Abstract
Purpose: Intensive chemotherapy with daunorubicin (DNR) is associated with serious side effects in acute myeloid leukemia (AML) patients. In this study the effect of small-molecule BH3-mimetic, ABT-737, on the sensitivity of HL60 and U937 AML cell lines was investigated.
Methods: The cytotoxic effects of DNR and ABT-737, alone or in combination were assessed using MTT assay and combination index analysis. The effects of treatments on the cell proliferation was determined by trypan blue assay. ELISA cell death assay was used for measurement of apoptosis.
Results: IC50 values of DNR and ABT-737 were 2.52 and 0.59 µM for HL-60 cells line and 1.31 and 0.80 µM for U937 cell line at 24 h, respectively. Surprisingly, combination treatment significantly lowered the IC50 values in a synergic manner in both cell lines. Moreover, treatment with a mixture of two agents had more growth inhibition effect relative to the monotherapy. Results of apoptosis assay showed that the cytotoxic effects are related to the enhancement of apoptosis.
Conclusion: Our study suggests that ABT-737 synergistically enhances the cytotoxic effect of DNR in AML cell lines and therefore may be useful to overcome chemoresistance of leukemia patients.
Keywords: Acute myeloid leukemia, Daunorubicin, ABT-737, Combination, Apoptosis
Introduction
Acute myeloid leukemia (AML) is an aggressive blood disorder that known with the accumulation of immature hematopoietic stem cells in bone marrow.1 AML is the most common type of leukemia in adults with lowest survival rate of all leukemias.2,3 AML treatment includes at least one course of induction chemotherapy including daunorubicin (DNR) and cytarabine.4 More than 50% of patient with AML do not achieve complete remission or show relapse after high-dose induction chemotherapy.5 In addition, the cardiotoxicity and nephrotoxicity of anthracyclines remain as a major problem in clinical treatment of AML.6 Studies have shown that the use of biological modifiers in combination with conventional cytotoxic agents is useful to reduce undesirable toxicity.7
Mitochondria play a central role in the regulation of apoptosis (programmed cell death).8 B-cell lymphoma-2 (Bcl-2) family of proteins are regulated the intrinsic pathway of apoptosis by the stabilization of the outer membrane of mitochondria (OMM). The members of this family are divided into three main groups based on function and regions of the Bcl-2 homology (BH) domains: multi-domain anti-apoptotic proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1) multi-domain pro-apoptotic proteins (Bax and Bak), and BH3-only pro-apoptotic proteins (Bid, PUMA, Bim and NOXA). Studies have showed that BH1, BH2 and BH3 domains of anti-apoptotic proteins interact with the α-helixes formed by BH3 domains of pro-apoptotic members. When the cells received the apoptosis signals, BH3-only pockets of anti-apoptotic proteins bind to the hydrophobic cleft formed by anti-apoptotic proteins resulting in release of Bax and Bak. Oligomerized Bax and Bak permeabilize OMM that cause release of cytochrome c and thereby execution of apoptosis.9-11
It is shown that the overexpression of anti-apoptotic Bcl-2 family of proteins have been correlated with survival and therapeutic resistance of tumor cells including leukemia.12,13 Moreover, others have demonstrated that targeting of anti-apoptotic Bcl-2 family members can induce apoptosis and reverse multi-drug resistance of cancer cells.14 Since, the BH3 binding pockets of anti-apoptotic proteins are essential for their functions, it is hypothesized that the small molecules that bind to these pockets may be able to block the hetero-dimerization of anti-apoptotic and pro-apoptotic proteins and trigger apoptosis.15
The aims of this study were to investigate the anti-tumor effect of anthracycline DNR on AML cells and to determine whether this effect can be enhanced by ABT-737. To this end, we have examined the effects of either agent, alone and in combination, in HL-60 and U937 cell lines.
