Abstract
Purpose: Nearly all of flavonoids are good metal chelators and can chelate many metal ions to form different complexes. This article describes a synthesis of Quercetin–Tb(III) in methanol, characterized by using elemental analysis, UV–visible and evaluation of its antioxidant properties.
Methods: The formation of complexes is realized from the UV–visible spectra which shows that the successive formation of Quercetin–Tb(III) occurs. To find out the antioxidant activity variation and the role of Tb(III) ion on the antioxidant activity of the complexes different radical scavenging methods such as: 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and 2,2′-azinobis 3-ethylbenzothiazoline-6-sulphonic acid (ABTS) were used.
Results: The results from DPPH, ABTS and FRAP methods showed that Quercetin and Quercetin–Tb(III) complex are capable of donating electron or hydrogen atom, and consequently could react with free radicals or terminate chain reactions in a time- and dose-dependent manner.
Conclusion: This study showed that the chelation of metal ions by Quercetin decrease the redox potential of Quercetin-metal complex.
Keywords: Flavonoid, Antioxidant, Quercetin–Tb(III) complex, DPPH, FRAP, ABTS
Introduction
Flavonoids are antioxidants, which are recognized to affect bio-availability of the metal in the body. They have a basic structure of 2-phenyl-benzo-γ-pyrones, frequently polyphenolic in nature. A large number of substitution patterns in the two benzene rings (A and B) of the basic structure occur in nature. Variations in their heterocyclic rings give rise to flavonols, flavones, catechins, flavanones, anthocyanidins and isoflavones.1-4
Flavonoids, and particularly quercetin derivatives, have received more attention as dietary constituents during the last few years. Experimental studies showed that they have frequent beneficial effects on human health, including cardiovascular protection, anticancer activity, antiulcer effects, and antiallergic, antiviral, and anti-inflammatory properties. These health-promoting activities seem to be related to the natural antioxidant (free-radical scavenging) activity of flavonoids.2
Reactive oxygen species (ROS) induce oxidative damage to biomolecules and organelles, and then lead to many diseases like Parkinson‘s disease, heart disease, and cancer. Researches demonstrated that exogenous antioxidants or free radical scavengers can scavenge the excess free radicals and be benefic to those diseases effectively. Thus, evaluating and developing novel antioxidant is becoming one of the noteworthy topics recently.2,5 Most of flavonoids are strong metal chelators which can chelate many metal ions to form different complexes. Those complexes are reported to have various important biological activities, and most of them exhibit higher antioxidant abilities than the ligand flavonoids. However, the antioxidant mechanism of the complexes has not been exactly elucidated so far.5,6 The aim of this study, was to compare the efficiency of DPPH, FRAP and ABTS assays to estimate antioxidant activities of Quercetin–Tb(III) complex and to find the role of Tb(III) ion on the antioxidant activity of Quercetin.
Materials and Methods
Chemical and Materials
All reagents used for experiments were analytical reagent grade. Extra pure methanol was purchased from Scharlau chemical company. Quercetin and DPPH (2,2-diphenyl-1-picrylhydrazyl) was purchased from Sigma.
The standard solutions at 1.0 × 10−3 M concentration of antioxidant compounds were all prepared in 100% MeOH. All working solutions of antioxidant compounds were freshly prepared.
Instrumentation
Spectroscopic study of Quercetin and its metal complex was performed using analytic jena UV-visible spectrophotometer specord 40 for obtaining UV spectra.
