr-EES could be attributed to greater biocompatibility of the fluorinated copolymer, although limited sample size in the current study must be taken into consideration. In porcine coronary model, CoCr-EES showed similar or even greater inflammation as compared to SES at 28 and 90 days following stent placement; however, decreasing inflammatory response was observed in CoCr-EES over time while SES showed escalating amount of inflammation up to 1 year.27 On the other hand, malapposition with excessive fibrin deposition is known to be associated with PES thrombosis.4 Considering that a cytotoxic drug paclitaxel showed a dose-dependent increase in fibrin deposition in preclinical animal models,28 reduced fibrin deposition in CoCr-EES might be partly attributable to optimal dose of a cytostatic drug everolimus and its better release kinetics. Clinical imaging studies have demonstrated that VLST following 1st-generation DES placement is associated with late acquired malapposition and positive vessel remodeling.29 In the current study, reduced inflammation and less fibrin deposition in CoCr-EES was accompanied by a lower frequency of malapposition, which may contribute to the decrease in LST/VLST. CoCr-EES failed to show a reduction in the prevalence of neoatherosclerosis versus SES and PES in this study population, although the morphology of neoatherosclerosis in CoCr-EES was characterized mostly by foamy macrophage infiltration in comparison to SES. It has been reported that 1st-generation DES develop neoatherosclerosis rapidly and more frequently as compared to BMS, where no neoatherosclerosis was identified in BMS implanted for ≤2 years.5 It is believed that accelerated neoatherosclerosis in 1st-generation DES might be secondary to incompetent re-generated endothelium with poor cell-to-cell junctions that characterize impaired endothelial barrier function.5, 30 In rabbit iliac arteries, CoCr-EES showed greater expression of platelet endothelial cell adhesion molecule-1 (PECAM-1), a transmembrane protein, versus SES and PES at 14 days following stent implantation.30 However, all DES showed decreased expressions of PECAM-1 and antithrombotic cofactor thrombomodulin as compared to BMS, which may at least partly indicate that endothelial maturation is still insufficient in CoCr-EES as compared to BMS.30
Differential stent design, distortion or acquired under-expansion, along with straightening of the artery and motion, enhance the possibility of fracture. The incidence of CoCr-EES fracture as clinically assessed by fluoroscopy or intravascular ultrasound has been recently reported to be 2.9%, where lesions with fracture versus those without fracture showed significantly greater prevalence of TLR (25.6% versus 2.0%) and stent thrombosis (5.1% versus 0.4%) at 9 months following stent placement.31 The higher observed frequency of CoCr-EES fracture in our autopsy lesions (13%) versus the clinical study could be in part explained by the superior resolution of the high-contrast film-based radiography (80 μm) versus fluoroscopy (300 μm) or intravascular ultrasound (200 μm), or may represent the very selected sample enriched with increased stent-related adverse events in the current study. Our radiographic analysis demonstrated that the majority of CoCr-EES fracture occurs in the non-linear link, which are intended to provide greater flexibility and conformability to the stents but at the same time could be a nidus for fatigue fracture, which may induce thrombosis or restenosis. Stent design, type of metal, and conformability of the stent to the artery curvature all contribute to stent fracture, therefore a more sensitive assessment may be needed by designing a more strenuous and clinically relevant method such as bending fatigue test rather than radial pulsatile fatigue testing that is required by the FDA.
Considering that neoatherosclerosis develops and progresses over time, similarly the frequency of stent fracture increases with advancing duration of implant probably due to continuous stress on the stents and metal fatigue,5, 8 contribution of these factors to vascular complications are required at later time point. Progressive increase in neointimal thickness together with substantial prevalence of neoatherosclerosis and fracture-related restenosis in CoCr-EES observed in the current study indicate that careful long-term follow-up is still required even after CoCr-EES placement, and further improvement in stent technologies are needed to overcome these issues.
Study Limitations
There is an inherent bias in studies involving an autopsy population with a relatively greater number of patients dying from DES complications as compared to clinical studies which have a defined large population of living patients. Nevertheless, clinical studies lack the precise nature of the complication as resolution of imaging modalities are limited and the nature of the defect at the time of death usually cannot be studied and are only surmised from history. Our study population consisted of consecutive lesions with DES from the all-comer autopsy registry where the duration of implant could not be completely matched, although the dependence of vascular responses on duration of implant was corrected with multiple regression analyses. In the current study, detailed clinical information including risk factors and dual antiplatelet therapy were available only in limited number of cases. Although greater stent healing in CoCr-EES versus 1st-generation DES were consistently observed across different lesion characteristics and duration of implant, how these finding can be extrapolated to living patients is difficult to ascertain but may be linked. The present study did not show direct evidence for linking the development of neoatherosclerosis with late vascular complications in CoCr-EES, because the duration of implant was relatively short (median=200 days). A substantial number of the autopsy cases come from noncardiac or nonstent-related cardiac deaths and the relationship of the pathologic findings observed in those lesions to clinical events cannot be determined.
