Abstract
Purpose: Oxidative stress and renin- angiotensin system are both involved in the pathophysiology of most of the systemic and central disorders as well as in aging. Angiotensin converting enzyme (ACE) inhibitors, well known for their cardiovascular beneficial effects, have also shown antioxidant properties in pathologic conditions. This study aimed to evaluate the central effect of ACE inhibitors on oxidative status under no pathologic condition.
Methods: Adult male rats were divided into four groups of 9 rats each. Groups were treated orally by perindopril at the doses of 1, 2, 4 mg/kg/day or normal saline as the control for four consecutive weeks. At the end of the treatment period the reduced and oxidized glutathione (GSH and GSSG respectively) and malondialdehyde (MDA), the product of lipid peroxidation, were measured in the rats’ hippocampus.
Results: The GSH increased dose dependently and was significantly higher in the 2 mg/kg perindopril treated group than the control group (p<0.05) while the GSSG level remained unchanged. As a consequent, the ratio of GSH to GSSG increased significantly in a dose dependent manner. There was not any significant change in MDA.
Conclusion: This study demonstrated that ACE inhibition may cause an increase in GSH as an anti- oxidant defense in the hippocampus.
Keywords: ACE inhibitor, Rat, Perindopril, Glutathione, Hippocampus
Introduction
Free radicals generated mainly from mitochondria induce oxidative stress, being involved in pathogenesis of several organ diseases. In the central nervous system, oxidative stress was found to be associated with a lot of brain disorders such as, depression,1 mental disorders due to severe life stress,2 Parkinson’s and Alzheimer’s diseases.3,4 In addition, oxidative stress is a well-documented cause of tissue damages leading to aging.5 Oxidative stress also hypothesized to lie in the upstream of neurodegenerative processes ending in Parkinson’s and Alzheimer’s diseases during normal life time.6
Angiotensin converting enzyme (ACE) inhibitors are common drugs used in treatment of hypertension and heart failure. They reduced high blood pressure and vascular resistance by decreasing angiotensin II (Ang II) production.7 Due to the existence of a unique renin-angiotensin system in the brain, ACE inhibitors have recently revealed neuroprotective effects in the central nervous system disorders including neurodegenerative diseases.8-10 In some tissues including the brain, it has been found that ACE inhibitors interfere with oxidative damages induced experimentally.11,12 However, to date it is not clear if the oxidative status under normal circumstances can also be affected by ACE inhibitors. Chronically administration of ACE inhibitors demonstrated through unknown mechanism an improvement in learning and memory of rats.13 Considering the unknown mechanisms of ACE on improvement of physiologic behavioral functions like learning and memory,13 together with the existence of oxidative stress in the upstream of neurodegenerative processes leading to aging,5 Alzheimer’s and Parkinson’s diseases,6 this study aimed to evaluate the basal oxidative status and anti-oxidant defense of neuronal tissue under ACE inhibition. For this purpose, we chronically administered different doses of perindopril to adult male rats and analyzed the oxidative parameters in their hippocampus.
Materials and Methods
Chemicals
Tricloroacetic acid (TCA), 2,3 thiobarbitoric acid (TBA), 1, 1, 3, 3-tetraethoxy propane (TEP), Coomassie brilliant blue G-250, bovine serum albumin (BSA), and phosphate buffer saline (PBS) tablets were purchased from Sigma- Aldrich St. Louis, MO, USA. Ellman's reagent (5-5'-dithiobis-(2-nitrobenzoic acid; DTNB), NADPH, reductase, scavenger, glutathione standard, meta phosphoric acid, and assay buffer provided by a Reduced/ Oxidized Glutathione Cuvette Assay kit # GSH39-K01 purchased from Eagle Biosciences Inc., Boston, USA. Other chemicals were commercially available.
Animals
Male Sprague–Dawley rats aging three to four months were obtained from Animal Care Center of Shiraz University of Medical Sciences. The rats were housed two per cage under controlled (12 h/12 h) light/dark cycle and constant temperature of 24±2 °C; they had free access to food and water. All experiments were carried out during the light phase and in accordance to the guideline of the animal ethics committee of Shiraz University of Medical Sciences.
Drug treatments
The rats were randomly divided into four groups containing nine animals each. Test groups received perindopril (Coversyl®, Servier, Cedex, France) at a dose of 1, 2 or 4 mg/kg/ml. Control group received normal saline at the same volume. Drugs were administered orally via an intragastric gavage tube once daily for four consecutive weeks.