Synthesis of the complex
The synthesis of the Quercetin–Tb(III) complex had been carried out according to our previous work.6 Briefly, in a 50-cm3 two-necked round-bottomed flask equipped with an electromagnetic stirrer and thermometer, quercetin·2H2O (0.05 g, 0.008 mol) dissolved in MeOH (20 ml) within 15 min, the color of the solution was light yellow. Consequently, TbCl3.6H2O (0.12 g, 0.016mol) was added quickly in the reaction mixture, now the color of the solution was brownish yellow and the solution was stirred at room temp for 4 h. After stirring the reaction mixture was filtered, and the filtrate was evaporated slowly at room temperature. The resulting dark brownish yellow product was washed with t-butanol and dried in a vacuum desiccator. A brownish yellow product, Quercetin–Tb(III) complex, was obtained in 82% yield.2,6-8
Antioxidant activity of the complex by DPPH method
Free radical scavenging capacity of the complex was determined by previously reported procedure using the stable 2,2-diphenyl-1-picrylhydrazylradical (DPPH•).1,9 Methanol solution (0.1 ml) containing different concentrations of standards (0, 5, 10, 15, 20, 25 µmol) was added to 1.95 ml of freshly prepared (57.65µmol) DPPH in methanol. The reduction of the DPPH was followed by monitoring the decrease in absorbance at 517 nm in each 5 min for about 25 min (AS). As a control, the absorbance of blank solution of DPPH (2 ml) was also determined at 515 nm (AC). The following equation has been used for the calculation of the percentage of radical scavenging activity (RSA%):1,9
FRAP assay of total antioxidant capacity
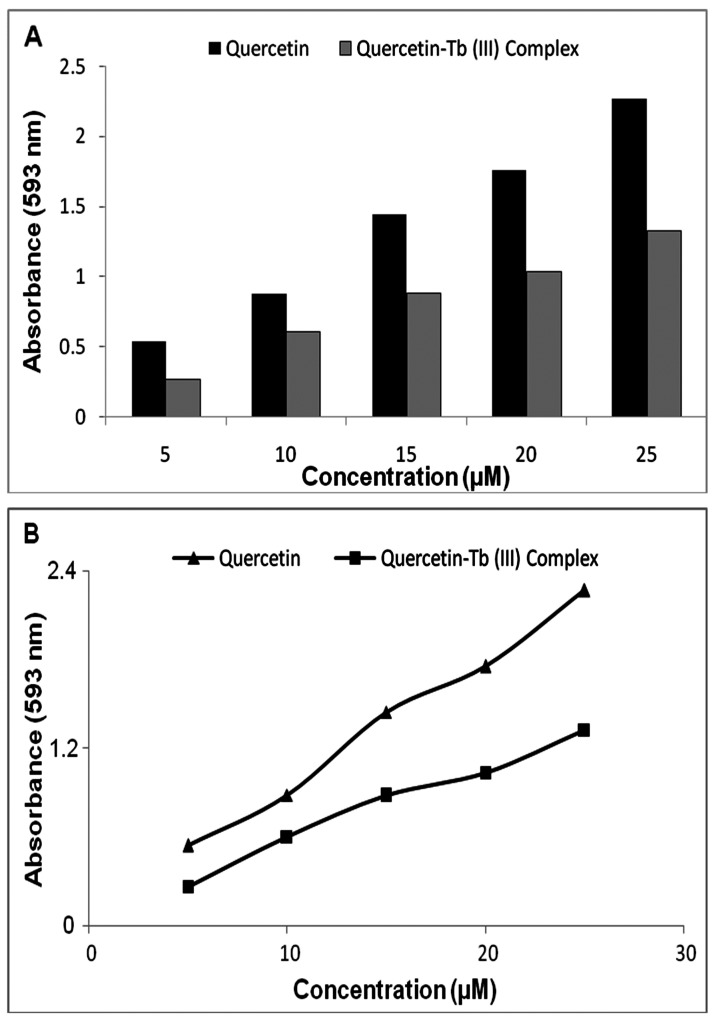
FRAP assay was carried out by the method of Benzie and Strain with minor modifications. The method is based on the reduction of a ferric 2,4,6-tripyridyl-s triazine complex (Fe3+-TPTZ) by antioxidants to the ferrous form (Fe2+-TPTZ). FRAP reagent was prepared freshly by mixing 2.5 ml of solutions TPTZ (10 mM, dissolved in 40 mM HCl) and FeCl3 (20 mM) in 25 ml of acetate buffer (300 mM concentration and pH 3.6), the light blue reagent contains Fe3+–TPTZ that changes to dark blue after interaction with antioxidants, which is clarified by the presence of Fe2+–TPTZ in the reagent. These changes were due to the absorbance increase as monitored at a wavelength of 593 nm for different concentrations (5, 10, 15, 20 and 25 mM) of the Quercetin and Quercetin–Tb(III) complex in FRAP reagent. The standard calibration curve obtained by using different concentrations of FeSO4·7H2O as standard for calculation of the FRAP values for both Quercetin and complex.9
