Abstract
Social animals frequently share decisions that involve uncertainty and conflict. It has been suggested that conflict can enhance decision accuracy. In order to judge the practical relevance of such a suggestion, it is necessary to explore how general such findings are. Using a model, I examine whether conflicts between animals in a group with respect to preferences for avoiding false positives versus avoiding false negatives could, in principle, enhance the accuracy of collective decisions. I found that decision accuracy nearly always peaked when there was maximum conflict in groups in which individuals had different preferences. However, groups with no preferences were usually even more accurate. Furthermore, a relatively slight skew towards more animals with a preference for avoiding false negatives decreased the rate of expected false negatives versus false positives considerably (and vice versa), while resulting in only a small loss of decision accuracy. I conclude that in ecological situations in which decision accuracy is crucial for fitness and survival, animals cannot ‘afford’ preferences with respect to avoiding false positives versus false negatives. When decision accuracy is less crucial, animals might have such preferences. A slight skew in the number of animals with different preferences will result in the group avoiding that type of error more that the majority of group members prefers to avoid. The model also indicated that knowing the average success rate (‘base rate’) of a decision option can be very misleading, and that animals should ignore such base rates unless further information is available.
Keywords: collective behaviour, conflict resolution, cooperation, quorum decision, shared decisions, social choice
1. Introduction
Social animals frequently need to make collective decisions that are vital to the survival and fitness of individual group members [1]. Such decisions often involve uncertainty [2–12] and conflict [13–29]. By sharing decision-making through majority vote, several decision-makers can pool personal information and eliminate individual errors, often resulting in very accurate decision outcomes (termed ‘swarm intelligence’; [2–4,10,12,30–33]).
Recently, it has been suggested that conflicting interests among decision-makers might enhance the advantages of decision sharing by further increasing decision accuracy [30,34]. If such benefits of including factions with vested interests among decision-makers are general, they could also be relevant to humans and, thereby, have valuable practical implications [22,24,32,34]. However, in order to judge their practical relevance, it is first necessary to explore how general such findings are. Therefore, I am interested whether accuracy benefits can be observed in situations with a very common form of preference conflict, namely that between a preference for avoiding false positives versus a preference for avoiding false negatives [2,35,36].
Imagine a group of foraging animals deciding whether to travel to a particular food patch, but the animals are unsure whether the patch is productive and food is available. If the patch was productive, it would be of advantage to all group members to travel to the patch in order to forage (correct positive), whereas if the patch was not productive, it would be of advantage to all group members not to travel to it (correct negative). However, if the patch was productive and the group were to decide not to travel to it (false negative), then some animals (individuals with relatively low energy reserves) would pay a higher cost than other animals in forgoing the available foraging opportunity [13,15,19]. On the other hand, if the patch was not productive but the group would erroneously travel to it (false positive), some animals (e.g. smaller animals) might pay a higher cost than other animals through the unnecessary, unrewarding and dangerous travel [23,31,37].
In such situations, the trade-off for an individual animal between avoiding false negative versus avoiding false positive decisions usually depends on the individual's energy reserves, its vulnerability to predation, dominance status and other physiological variables that are likely to differ between individuals [17,19,20,23]. Therefore, it can be in the interest of some group members to more strongly avoid false negatives, while it is in the interest of other group members to more strongly avoid false positives. As a consequence, there often is a conflict of interest between group members, even in decisions when the actual goals (e.g. finding a productive patch, avoiding an unproductive patch) are the same [36]. Relevant decision scenarios are common: e.g. collective decisions concerning the detection of (i) predators [12,17,19,35]; (ii) food patches [13,15,19]; (iii) suitable nest sites [21,29]; (iv) homing and migration routes [31,37], and in humans in a wide range of medical and jurisdictive decision-making, evaluations and assessments [2,36,38,39].