Conclusions
CoCr-EES as compared to SES and PES showed less LST/VLST with fewer uncovered struts, less inflammation (with no hypersensitivity reaction), and less fibrin deposition in humans, where greater strut coverage of CoCr-EES was consistently observed irrespective of lesion characteristics and indications for stenting. Our results support greater clinical safety of CoCr-EES as compared to 1st-generation DES, whereas the importance of long-term clinical follow-up should also be emphasized with appropriate tools to investigate outcomes since progressive neointimal growth with similar frequency of neoatherosclerosis and fracture-related adverse events were observed even after CoCr-EES placement.
Clinical Perspective Summary.
Clinical trials have demonstrated that second-generation cobalt-chromium everolimus-eluting stent (CoCr-EES) is superior to first-generation paclitaxel-eluting stent (PES) and is non-inferior or superior to sirolimus-eluting stent (SES) in terms of safety and efficacy. However, histologic vascular responses to CoCr-EES versus SES and PES have not been reported in humans. The current autopsy study for the first time demonstrated that CoCr-EES exhibit significantly greater strut coverage with less inflammation (with no case of hypersensitivity) and fibrin deposition, and a decrease in late stent thrombosis as compared to first-generation drug-eluting stents (DES) in humans. In addition, greater strut coverage in CoCr-EES versus SES and PES was consistently observed irrespective of lesion characteristics and indications for stenting. These findings support greater clinical safety of CoCr-EES versus first-generation DES, even for “off-label” indications. On the other hand, the current study also revealed that CoCr-EES showed progressive neointimal growth up to 3 years, which was similar to first-generation DES. Moreover, the prevalence of neoatherosclerosis and fracture-related adverse events (restenosis or thrombosis) in CoCr-EES were comparable to SES and PES. It is believed that neoatherosclerosis develops and progresses over time, and similarly the frequency of stent fracture increases with advancing duration of implant due to continuous stress on the stents and metal fatigue. The current pathologic findings indicate that careful long-term follow-up is still required even after CoCr-EES placement.
Acknowledgments
Funding Sources: CVPath Institute Inc., Gaithersburg, Maryland, USA provided full support for this work. Dr. Otsuka is supported by a research fellowship from the Uehara Memorial Foundation, Tokyo, Japan.
Footnotes
Conflict of Interest Disclosures: Dr. Virmani receives research support from Abbott Vascular, BioSensors International, Biotronik, Boston Scientific, Medtronic, MicroPort Medical, OrbusNeich Medical, SINO Medical Technology, and Terumo Corporation; has speaking engagements with Merck; receives honoraria from Abbott Vascular, Boston Scientific, Lutonix, Medtronic, and Terumo Corporation; and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. Dr. Finn is supported by the NIH grant HL096970-01A1, the American Heart Association, the Woodruff Sciences Health Center and Carlyle Fraser Heart Center both at Emory University, sponsored research agreement with Medtronic and Boston Scientific, and is a consultant for Medtronic. Dr. Sakakura has received speaking honorarium from Abbott Vascular, Boston Scientific, and Medtronic CardioVascular. The other authors report no conflicts.
References
- 1.Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- 2.Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Iida O, Sera F, Nanto S, Hori M, Nagata S. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116:910–916. doi: 10.1161/CIRCULATIONAHA.105.609057. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–1145. doi: 10.1161/CIRCULATIONAHA.107.762047. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57:390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants: bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popma JJ, Tiroch K, Almonacid A, Cohen S, Kandzari DE, Leon MB. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol. 2009;103:923–929. doi: 10.1016/j.amjcard.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Umeda H, Gochi T, Iwase M, Izawa H, Shimizu T, Ishiki R, Inagaki H, Toyama J, Yokota M, Murohara T. Frequency, predictors and outcome of stent fracture after sirolimus-eluting stent implantation. Int J Cardiol. 2009;133:321–326. doi: 10.1016/j.ijcard.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 8.Nakazawa G, Finn AV, Vorpahl M, Ladich E, Kutys R, Balazs I, Kolodgie FD, Virmani R. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol. 2009;54:1924–1931. doi: 10.1016/j.jacc.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 9.Kukreja N, Onuma Y, Serruys PW. Xience V everolimus-eluting coronary stent. Expert Rev Med Devices. 2009;6:219–229. doi: 10.1586/erd.09.1. [DOI] [PubMed] [Google Scholar]