ABTS radical scavenging activity of Quercetin and Quercetin–Tb(III) complex
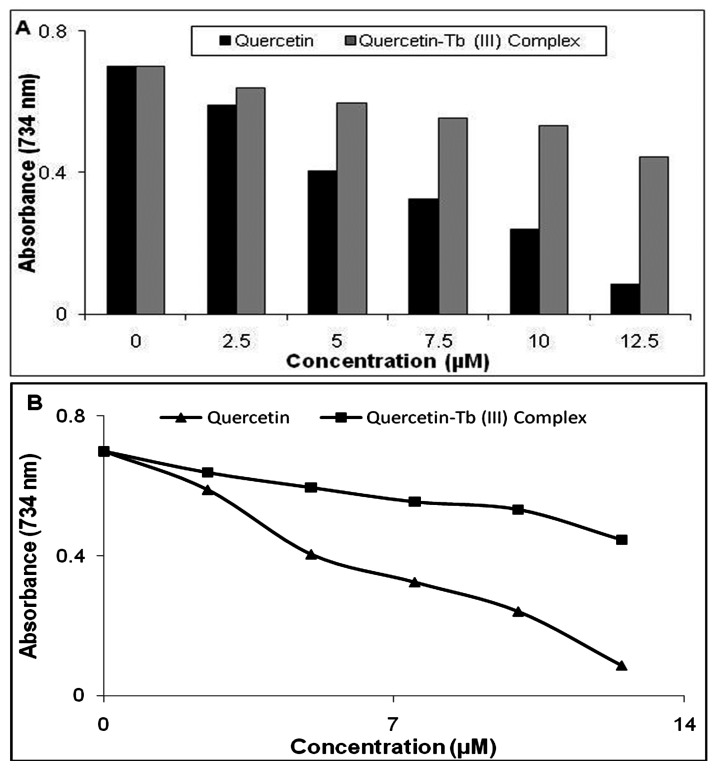
ABTS radical scavenging activity was based on the method of Dehghan et al. Briefly, 54.2 mg of ABTS powder was dissolved in 10 ml of phosphate buffer (5 mM, pH 7.0) and then mixed with 1 g of MnO2 and incubated in room temperature within 30 min for generation of green colored ABTS+. After that the prepared solution was centrifuged for 5 min and after filtration; the filtrate was diluted with phosphate buffer until the absorbance of solution equals with 0.70 ± 0.01 in 734 nm. Different concentrations (0–12.5 μM) of Quercetin and its Quercetin–Tb(III) complex were mixed with 2 ml of ABTS solution and incubated for 10 min at room temperature. The decrease of absorbance was monitored at 734 nm after 10 min.9
Results
Interaction of quercetin with Tb(III)
The changes in UV–vis absorption of Quercetin in the presence of Tb(III) were examined in the methanol solution. The UV–vis spectra of Quercetin showed an intense absorbance at 280 and 372 nm. When the solution of Tb(III) was added, decrease in absorption observed. The results indicated formation of a complex between Quercetin and Tb(III) (Figure 1).1,6,10
Figure 1 .
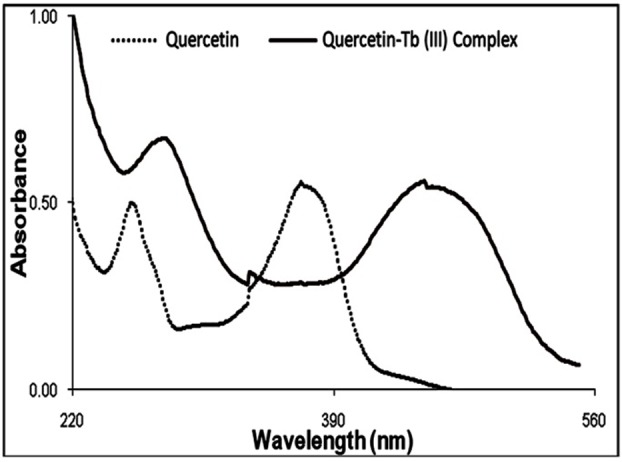
UV-vis spectrum of Quercetin and Quercetin-Tb (III) complex in methanol.
DPPH radical scavenging analysis
The results of DPPH radical scavenging analysis can be seen in Figure 2. Quercetin and Quercetin–Tb(III) complex can scavenge DPPH radical effectively. The scavenging activity of Quercetin–Tb(III) complex is obviously less than that of Quercetin, indicating that this complex is a much weaker free radical scavenger and antioxidant than Quercetin.1,10 Radical scavenging activity of Quercetin was increased in a dose- and time-dependent manner, at the same time, antioxidant ability of Quercetin decreased after chelation of stannous cation (Figure 3). The antioxidant activity of flavonoids was owing to their molecular structure. The higher antioxidant activity of Quercetin may be because of the highly contribution of the 3ʹ, 4ʹ hydroxyl groups on the B-ring. Besides, it may be supposed that 3ʹ, 4ʹ hydroxyl groups are mainly involved in H-atom transfer reactions to DPPH.
Figure 2 .