In the following, I examine whether conflicts between individuals in a group with respect to preferences for avoiding false positives versus false negatives could, in principle, enhance decision accuracy in a manner similar to that suggested for groups with more direct goal conflicts [30]. In particular, I ask whether a group in which some decision-makers are trying to avoid false positives more strongly, and others are trying to avoid false negatives more strongly (i.e. ‘a group with conflicting preferences’), is overall more accurate than a group in which most animals are trying to avoid either false positives or false negatives (i.e. a homogeneous group with no conflict), and how such groups compare to groups in which all individuals are trying to maximize accuracy irrespective of the rate of false negatives versus false positives (‘Condorcet jury’) [2,9]. It is assumed that individuals have previous experience with the decision situation, so that they can use their experience to adaptively improve the accuracy of their personal choice. This assumption differs from, and is more realistic than, the commonly used assumption of a one-shot decision set-up whereby individuals have a fixed ad hoc probability to make a correct personal choice [2].
2. Methods: the decision model
2.1. Decision options
I consider collective decisions between two mutually exclusive decision options: option PLUS and option MINUS (e.g. travelling/not travelling to a foraging patch, fleeing/not fleeing from a suspected predator, using/not using a particular migration route [15,20,31,37,40,41]). At any time, one of the options is the ‘correct’ option (e.g. a correctly identified non-productive patch; a correctly detected predator, etc.) and the other option is ‘incorrect’.
2.2. Uncertainty and environmental clues
I assume that there are K different environmental situations and that p(k) is the probability that in the kth environmental situation, option PLUS is the correct option and 1− p(k) is the probability that option MINUS is the correct option (1 ≤ k ≤ K). In an uncertain world, the probability p(k) is unknown to animals [41]. However, each animal can from its past experience estimate whether option PLUS might be the correct option in the kth environmental situation or not, as follows.
2.3. Personal choice under uncertainty and decision thresholds
Assume the ith animal had nik past experiences in the kth environmental situation, and of those the animal observed aik times that option PLUS was the correct option, and nik-aik times that option MINUS was the correct option. The animal can use this information to make a personal (individual) choice between options PLUS and MINUS. In particular, it can use a strategy ti(nik), so that in the kth situation, the animal chooses option PLUS if in its past experiences aik ≥ ti(nik); or else it chooses option MINUS (note, that ti does not depend on k other than through the size of nik, because it is assumed that the kth situation gives no further decision cues other than aik and nik). ti(nik) is termed the animal's ‘decision threshold’. It follows that the probability Pik that the ith animal chooses option PLUS in the kth situation, is equal to the probability that the animal has observed more than ti(nik) occasions in the past, on which option PLUS was correct. That is,
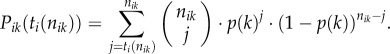 |
2.1 |
Note that Pik decreases monotonically with ti(nik) and increases with p(k). To simplify, I assume, without much loss of generality, that nik = ni (i.e. that animals have equal experience/expertise in each environmental situation). To simplify further, I assume that all animals are about equally experienced, so that ni = n for all animals (to avoid ties, I assume that n is uneven; note, to explore the influence of diversity in experience/expertise between individuals would be beyond the scope of this study). The size of n defines the ‘level of expertise of animals’. Equation (2.1) can now be simplified and the probability that an animal with strategy ti will choose option PLUS in the kth environmental situation is thus
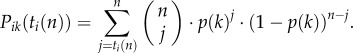 |
2.2 |
2.4. PLUS-, MINUS- and CONDORCET-animals
I consider decisions by animals that have conflicting preferences with respect to avoiding false negatives versus avoiding false positives. In particular, ‘PLUS-animals’ have a stronger preferences to avoid false negatives, and ‘MINUS-animals’ have a stronger preference to avoid false positives. For reasons of comparison, I also consider animals that do not have a particular preference to avoid false positives or false negatives, but maximize decision accuracy, as jurors do in a Condorcet jury (termed ‘CONDORCET-animals’ [2]).