ABT-737 is a potent small molecule inhibitor of the Bcl-2, Bcl-xL and Bcl-w proteins, developed by Abbott laboratories. This compound, like BH3-only proteins, binds to anti-apoptotic Bcl-2 family members and antagonizes their effects, thereby diminishing their ability to inhibit apoptosis.16 Furthermore, ABT-737 was found to exhibit chemosensitization effect, and single anti-cancer activity was observed in lymphoma and small-cell lung carcinoma (SCLC) tumor cells with low toxicity.17
The aims of this study were to investigate the anti-tumor effect of anthracycline DNR on AML cells and to determine whether this effect can be enhanced by ABT-737. To this end, we have examined the effects of either agent, alone and in combination, in HL-60 and U937 cell lines.
Materials and Methods
Cell lines and culture
HL-60 (acute promyelocytic leukemia) and U937 (human leukemic monocyte leukemia) cell lines were purchased from Pasteur Institute Cell Bank of Iran. RPMI-1640 medium (Sigma, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco, Invitrogen, USA), 2 mg/ml sodium bicarbonate, 0.05 mg/ml penicillin G (Serva co, Germany) and 100 µg/ml streptomycin (Gibco) was used for cell culture. The cell lines were cultured in 25 cm2 flasks and maintained in a humidified incubator containing 5% CO2 at 37 °C.
In vitro cytotoxicity
The cytotoxicity of treatments was determined by MTT assay. This test detects the reduction of yellow MTT [3-(4, 5-dimethylthiazolyl)-2, 5-diphenyl-tetrazolium bromide] into purple formazan crystals by mitochondrial dehydrogenases, which reflects the normal function of mitochondria. Just before treatments, leukemia cell lines were cultured in complete medium at a density of 3×104 cells/well in 96-well U-shape bottom tissue culture plates (Nunc, Denmark) and incubated overnight at 37 °C. The next day, the culture medium was replaced with 200 µl of fresh complete medium and then the cells were treated with different concentrations (0.001, 0.01, 0.1, 0.5, 1 and 2 µM) of either ABT-737 (Active Biocheminals, HongKong) or DNR (Sigma, Germany) alone. Moreover, a combination treatment with equal concentrations of two agents was performed. Treatments with 1% DMSO (solvent of ABT-737) and RPMI (solvent of DNR) without drugs were also considered as a blank control. After 24 h of incubation, the culture medium was removed and the cells were incubated with MTT solution (Sigma) (0.2 mg/ml, 200 µl) for 4 h at 37 °C in a humidified atmosphere. The cell culture plates were centrifuged at 1500 g for 5 min and the supernatants were discarded. Subsequently, 200 µl of DMSO and 25 µl of Sorenson's glycine buffer were added to the wells to dissolve the formazan crystals. Finally, the amount of soluble formazan was determined by quantification of the absorbance at 570 nm (with a reference wavelength of 650 nm) using EL × 800 ELISA plate reader (Bio Tech Instruments, USA). After correction of the background absorbance, the percentage of cell viability was determined using the following formula:
Cell viability (%) = AbsorbanceTest / AbsorbanceControl × 100.
The IC50 values (concentrations that induced 50% cytotoxicity) were calculated using GraphPad Prism 6.01 software (GraphPad Software Inc., USA).
Combination index analysis
To investigate the interaction effect between ABT-737 and DNR, combination index analysis, based on Chou and Talalay method was performed.18 The combination index (CI) was calculated using the following equation: CI = (A/B) + (A/C), which A, B and C are the IC50 values of the combination treatment, ABT-737 and DNR, respectively. The values of CI less than 1, equal to 1 and bigger than 1, indicate synergistic, additive and antagonist effects respectively.
Cell proliferation assay
Trypan blue exclusion assay was used to determine anti-proliferative effects of treatments. In brief, the cells (5×104 cells/well) were treated with the IC50 doses of either ABT-737 or DNR and their combination in 24-well tissue culture plate (Nunc). Following treatments, the cells were incubated in appropriate culture conditions for 24-96 h. At the end of each day, the cells were stained with 0.4% trypan blue dye (Merck KGaA, Germany) and then the number of viable cells was counted using neubauer chamber under an Olympus inverted microscope.