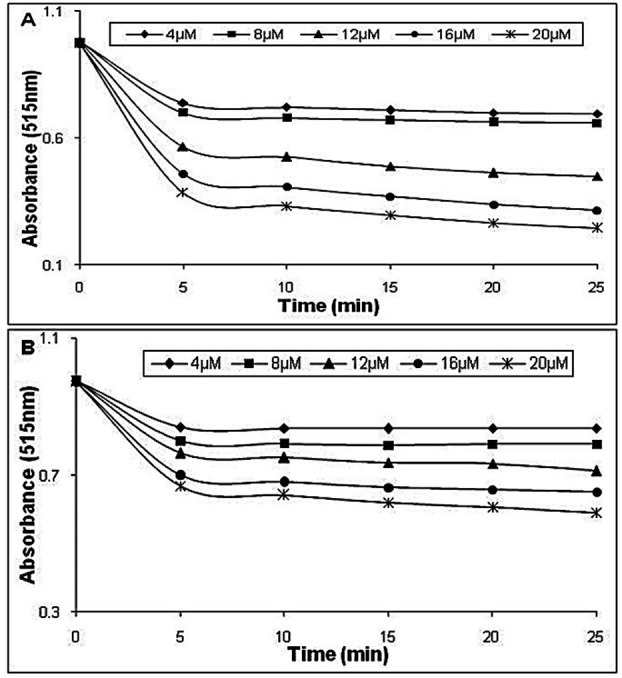
Decrease in absorbance at (λ=517 nm) of DPPH methanol solution in the presence of different concentrations of (A) Quercetin and (B) Quercetin-Tb (III) complex (0–20µM).
Figure 3 .
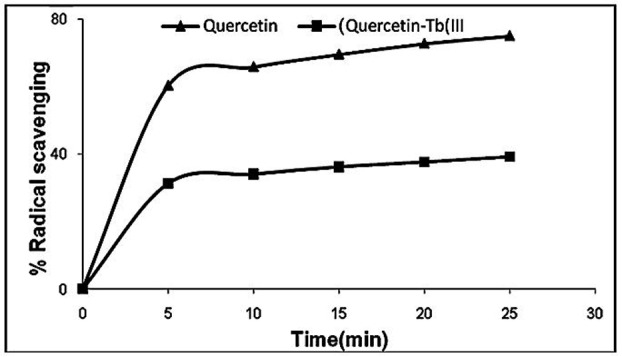
Dependence of the ligand Quercetin and Quercetin-Tb(III) complex concentration in the DPPH, scavenging activities evaluated through the absorbance decrease at 517 nm caused by the addition of Quercetin and Quercetin-Tb (III) complex.
Ferric reducing antioxidant power of Quercetin and Quercetin–Tb(III) complex
Antioxidants may exert their protective effects by reduction of metal ions and therefore influencing the oxidative stress caused by these metal ions. This reduced ability can be assessed for antioxidants such as flavonoids according to the FRAP method. The ability of antioxidants to reduce Fe3+ to Fe2+ in the presence of TPTZ and forming an intense blue Fe2+–TPTZ complex with an absorption maximum at 593 nm is the basic theory of the FRAP assay. The absorbance increase is proportional to the antioxidant content. Herein, the FRAP results for the Quercetin and Quercetin–Tb(III) complex were measured via absorbance variations during 10 min of interaction of subjected compounds with FRAP reagent and the relevant data are shown in Figure 3, which again confirm the decline of antioxidant power of the resultant complex in comparison with free Quercetin. These findings propose that Quercetin and Quercetin–Tb(III) complex are capable of donating electrons, and could therefore react with free radicals or terminate chain reactions, whereas metal chelation reduces electron transfer from Quercetin after complex formation. It is reasonable that the chelation of metal ions by Quercetin decreases the redox potential of metal-Quercetin complex.9
ABTS radical scavenger activity of Quercetin and Quercetin–Tb(III) complex
For confirming the anti-radical potential of the synthesized complex, we used the ABTS assay as well. The effect of Quercetin and Quercetin–Tb(III) complex on ABTS radical is shown in Figure 4. Absorption of active ABTS solution at 734 nm obviously decreased in the presence of different concentrations of both Quercetin and Quercetin–Tb(III) complex (Figure 5). While, similar to previous methods, radical scavenging activity of free Quercetin was better than Quercetin–Tb(III) complex.
Figure 4 .
Time dependent ferric reducing antioxidant power (mmolFe2+/L) of Quercetin and the Quercetin-Tb (III) complex.
Figure 5.
Decrease in absorbance at (λ=734 nm) of ABTS at different concentrations of Quercetin and the Quercetin-Tb (III) complex.