A ‘CONDORCET-animal’ chooses a strategy ti(n) = tCONDORCET that maximizes the probability of a correct personal choice Pcorrect(tCONDORCET) Assuming that the animal has no further information other than its personal experiences defined by n and aik, the strategy t = (n + 1)/2 is the strategy that maximizes an animal's expected probability of a correct personal choice (see [41] for a proof). Therefore, I assume that a CONDORCET-animal has a personal choice strategy tCONDORCET = (n + 1)/2.
Furthermore, PLUS-animals try to avoid false negatives more than they try to avoid false positives. Using equation (2.2), the probability of an animal to make a false negative personal choice is p(k) · (1 − Pik (ti(n))). This probability decreases monotonically with each Pik(ti(n)) and, thus, increases monotonically with ti(n) (see equation (2.2)), and t = 0 would minimize false negatives. However, minimizing false negatives usually come at a very high cost in terms of individual accuracy [35] and would nearly always be a detrimental strategy to follow. Therefore, it is not realistic to assume that an animal tries to minimize false negatives (unless it is in an accordingly trivial environmental situation). It is most realistic to assume that PLUS-animals decrease false negatives, without decreasing overall accuracy too drastically, by just slightly staying below the threshold that maximizes accuracy (tCONDORCET; see above). Therefore, I assume that PLUS-animals decrease their threshold ti(n) relative to that of CONDORCET-animals by the smallest possible amount, namely tPLUS = tCONDORCET − 1.
Finally, MINUS-animals try to avoid false positives more than they try to avoid false negatives. Following a similar rationale as that in the last paragraph for PLUS-animals, I thus assume that MINUS-animals keep the probability of false positives low by following a personal choice strategy of increased ti(n) relative to that of CONDORCET-animals, namely: tPLUS = tCONDORCET + 1.
2.5. Collective decision: choice aggregation
The personal choice of an individual is given by equation (2.2). In a collective decision by a group, the personal choices of all group members are aggregated by majority vote into a collective decision outcome [2,32]. Thus, the probability that a group of CONDORCET-animals (i.e. a Condorcet jury: [2,9]) decides collectively in favour of option PLUS in the kth environmental situation is (using Pik(ti(n)) = Pk (tCONDORCET) from equation (2.2))
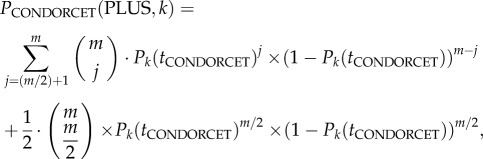 |
2.3 |
where m is the group size (i.e. the number of decision-makers; for reasons of symmetry, m is assumed to be even).
The probability that a group consisting of PLUS- and MINUS-animals (i.e. a group with conflicting preferences) decides collectively in favour of option PLUS in the kth environmental situation is (using for PLUS-animals: Pik(ti(n)) = Pk (tCONDORCET − 1) and for MINUS-animals: Pik(ti(n)) = Pk(tCONDORCET + 1) from equation (2.2))
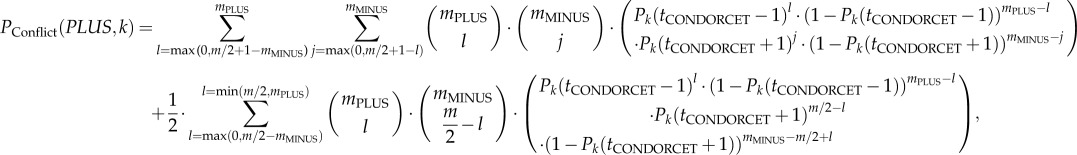 |
2.4 |
whereby mPLUS and mMINUS are the number of PLUS- and MINUS-animals, respectively, within the group (i.e. the number of decision-makers: mPLUS + mMINUS = m; for reasons of symmetry, m is assumed to be even).