Tissue preparation
The rats were decapitated under light ether anesthesia. The skull was cut open and the whole brain was quickly removed. The hippocampi were dissected and cleaned with chilled normal saline on the ice, frozen quickly in liquid nitrogen and stored separately under -70 °C for future use.
Biochemical assay
Estimation of malondialdehyde (MDA) and protein
One of the right or left hippocampus of each rat was selected randomly (n=9) and homogenized in 10% (w/v) of PBS (pH 7.4) with an ultrasonic homogenizer (BANDELIN, Sonoplus, Berlin) for 20 seconds with 3/2 seconds on/off periods. The homogenate was centrifuged at 10000 g for 20 min at 4°C and the supernatant was used for the measurement of MDA and protein. The concentration of protein in the supernatant was measured by spectrophotometer at 595 nm by the Coomassie brilliant blue G-250 dye-binding technique of Bradford14 using serial dilution of BSA (1mg/1ml) as standard.
MDA as a marker of lipid peroxidation was estimated according to its reaction with TBA as a TBA reactive substance15 with modification. Briefly, 0.5 ml of the supernatant was added to 2 ml TBA reagent containing 0.375% TBA, 15% TCA and 0.25 mol/L HCl. The mixture was boiled in a water bath at 95°C for 30 minutes and after fast cooling it was centrifuged at 8000 g for 15 min at 4°C. The absorbance of the pink color supernatant was measured at 532 nm. MDA concentration was calculated using TEP as standard and expressed as nmol/mg protein.
Glutathione analyses
The second hippocampus of the corresponding rat (n=7) was weighed precisely and homogenized the same way as that for MDA. The reduced and the oxidized glutathione content, GSH and GSSG respectively, and the ratio of GSH to GSSG were measured according to an eagle bioscience GSH/GSSG cuvette assay kit. Briefly, two samples were prepared from the homogenate, one for GSH and the second mixed with scavenger for GSSG assessment. After protein denaturation by metaphosphoric acid, the samples were centrifuged at 10000 g for 10 minutes at 4°C and the supernatant was diluted with assay buffer. The samples were then mixed with DTNB, reductase and NADPH at equal volumes (200µL) to yield a yellowish color as a result of thiol-DTNB enzymatic reaction. The absorbance was measured kinetically at 412 nm for 5 minutes. The glutathione concentrations were calculated using GSH and GSSG standard curves and the ratio of GSH/GSSG was calculated by (GSH-2*GSSG)/GSSG equation. All reagents and formulas were provided by the kit. The results were expressed as µmol/ gram wet tissue.
Data analysis
All statistical analyses were performed by SPSS version 15. Data were presented as mean ±SEM. Because of the normal distribution of data confirmed by Kolmogrov Esmirnov test; comparisons between the studied groups were made using one way ANOVA followed by Tukey’s post hoc test. The P values less than 0.05 were considered as significant.
Results
Table 1 shows the results of GSH and GSSG analyses in the hippocampus of perindopril and normal saline treated rats. The data indicate that treatment of rats with perindopril induced enhancement of GSH in a dose dependent manner [F (3, 24) = 4.34 P=0.014] that was significantly about 66.6 % higher (p<0.01) in 2 mg/kg perindopril treated group in comparison to the control rats.
Table 1. Effect of normal saline (N/S) or perindopril (P) on GSH and GSSG .
| Treatments | GSH(µMol/mg tissue) | GSSG(µMol/mg tissue) |
| N/S as control | 1.8±0.2 | 0.04±0.004 |
| P 1 mg/kg | 2.5±0.2 | 0.04±0.005 |
| P 2 mg/kg | 3±0.25* | 0.035±0.003 |
| P 4 mg/kg | 2.6±0.25 | 0.04±0.005 |
| Effect of perindopril (P 1, 2, 4 mg/kg/day orally) compared to normal saline (N/S) as controlon GSH and GSSG (n= 7). Results are expressed as mean± SEM and data were analyzed by one way ANOVA followed by Tukey multiple comparison test. * Significantly different from the control, p<0.05 | ||
On the other hand the data in Table 1 demonstrate that the GSSG level remained unchanged in all groups as compared to the control groups [F (3,24)= 0.74 P=0.538]. Therefore the GSH/GSSG ratio showed a significant increase [F (3,24) = 3.16 P=0.043] as an antioxidant marker and this ratio was higher in 2 mg/kg perindopril treated group, showing 82.8 % enhancement as compared to the control group (P<0.05) as shown in Figure 1.