Discussion
Antioxidant property of various flavonoids is more considerably related to their molecular structure. Hydrogen atom transferring and electron donation are two major mechanisms through which phenolic compounds can exert their antioxidative functions. Pure Quercetin showed more antioxidant activity in comparison with the Quercetin–Tb(III) complex. The higher antioxidant activity of the Quercetin may be due to the considerable contribution of the hydroxyl groups that their hydrogens are replaced by Tb (III), therefore decreasing their ability for hydrogen donation or radical scavenging of Quercetin.
It can be concluded that a radical inhibitory and metal reducing activity of Quercetin was decreased after chelation of cation, taking into consideration three methods: DPPH, ABTS radical scavenging activities and FRAPS assays. Therefore, this study proposed that metal ions significantly alter the chemical properties of Quercetin and influence its antioxidant activity. Also the results arisen from DPPH, FRAP and ABTS assays showed that Quercetin and Quercetin–Tb(III) complex can scavenge free radicals or reduce Fe3+ in a concentration- and time- dependent manner.
Conclusion
The complex of Tb(III) with Quercetin was prepared and characterised by several spectroscopic techniques. Using UV–vis the coordination to the carbonyl group of the ligand and one of the adjacent hydroxyl groups is assumed. Spectroscopic data suggest that Quercetin molecule can chelate lanthanide cations such as Tb from both 3-hydroxy-carbonyl and the 3′, 4′-dihydroxyl (catechol) chelation sites. The results from DPPH, ABTS and FRAP methods demonstrated that Quercetin and Quercetin–Tb(III) complex are capable of donating electron or hydrogen atom, and consequently could react with free radicals or terminate chain reactions in a time- and dose-dependent manner. The results of this study showed that the chelation of metal ions by Quercetin decrease the redox potential of Quercetin-metal complex and the metal ions Tb(III) considerably change the chemical properties of the Quercetin.
Acknowledgments
The Authors are grateful for financial support from the Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences and University of Tabriz.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Bukhari SB, Memon S, Mahroof-Tahir M, Bhanger MI. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim Acta A Mol Biomol Spectrosc . 2009;71(5):1901–6. doi: 10.1016/j.saa.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi SM, Dehghan G, Hosseinpourfeizi MA, Dolatabadi JE, Kashanian S. Preparation, characterization, and DNA binding studies of water-soluble quercetin--molybdenum(VI) complex. DNA Cell Biol . 2011;30(7):517–23. doi: 10.1089/dna.2010.1205. [DOI] [PubMed] [Google Scholar]
- 3.Jamali A, Tavakoli A, Ezzati Nazhad Dolatabadi. Analytical overview of DNA interaction with Morin and its metal complexes. Eur Food Res Technol . 2012;235:367–73. [Google Scholar]
- 4.Hu YJ, Yue HL, Li XL, Zhang SS, Tang E, Zhang LP. Molecular spectroscopic studies on the interaction of morin with bovine serum albumin. J Photochem Photobiol B . 2012;112:16–22. doi: 10.1016/j.jphotobiol.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen WJ, Sun SF, Cao W, Liang Y, Song JR. Antioxidant property of quercetin-Cr(III) complex: The role of Cr(III) ion. J Mol Struct . 2009;918:194–7. [Google Scholar]
- 6.Dehghan G, Dolatabadi JE, Jouyban A, Zeynali KA, Ahmadi SM, Kashanian S. Spectroscopic studies on the interaction of quercetin-terbium(III) complex with calf thymus DNA. DNA Cell Biol . 2011;30(3):195–201. doi: 10.1089/dna.2010.1063. [DOI] [PubMed] [Google Scholar]
- 7.Xie WL, Yang PH, Cai JY. Synthesis, Characterization and Antioxidation Activity of Germanim(IV)-Quercetin Complex. Chinese J Anal Chem . 2010;38:1809–12. [Google Scholar]
- 8.Cornard JP, Merlin JC. Spectroscopic and structural study of complexes of quercetin with Al(III) J Inorg Biochem . 2002;92(1):19–27. doi: 10.1016/s0162-0134(02)00469-5. [DOI] [PubMed] [Google Scholar]
- 9.Dehghan G, Khoshkam Z. Tin(II)-quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem . 2012;131:422–6. [Google Scholar]
- 10.Zhang GW, Guo JB, Pan JH, Chen XX, Wang JJ. Spectroscopic studies on the interaction of morin-Eu(III) complex with calf thymus DNA. J Mol Struct . 2009;923(1-3):114–9. [Google Scholar]