Apoptosis assay
HL-60 and U937 cells were seeded at a density of 5×104 cells/well in 96-well plates and treated with drugs as described in the cytotoxicity assay section. Following the treatments, apoptosis was detected using an apoptosis ELISA assay kit (Roche Diagnostics GmbH, Germany) according to the manufacturer's protocol. This test is based on the identification of mono and oligonucleosomes in the cytoplasmic fraction of apoptotic cell lysates. In brief, 24 h after treatments, the cells were centrifuged and lysed. Then, 20 µl of supernatants and 80 µl of immunoreagent containing monoclonal antibodies directed against DNA and histones were transfered to each wells of ELISA microplate. After 2 h of incubation, the wells were washed and 100 µl of ABTS solution was added. The resulting colors were quantified using a plate reader at 405 nm (with reference wavelength in 490 nm).
Statistical analysis
Statistical analyses were performed with GraphPad Prism 6.01 software. Results were expressed as the mean ± standard deviation (SD). Statistical differences were assessed by unpaired student t-test; and a value of P less than 0.05 was considered significant.
Results
ABT-737, synergistically enhanced the cytotoxic effects of DNR in leukemia cells
To analyze the effect of ABT-737 on sensitivity of leukemic cells to DNR, a combination treatment of two agents was investigated. HL-60 and U937 leukemia cells were exposed to the various concentrations of drugs (0.001-2 µM), alone or in combination, and cytotoxicity was measured by MTT assay after 24 h. As shown in Figure 1a and b, monotherapy with BAT-737 or DNR markedly decreased the viability of the two cell lines in a dose-dependent manner. Results of MTT assay showed that, compare with the single agent treatment, the combination therapy further decreased the percentage of viable cells (P<0.05, Figure 1a and b). Moreover, combination of two agents resulted in significant decrease in the IC50 values relative to the monotreatment (Table 1). Results of combination index analyses indicated that the interaction effect between drug combinations was synergistic with CI values of 0.93 and 0.80 in HL-60 and U937 cell lines, respectively.
Table 1. The IC50 values determined by MTT assay .
| IC50 (µM) | ||
| - | HL-60 | U937 |
| ABT-737 | 0.59 | 0.80 |
| DNR | 2.52 | 1.31 |
| Combination | 0.45* | 0.41* |
| * P<0.05 relative to single agent reatments | ||
ABT-737 enhanced the growth inhibitory effect of DNR
We investigated whether treatment with DNR, ABT-737 or the combination of them affects cell proliferation. Cell proliferation was assessed by trypan blue exclusion assay at 24-96 h after treatments. The cell growth curves of HL-60 and U937 cells showed that the treatment with DNR or ABT-737 alone, significantly suppressed cell growth over a period of 5 days (relative to the blank control) (Figure 2a and b). Moreover, two treatments with DNR or ABT-737 further inhibited the growth of two cell lines (P<0.05).
Figure 1 .
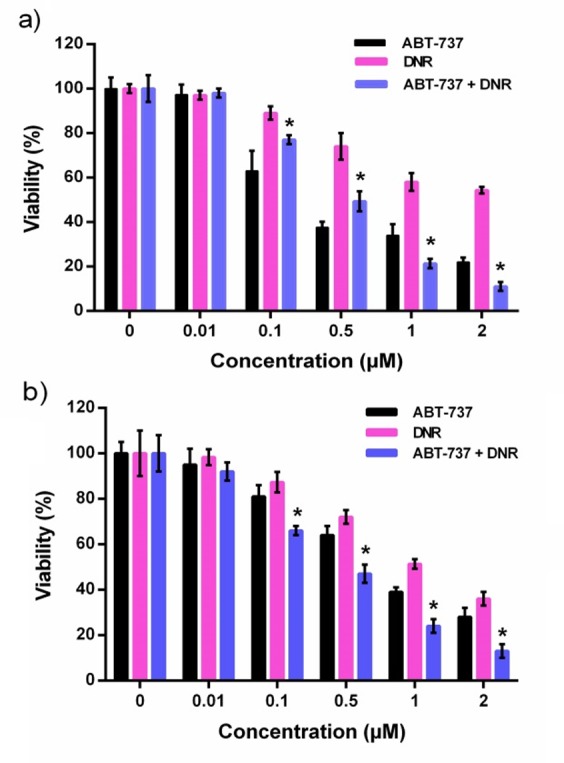
The cytotoxic effects of ABT-737, DNR and their combination on HL-60 (a) and U937 (b) cells were determined by MTT assay. Results expressed as the mean ± SD (n=3), *p<0.05 versus DNR or ABT-737 alone.