Figure 1 .
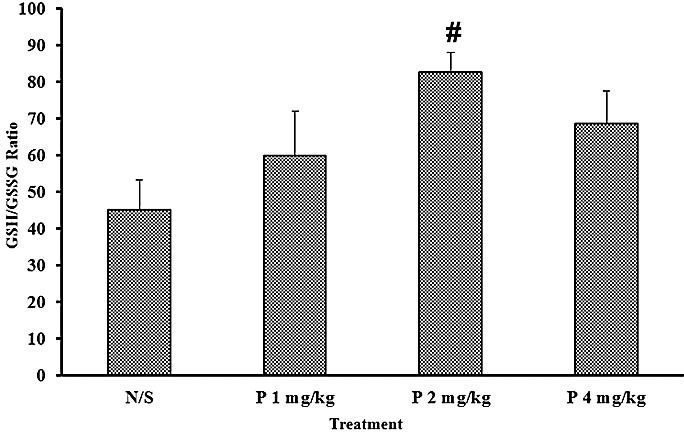
Effect of perindopril (P 1, 2, 4 mg/kg/day orally) compared to normal saline (N/S) as control on GSH/GSSG ratio (n= 7). Results are expressed as mean± SEM and data were analyzed by one way ANOVA followed by Tukey multiple comparison test. # Significantly different from the control, p<0.05
The data in Figure 2 demonstrate that treatment of normal rats with perindopril recorded an insignificant reduction in brain MDA level [F (3, 30) = 1.693, P=0.19] from 2.6±0.5 in control group to 1.7±0.2 in 1 mg/kg perindopril treated group. However the MDA level rises again, although not significant, to 2.9±0.4 in 4 mg/kg perindopril treated group.
Figure 2 .
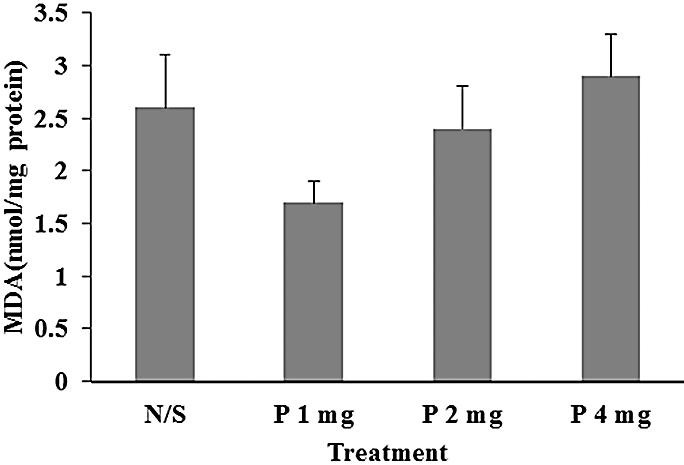
Effect of perindopril (P 1, 2, 4 mg/kg/day orally) compared to normal saline (N/S) as control on MDA (n=8-9). Results are expressed as mean± SEM and data were analyzed by one way ANOVA followed by Tukey multiple comparison test.
Discussion
In the present study GSH and GSH/GSSG ratio increased dose dependently in the hippocampus of perindopril treated groups. Brain is a susceptible organ to oxidative stress due to high metabolic rate, high poly-unsaturated fatty acids and low antioxidant demand.16 To combat with oxidative conditions the cells are provided with several antioxidants such as GSH which is a major and strong intracellular antioxidant. GSH acts as a donor of electron to free radicals, giving rise to GSSG which then reduced and recycled again to GSH through enzymatic reactions.17 GSH also acts as a scavenger of reactive oxygen species (ROS) and an inhibitor of H2O2-induced hydroxyl radical formation. Thereby, GSH and the GSH/GSSG ratio levels determine the defensive potency of the cells against oxidative stress in tissues like the brain.18
The glutathione analysis technique in this study support directly analysis of both reduced and oxidized glutathione. Previous studies showed that the inappropriate changes of total or oxidized glutathione recovered by ACE inhibitors such as ramipril or captopril in the experimentally models of oxidative damages.12,19 In our study although perindopril did not exert any beneficial effect on physiologic amount of GSSG, but it suggested that even physiologic amounts of GSH, the reduced form, may increase in response to ACE inhibitors. In the current study, all of the perindopril –treated groups showed higher amounts of GSH and GSH/GSSG ratio. However, a significant rise was only seen in 2 mg/kg perindopril-treated group indicating that there might be an optimum dose for perindopril to effectively cause an increase in the reduced glutathione level.