- 10.Smits PC, Kedhi E, Royaards KJ, Joesoef KS, Wassing J, Rademaker-Havinga TA, McFadden E. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial) J Am Coll Cardiol. 2011;58:11–18. doi: 10.1016/j.jacc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, Maddux JT, Cutlip DE, Simonton CA, Sood P, Kereiakes DJ. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol. 2011;58:19–25. doi: 10.1016/j.jacc.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Jensen LO, Thayssen P, Hansen HS, Christiansen EH, Tilsted HH, Krusell LR, Villadsen AB, Junker A, Hansen KN, Kaltoft A, Maeng M, Pedersen KE, Kristensen SD, Botker HE, Ravkilde J, Sanchez R, Aaroe J, Madsen M, Sorensen HT, Thuesen L, Lassen JF. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV) Circulation. 2012;125:1246–1255. doi: 10.1161/CIRCULATIONAHA.111.063644. [DOI] [PubMed] [Google Scholar]
- 13.Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, Tanabe K, Morino Y, Akasaka T, Takatsu Y, Nishikawa H, Yamamoto Y, Nakagawa Y, Hayashi Y, Iwabuchi M, Umeda H, Kawai K, Okada H, Kimura K, Simonton CA, Kozuma K. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting versus Everolimus-eluting stent Trial (RESET) Circulation. 2012;126:1225–1236. doi: 10.1161/CIRCULATIONAHA.112.104059. [DOI] [PubMed] [Google Scholar]
- 14.Räber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, Wenaweser P, Daemen J, Meier B, Juni P, Serruys PW, Windecker S. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–1121. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 15.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, Kimura T, Briguori C, Sabate M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 16.Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, Bhatt DL, Slater J. Short-and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 17.Inoue T, Shite J, Yoon J, Shinke T, Otake H, Sawada T, Kawamori H, Katoh H, Miyoshi N, Yoshino N, Kozuki A, Hariki H, Hirata K. Optical coherence evaluation of everolimus-eluting stents 8 months after implantation. Heart. 2011;97:1379–1384. doi: 10.1136/hrt.2010.204339. [DOI] [PubMed] [Google Scholar]
- 18.Marroquin OC, Selzer F, Mulukutla SR, Williams DO, Vlachos HA, Wilensky RL, Tanguay JF, Holper EM, Abbott JD, Lee JS, Smith C, Anderson WD, Kelsey SF, Kip KE. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358:342–352. doi: 10.1056/NEJMoa0706258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 20.Simon C, Palmaz JC, Sprague EA. Influence of topography on endothelialization of stents: clues for new designs. J Long Term Eff Med Implants. 2000;10:143–151. doi: 10.1615/jlongtermeffmedimplants.v10.i12.120. [DOI] [PubMed] [Google Scholar]
- 21.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planer D, Smits PC, Kereiakes DJ, Kedhi E, Fahy M, Xu K, Serruys PW, Stone GW. Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (A Trial of Everolimus-Eluting Stents and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice) Trials. JACC Cardiovasc Interv. 2011;4:1104–1115. doi: 10.1016/j.jcin.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Kalesan B, Stefanini GG, Raber L, Schmutz M, Baumgartner S, Hitz S, Baldinger SH, Pilgrim T, Moschovitis A, Wenaweser P, Bullesfeld L, Khattab AA, Meier B, Juni P, Windecker S. Long-term comparison of everolimus- and sirolimus-eluting stents in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2012;5:145–154. doi: 10.1016/j.jcin.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Claessen BE, Smits PC, Kereiakes DJ, Parise H, Fahy M, Kedhi E, Serruys PW, Lansky AJ, Cristea E, Sudhir K, Sood P, Simonton CA, Stone GW. Impact of lesion length and vessel size on clinical outcomes after percutaneous coronary intervention with everolimus- versus paclitaxel-eluting stents pooled analysis from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) and COMPARE (Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice) Randomized Trials. JACC Cardiovasc Interv. 2011;4:1209–1215. doi: 10.1016/j.jcin.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Valenti R, Migliorini A, Parodi G, Carrabba N, Vergara R, Dovellini EV, Antoniucci D. Clinical and angiographic outcomes of patients treated with everolimus-eluting stents or first-generation Paclitaxel-eluting stents for unprotected left main disease. J Am Coll Cardiol. 2012;60:1217–1222. doi: 10.1016/j.jacc.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A, Vazquez N, Gomez-Hospital JA, Baz JA, Martin-Yuste V, van Geuns RJ, Alfonso F, Bordes P, Tebaldi M, Masotti M, Silvestro A, Backx B, Brugaletta S, van Es GA, Serruys PW. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa G, Finn AV, Ladich E, Ribichini F, Coleman L, Kolodgie FD, Virmani R. Drug-eluting stent safety: findings from preclinical studies. Expert Rev Cardiovasc Ther. 2008;6:1379–1391. doi: 10.1586/14779072.6.10.1379. [DOI] [PubMed] [Google Scholar]
- 28.Farb A, Heller PF, Shroff S, Cheng L, Kolodgie FD, Carter AJ, Scott DS, Froehlich J, Virmani R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation. 2001;104:473–479. doi: 10.1161/hc3001.092037. [DOI] [PubMed] [Google Scholar]
- 29.Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115:2426–2434. doi: 10.1161/CIRCULATIONAHA.106.658237. [DOI] [PubMed] [Google Scholar]
- 30.Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–342. doi: 10.1016/j.jacc.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Kuramitsu S, Iwabuchi M, Haraguchi T, Domei T, Nagae A, Hyodo M, Yamaji K, Soga Y, Arita T, Shirai S, Kondo K, Ando K, Sakai K, Goya M, Takabatake Y, Sonoda S, Yokoi H, Toyota F, Nosaka H, Nobuyoshi M. Incidence and clinical impact of stent fracture after everolimus-eluting stent implantation. Circ Cardiovasc Interv. 2012;5:663–671. doi: 10.1161/CIRCINTERVENTIONS.112.969238. [DOI] [PubMed] [Google Scholar]