Figure 2 .
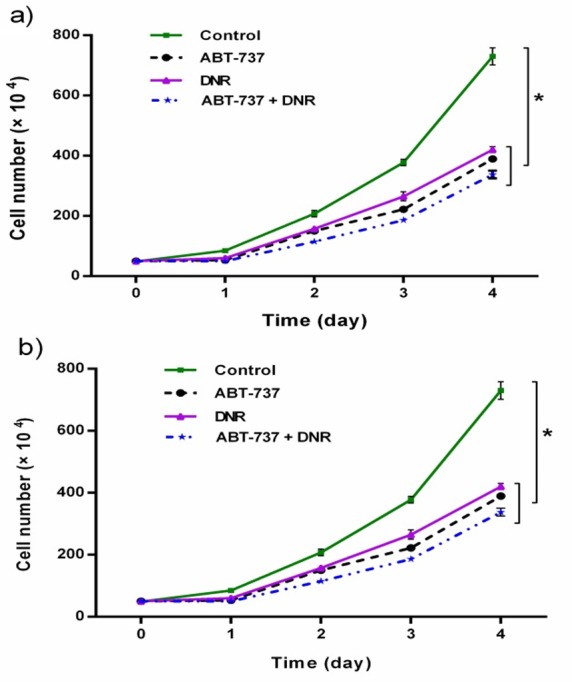
Effects of ABT-737, DNR and their combination on the proliferation of HL-60 (a) and U937 (b) cell lines. The growth curves of leukemic cells were constructed from the results of trypan blue dye exclusion assay. Results expressed as the mean ± SD (n=3), *p<0.05 versus control.
ABT-737 increased the sensitivity of leukemia cells to DNR-mediated apoptosis
To investigate whether the cytotoxic effects of treatments are linked to the enhancement of apoptosis, an apoptosis ELISA assay was performed. HL-60 and U937 tumor cells were treated with both IC50 dose of ABT-737 or DNR alone and IC50 dose of combination for 24 h, and then validated for apoptosis. Results showed that, Compare with the blank control, treatment with ABT-737 resulted in 5.56 and 7.25 fold increase in apoptosis in HL-60 and U937 cells, respectively (P<0.05, Figure 3). In addition, exposure of HL-60 and U937 cells with DNR significantly enhanced the extent of apoptosis to 4.42 and 5.39 fold, respectively. Moreover, with the combination treatment of two agents, fold increases in apoptosis were 5 for HL-60 cells and 7.59 for U937 Cells.
Figure 3 .
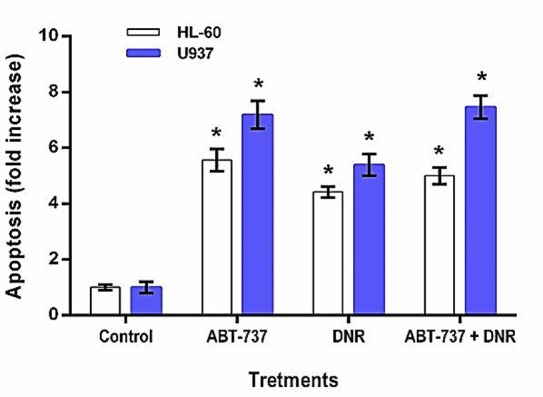
ABT-737 enhanced the sensitivity of leukemia cells to DNR-mediated apoptosis. HL-60 and U937 cells were treated with the drugs and apoptosis was measured by ELISA cell death assay. Results expressed as the fold increase in apoptosis compare with the blank control. Data are the mean ± SD of independent experiments (n=3), *p<0.05 versus blank control.