2.6. Degree of preference conflict
To quantify the ‘degree of conflict’ within a group, one simple and appropriate measure is to determine, if you pick two group members at random, how likely it is that you observe a conflict between them: 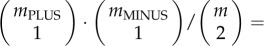
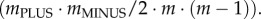 However, the range of this measure changes with group size m. Thus, instead, I use a measure for the degree of conflict that is strictly monotonically correlated with the proportion of randomly drawn pairs of group members that have conflicting preferences (i.e. with mPLUS · mMINUS/2 · m · (m − 1)), is easy to calculate and has the advantage of a fixed range that is independent of the size m of the group. Such a measure for the degree of conflict, conflict, in a group is conflict = min (mPLUS, mMINUS)/((mPLUS + mMINUS)/2). Conflict can range from 0 (no conflict; all group members are either PLUS- or MINUS-animals) to 1 (maximal conflict, half of group members are PLUS-animals, the other half are MINUS-animals).
However, the range of this measure changes with group size m. Thus, instead, I use a measure for the degree of conflict that is strictly monotonically correlated with the proportion of randomly drawn pairs of group members that have conflicting preferences (i.e. with mPLUS · mMINUS/2 · m · (m − 1)), is easy to calculate and has the advantage of a fixed range that is independent of the size m of the group. Such a measure for the degree of conflict, conflict, in a group is conflict = min (mPLUS, mMINUS)/((mPLUS + mMINUS)/2). Conflict can range from 0 (no conflict; all group members are either PLUS- or MINUS-animals) to 1 (maximal conflict, half of group members are PLUS-animals, the other half are MINUS-animals).
2.7. Decision accuracy and the probabilities of false positives and false negatives
I investigate how the distribution of conflicting preferences within a group of PLUS- and MINUS-animals affects decision accuracy. In particular, I examine whether decision accuracy increases with the degree of conflict in the group, as has been suggested for other conflict situations [30]. Furthermore, I compare the decision accuracies between conflict groups and groups of CONDORCET-animals that simply maximize accuracy rather than have preferences with respect to avoiding a particular form of false decisions (Condorcet juries: [2]). Decision accuracy is defined as the probability of a correct decision outcome. The decision accuracy PCondorcet(correct) of a group of m CONDORCET-animals (i.e. a Condorcet jury) equals the probability that the group makes a correct choice. Using equations (2.2) and (2.3), it is
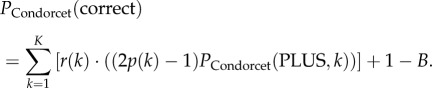 |
2.5 |
The decision accuracy PConflict(correct) of a ‘conflict group’ of PLUS- and MINUS-animals is (using equation (2.4)):
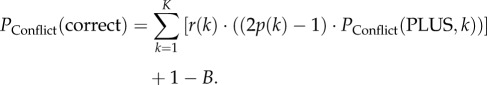 |
2.6 |
Similarly, the probability of a Condorcet jury to make a false positive decision PCondorcet(false positive) is
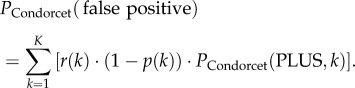 |
2.7 |
The probability of a conflict group to make a false positive decision PConflict(false positive) is
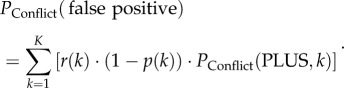 |
2.8 |
The probability of a Condorcet jury to make a false negative decision PCondorcet(false negative) is
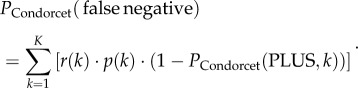 |
2.9 |
Finally, the probability of a conflict group to make a false negative decision PConflict(false negative) is
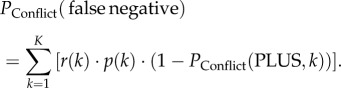 |
2.10 |
2.8. Investigated parameter ranges
To explore the robustness and generality of results, I cover a biologically extensive range of parameter combinations, as follows:
-
— animal's ‘expertise’ (i.e. their size of past experience):
low (n = 3), medium (n = 7) and large (n = 25);
-
— proportion prop of PLUS-animals in conflict groups:
none (prop = 0), low (prop = 0.25), medium (prop = 0.5), high (prop = 0.75) and all (prop = 1);
-
— degree of conflict of decision-makers in conflict groups:
low (conflict = 0), medium (conflict = 0.5) and large (conflict = 1);
-
— group size in collective decisions:
small (m = 4), medium (m = 8) or large (m = 24); and
-
— distribution of the probability p(k) that option PLUS is the correct choice in the kth situation.