An insignificant decrease was observed in MDA analysis which was recovered as the dose of perindopril increased. MDA, a by-product of lipid peroxidation, is a major biomarker of the membrane lipid peroxidation by excessive ROS in the brain tissue that composes high poly unsaturated fatty acids.16 In the oxidative condition induced by chronic cerebral hypoperfusion in rats, the elevated MDA decreased after captopril treatment.11 In the present study there was also a tendency for MDA to be lessened in 1 mg/kg perindopril treated group. MDA then increased progressively to a higher level than control in 4 mg/kg perindopril treated group, indicating a probable dual action of perindopril: the antioxidant effects at low doses and oxidative effects at high doses. The insignificant decrease in GSH level in 4 mg/kg perindopril treated group, in accordance with enhancement of MDA level at 4 mg//kg perindopril treated group, also may result from conversion of benefit to toxic effects of perindopril through increasing the dose that should be confirmed by further studies.
In another point of view, the insignificant alteration in MDA and GSSG in this study may also indicate first: under physiologic condition, the basal lipid peroxidation may be continued normally and might not be changed prominently by perindopril. Second: the increase of reduced glutathione may not be the outcome of a compensatory response to increased free radicals as indicated previously,20 but it is potentially an improvement in anti-oxidant defense in response to perindopril. This may strengthen the antioxidant pool of the tissues like the brain as a sensitive organ to oxidative stress.
The main mechanism by which perindopril caused the increase of reduced glutathione in this study might be the inhibition of ACE. Hippocampus is a large structure in the brain and involved in advanced brain functions like learning and memory.21 It has been found to possess all of the renin-angiotensin components including ACE, which is abundant both in the vessels and neuronal cells, its active product, angiotensin II, and all of the angiotensin receptors.22 Perindopril is a lipophilic brain penetrating ACE inhibitor with an effective potency of ACE inhibition in structures like hippocampus. In this study we used the similar doses of perindopril which have been demonstrated previously to inhibit more than 50% of hippocampal ACE.10,23
ACE inhibition by perindopril in turn leads to attenuation of Ang II formation and AT1 receptor expression.11 Ang II through stimulation of AT1 receptor exerts its potent vasoconstrictory activity.7 Circulatory disturbance, as seen in hypo-perfusion or ischemia reperfusion, resulted in severe oxidative stress in several tissues as well as the brain.12,24,25 Therefore, ACE inhibition may lead to better circulation and prevent minor oxidative stresses caused by transient vasoconstrictions that may happen during normal life or probably during one month treating with perindopril in this study. Attenuation of AngII by ACE inhibition, additionally, may diminish the activation of NADPH oxidase26 which is a pivotal enzyme in the production of free radicals.27 ACE inhibitors were also evidenced to directly act as a scavenger of free radicals.28,29 All these mechanisms may lower the amount of free radicals and the consequent GSH consumption. On the other hand, ACE inhibitors was found to augment the activity of glutathione reductase, the enzyme that catalyze recycling of GSSG to GSH19 and may result in more GSH production. Further studies should address this issue and also the probable effect of ACE inhibitors on the activity of the enzymes that catalyze GSH synthesis reactions and/ or intestinal absorption of GSH which are other GSH supplementary pathways.30
Conclusion
This study reports that ACE inhibition can improve the cellular anti-oxidant defense and may suggest ACE inhibitors, especially those that penetrate blood-brain barrier, as worthy drugs for prevention of neural damages induced by oxidative stress.
Acknowledgments
The results described in this paper were part of student thesis written by Tahereh Mashhoody and was financially supported by the office of Vice Chancellor of Research Affair, Shiraz University of Medical Sciences (Grant No. 89-5231).
Conflict of Interest
The authors declare that they have no conflict of interest.