Discussion
The use of anthracyclines (daunorubicin or idarubicin) in combination with cytarabine is one of the effective induction treatments for AML patients.19 Since, intensive chemotherapy with DNR is associated with undesirable side effects such as cardiotoxicity; the use of lower doses of daunorubicin is one of great clinical interests.6 Overexpression of anti-apoptotic proteins plays a critical role in the inherent resistance of tumor cells to cytotoxic agents.12 Studies have indicated that the suppression of these mediators of apoptosis pathway by various strategies could induce apoptosis and overcome drug resistance of cancer cells.13,14 In this study, the effect of, BH3-memetic ABT-737, on the apoptosis and sensitivity of HL-60 and U937 AML cells to DNR investigated.
Our data demonstrated that the treatment with ABT-737 or DNR alone causes significant cytotoxic and growth inhibitory effects in leukemic cells. MTT assay and combination index analysis showed that ABT-737 synergistically enhances the cytotoxic effect of DNR in two cell lines. Moreover, apoptosis assay findings revealed that the chemosensitization effect of ABT-737 is contribute to the induction of apoptosis. These results were in agreement with the other reports describing the important role of mitochondrial anti-apoptotic proteins in drug resistance of tumor cells.12-14
Apoptosis can be triggered via two distinct pathways: the intrinsic and the extrinsic pathways. Anti-apoptotic Bcl-2 family of proteins commonly inhibit the intrinsic pathway of apoptosis by interaction with the BH3-only pro-apoptotic proteins (Bid, PUMA, Bim and NOXA), thereby release multi-domain pro-apoptotic proteins (Bax and Bak) to cytoplasm. Oligomerization of Bax and Bak proteins in OMM causes release of cytochrome c from the inner mitochondrial membrane space that lead to caspases activation and the next apoptosis events.8-11 Our study shows that treatment of leukemic cells with either DNR or ABT-737 triggers marked apoptotic cell death. Furthermore, ABT-737 enhances the apoptosis effect caused by DNR. Thus, it is conceivable that BH3-memetic ABT-737 that interacts with anti-apoptotic proteins, triggers apoptosis through the intrinsic pathway. Chemotherapeutic drug, DNR, is a DNA intercalating agent that induces apoptosis by the inhibition of DNA and RNA synthesis.4 However, the exact cellular mechanisms of DNR-mediated apoptosis and chemosensitization effect of ABT-737 remain unclear. Further investigations are needed.
Small-molecule BH3-mimetic, ABT-737, strongly inhibits Bcl-2, Bcl-xL, and Bcl-w pro-apoptotic proteins (Ki < 1 nM), but not Mcl-1(Ki > 1 µM).9,17 ABT-737 has shown promise for treatment of follicular B-cell lymphoma and small-cell lung cancer with low levels of Mcl-1 expression.20 In addition, it was confirmed that the cells with high levels of Mcl-1 are more resistance to ABT-737, indicates the correlation between the cellular levels of Mcl-1 and response to the compound.21-24 Our study demonstrated that HL-60 cells are more sensitive to ABT-737 relative to U937 cells (Table 1) that have higher levels of Mcl-1 expression.25 Our findings support the above-mentioned reports and further confirm the cytoprotective role of Mcl-1 against ABT-737.
Conclusion
In conclusion, the present study has demonstrated that targeted down-regulation of anti-apoptotic proteins by ABT-737 results in a significant single-agent activity against leukemic cells. Furthermore, combination of DNR with ABT-737 exhibited synergistic anti-tumor effect. These findings highlight anti-apoptotic Bcl-2 family of proteins as a relevant target and drug resistance factor in AML patients. Our findings underline the potential of ABT-737 to induce tumor cell apoptosis and reduce the serious side effects caused by high dose chemotherapy. We suggest that the specific suppression of Mcl-1 may further enhance the chemosensitization effect of ABT-737.