The probability that option PLUS is the correct choice is likely to depend on the environmental situation. To take as an example predator-avoidance decisions: in some, predator-rich habitats the likelihood that a predator is present (and option PLUS is the correct decision) might be higher than in other, predator-poor habitats. Thus, to make the present model widely relevant, a range of scenarios are considered for the distribution of the probability p(k) that option PLUS is the correct choice in the kth environmental situation, as follows:-
— Uniform distribution (p(k) = k/10; k = 1,2,3,4,5,6,7, 8,9). The base rate is B = 0.5.Here, habitats which have low, intermediate or high probability that option PLUS is correct (e.g. that a predator appears) are equally likely to be encountered. This would be the case in scenarios with very patchy and diverse habitats.
-
— Unimodal distributions with either a unbiased (B = 0.5) or a raised PLUS base rate (B = 0.6); and either a small (σx = 0.6) or high variance (σx = 1; x = ln(p(k)/(1 – p(k))) (table 1) note that results for a lowered base rate are symmetric and are therefore not considered).Here, habitats with an intermediate probability that option PLUS is correct are likely to be encountered. This would be the case if habitats were relatively homogeneous with respect to the relevant feature (e.g. the distribution of predators).
-
— Bimodal distributions that are either symmetric or asymmetric; have either an unbiased or raised base rate and have either a low or high variance σ (table 2).Here, habitats with either a relatively high or a relatively low probability that option PLUS is correct are likely to be encountered. This would be the case if there were largely two main habitat types (e.g. a predator-rich and a predator-poor habitat).
-
Table 1.
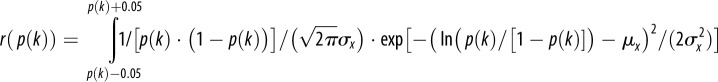 |
| µx | 0 | 0 | 0.4 | 0.4 |
|---|---|---|---|---|
| variance (σx) | low: 0.6 | high: 1 | low: 0.6 | high: 1 |
| PLUS base rate (B) | unbiased: 0.5 | unbiased: 0.5 | raised: 0.6 | raised: 0.6 |
| p(k) | r(p(k)) | r(p(k)) | r(p(k)) | r(p(k)) |
| 0.1 | 0 | 0.04 | 0 | 0.02 |
| 0.2 | 0.03 | 0.09 | 0.01 | 0.05 |
| 0.3 | 0.12 | 0.13 | 0.04 | 0.09 |
| 0.4 | 0.22 | 0.15 | 0.11 | 0.12 |
| 0.5 | 0.26 | 0.16 | 0.21 | 0.15 |
| 0.6 | 0.22 | 0.15 | 0.27 | 0.17 |
| 0.7 | 0.12 | 0.13 | 0.24 | 0.17 |
| 0.8 | 0.03 | 0.09 | 0.11 | 0.15 |
| 0.9 | 0 | 0.04 | 0.01 | 0.09 |
Table 2.
Frequencies r(p(k)) for bimodally distributed p(k) with different PLUS base rates B, low or high variance and symmetric or asymmetric distribution.