Abbreviations
Angiotensin II (Ang II), Angiotensin converting enzyme (ACE), malondialdehyde (MDA), reduced (GSH), oxidized glutathione (GSSG), angiotensin (Ang II), Tricloroacetic acid (TCA), thiobarbitoric acid (TBA), 1, 1, 3, 3-tetraethoxy propane (TEP), Ang II type1 (AT1)
References
- 1.Wang C, Wu HM, Jing XR, Meng Q, Liu B, Zhang H. et al. Oxidative parameters in the rat brain of chronic mild stress model for depression: relation to anhedonia-like responses. J Membr Biol. 2012;245(11):675–81. doi: 10.1007/s00232-012-9436-4. [DOI] [PubMed] [Google Scholar]
- 2.Schiavone S, Jaquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2013;18(12):1475–90. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS. et al. The role of free radicals in the aging brain and Parkinson's disease: convergence and parallelism. Int J Mol Sci. 2012;13(8):10478–504. doi: 10.3390/ijms130810478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labunskyy VM, Gladyshev VN. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid Redox Signal 2012. [DOI] [PMC free article] [PubMed]
- 6.Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1-2):254–62. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Ruilope LM, Rosei EA, Bakris GL, Mancia G, Poulter NR, Taddei S. et al. Angiotensin receptor blockers: therapeutic targets and cardiovascular protection. Blood Press. 2005;14(4):196–209. doi: 10.1080/08037050500230227. [DOI] [PubMed] [Google Scholar]
- 8.Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of the central renin-angiotensin system in Parkinson's disease. J Renin Angiotensin Aldosterone Syst. 2010;11(1):49–56. doi: 10.1177/1470320309347789. [DOI] [PubMed] [Google Scholar]
- 9.Duron E, Hanon O. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis. 2010;20(3):903–14. doi: 10.3233/JAD-2010-091552. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Uchida S, Takahashi S, Takayama M, Nagata Y, Suzuki N. et al. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res. 2010;1352:176–86. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Kumaran D, Udayabanu M, Kumar M, Aneja R, Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008;155(3):626–39. doi: 10.1016/j.neuroscience.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Yun I, Choi YB, Lee KS, Kim YI. Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J Clin Neurosci. 2008;15(2):174–8. doi: 10.1016/j.jocn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins TA, Chai SY. Effect of chronic angiotensin converting enzyme inhibition on spatial memory and anxiety-like behaviours in rats. Neurobiol Learn Mem. 2007;87(2):218–24. doi: 10.1016/j.nlm.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 15.Colado MI, O'shea E, Granados R, Misra A, Murray TK, Green AR. A study of the neurotoxic effect of MDMA ('ecstasy') on 5-HT neurones in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol. 1997;121(4):827–33. doi: 10.1038/sj.bjp.0701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull. 1993;49(3):577–87. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 17.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–6. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 18.Woltjer RL, Nghiem W, Maezawa I, Milatovic D, Vaisar T, Montine KS. et al. Role of glutathione in intracellular amyloid-alpha precursor protein/carboxy-terminal fragment aggregation and associated cytotoxicity. J Neurochem. 2005;93(4):1047–56. doi: 10.1111/j.1471-4159.2005.03109.x. [DOI] [PubMed] [Google Scholar]
- 19.De Cavanagh EM, Inserra F, Ferder L, Fraga CG. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol. 2000;278(3):R572–7. doi: 10.1152/ajpregu.2000.278.3.R572. [DOI] [PubMed] [Google Scholar]
- 20.Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol. 2001;428(2):185–92. doi: 10.1016/s0014-2999(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 21.Turgut YB, Turgut M. A mysterious term hippocampus involved in learning and memory. Childs Nerv Syst. 2011;27(12):2023–5. doi: 10.1007/s00381-011-1513-y. [DOI] [PubMed] [Google Scholar]
- 22.Von Bohlen Und Halbach O, Albrecht D. The CNS renin-angiotensin system. Cell Tissue Res. 2006;326(2):599–616. doi: 10.1007/s00441-006-0190-8. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins TA, Mendelsohn FA, Chai SY. Angiotensin-converting enzyme modulates dopamine turnover in the striatum. J Neurochem. 1997;68(3):1304–11. doi: 10.1046/j.1471-4159.1997.68031304.x. [DOI] [PubMed] [Google Scholar]
- 24.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4(6):461–70. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15(1):1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- 26.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74(6):1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S, Sato H, Shimada H, Takashima N, Arakawa M. Comparative free radical scavenging action of angiotensin-converting enzyme inhibitors with and without the sulfhydryl radical. Pharmacology. 1993;47(1):61–5. doi: 10.1159/000139079. [DOI] [PubMed] [Google Scholar]
- 29.Mira ML, Silva MM, Queiroz MJ, Manso CF. Angiotensin converting enzyme inhibitors as oxygen free radical scavengers. Free Radic Res Commun. 1993;19(3):173–81. doi: 10.3109/10715769309111600. [DOI] [PubMed] [Google Scholar]
- 30.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27(9-10):916–21. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]