Acknowledgments
This study was supported by a grant from Hematology and Oncology Research Center, Tabriz University of Medical Sciences. We acknowledge Dr. Hadi Karami, Molecular Medicine Research Center, Arak University of Medical Sciences, Arak, Iran, for technical assistance and critical review of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med . 1999;341(14):1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Kupsa T, Horacek JM, Jebavy L. The role of cytokines in acute myeloid leukemia: a systematic review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub . 2012;156(4):291–301. doi: 10.5507/bp.2012.108. [DOI] [PubMed] [Google Scholar]
- 3.Kersemans V, Cornelissen B, Minden MD, Brandwein J, Reilly RM. Drug-resistant AML cells and primary AML specimens are killed by 111In-anti-CD33 monoclonal antibodies modified with nuclear localizing peptide sequences. J Nucl Med . 2008;49(9):1546–54. doi: 10.2967/jnumed.107.047399. [DOI] [PubMed] [Google Scholar]
- 4.Rabbani A, Finn RM, Ausio J. The anthracycline antibiotics: antitumor drugs that alter chromatin structure. Bioessays . 2005;27(1):50–6. doi: 10.1002/bies.20160. [DOI] [PubMed] [Google Scholar]
- 5.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther . 2009;31 Pt 2:2349–70. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bardi E, Bobok I, A VO, Kappelmayer J, Kiss C. Anthracycline antibiotics induce acute renal tubular toxicity in children with cancer. Pathol Oncol Res . 2007;13(3):249–53. doi: 10.1007/BF02893506. [DOI] [PubMed] [Google Scholar]
- 7.Gu C, Ye T, Wells RA. Synergistic effects of troglitazone in combination with cytotoxic agents in acute myelogenous leukaemia cells. Leuk Res . 2006;30(11):1447–51. doi: 10.1016/j.leukres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) . 2005;70(2):231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 9.Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature . 1996;379(6565):554–6. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 10.Lutz RJ. Role of the BH3 (Bcl-2 homology 3) domain in the regulation of apoptosis and Bcl-2-related proteins. Biochem Soc Trans . 2000;28(2):51–6. doi: 10.1042/bst0280051. [DOI] [PubMed] [Google Scholar]
- 11.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature . 2005;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 12.Varin E, Denoyelle C, Brotin E, Meryet-Figuiere M, Giffard F, Abeilard E. et al. Downregulation of Bcl-xL and Mcl-1 is sufficient to induce cell death in mesothelioma cells highly refractory to conventional chemotherapy. Carcinogenesis . 2010;31(6):984–93. doi: 10.1093/carcin/bgq026. [DOI] [PubMed] [Google Scholar]
- 13.Akagi H, Higuchi H, Sumimoto H, Igarashi T, Kabashima A, Mizuguchi H. et al. Suppression of myeloid cell leukemia-1 (Mcl-1) enhances chemotherapy-associated apoptosis in gastric cancer cells. Gastric Cancer . 2013;16(1):100–10. doi: 10.1007/s10120-012-0153-6. [DOI] [PubMed] [Google Scholar]
- 14.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res . 2009;15(4):1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene . 2004;23(16):2934–49. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 16.Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB. et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res . 2007;13(23):7191–8. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 17.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med . 2008;8(2):138–47. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul . 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood . 2005;106(4):1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 20.Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC. et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res . 2012;18(3):783–95. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res . 2007;67(2):782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA. et al. 'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene . 2007;26(27):3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 23.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J. et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res . 2007;67(3):1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 24.Van Delfts MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE. et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell . 2006;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ugarenko M, Nudelman A, Rephaeli A, Kimura K, Phillips DR, Cutts SM. ABT-737 overcomes Bcl-2 mediated resistance to doxorubicin-DNA adducts. Biochem Pharmacol . 2010;79(3):339–49. doi: 10.1016/j.bcp.2009.09.004. [DOI] [PubMed] [Google Scholar]