| symmetric |
asymmetric |
|||
|---|---|---|---|---|
| variance (σ) | low | high | low | high |
| PLUS base rate (B) | unbiased: 0.5 | unbiased: 0.5 | raised: 0.55 | raised: 0.55 |
| p(k) | r(p(k)) | r(p(k)) | r(p(k)) | r(p(k)) |
| 0.1 | 0 | 0 | 0 | 0 |
| 0.2 | 0 | 0.05 | 0 | 0.05 |
| 0.3 | 0.05 | 0.4 | 0.05 | 0.4 |
| 0.4 | 0.4 | 0.05 | 0.4 | 0.05 |
| 0.5 | 0.1 | 0 | 0.05 | 0 |
| 0.6 | 0.4 | 0.05 | 0.05 | 0 |
| 0.7 | 0.05 | 0.4 | 0.4 | 0.05 |
| 0.8 | 0 | 0.05 | 0.05 | 0.4 |
| 0.9 | 0 | 0 | 0 | 0.05 |
3. Results
3.1. Decision thresholds
The strategy tCONDORCET = (n + 1)/2 led to the most accurate personal choice in a majority of investigated parameter combinations (22 of 27; note that a threshold t = (n + 1)/2 implies a majority of n past experiences). That is, under most circumstances, an animal has the highest chance to make a correct personal choice, if it uses the following strategy. If in the animal's past experience, option PLUS was the correct choice in a majority of cases in the given environmental situation, then the animal should choose option PLUS now. If in the animal's past experience, option PLUS was the correct choice in a minority of cases (i.e. option MINUS was the correct choice in a majority of cases) in the given environmental situation, then the animal should choose option MINUS now.
For three parameter combinations, the personal choice of an animal was most accurate with a slightly lower decision threshold of tmost accurate = (n + 1)/2 − 1 (i.e. the threshold was one less than the simple majority of past-experienced cases n). That is, here the animal has the highest chance to make a correct personal choice, if it chooses option PLUS already when option PLUS was the correct choice in one less than the majority, or in the majority, of past-experienced cases in the given environmental situation. It should choose option MINUS only when, in its past experience, option MINUS was the correct choice in at least one more than the majority of cases. Such lowered threshold tmost accurate = (n + 1)/2 −1 for option PLUS was found when the probability density distribution that option PLUS is the correct option across environmental situations (p(k)) was a unimodal frequency distribution with a low variance and raised PLUS base rate of B = 0.6, at all three levels of animal's expertise (low, medium and high). Here, a lowered threshold for option PLUS is intuitive since option PLUS is a priori more likely to be the correct choice than option MINUS (i.e. the PLUS base rate 0.6 is higher than the MINUS base rate of 0.4). However, a lowered decision threshold for option PLUS did not lead to more accurate decisions in the other investigated distributions of p(k) in which PLUS base rates were also higher than MINUS base rates (tables 1 and 2).
Finally, for two parameter combinations, the personal choice of an animal was most accurate for a slightly higher decision threshold of tmost accurate = (n + 1)/2 + 1 (i.e. the threshold was one more than the simple majority of past-experienced cases n). That is, here the animal has the highest chance to make a correct personal choice, if it chooses option PLUS only when option PLUS was the correct choice in at least one more than the majority of past-experienced cases in the given environmental situation. It should choose option MINUS already when, in its past experience, option MINUS was the correct choice in one less than the majority, or in the majority, of cases. Such raised threshold of tmost accurate = (n + 1)/2 + 1 for option PLUS was the case when the probability density distribution that option PLUS is the correct option across environmental situations (p(k)) was a bimodal and asymmetric frequency distribution and expertise was high (n = 25), at both variance levels (low and high; table 2). This is surprising, since here the base rate for option PLUS was higher than that for option MINUS, and option PLUS had therefore a higher a priori probability to be the correct choice than did option MINUS (PLUS base rate: B = 0.55; MINUS base rate: 1 – B = 0.45; table 2). Nevertheless, the most accurate decision threshold was higher than the expected simple majority threshold t = (n + 1)/2. Thus, here an animal should still favour option MINUS, even when in its past experience option PLUS was the correct choice in one more case than was option MINUS, and despite the fact that the a priori probability that option PLUS is correct is higher than the a priori probability that option MINUS is correct (the reasons for this are discussed in detail in the Discussion section).
Parameter combinations with an a priori higher accuracy rate of option MINUS were not investigated, since results would simply be symmetric.
3.2. Decision accuracy
In groups with preference conflict, collective decision accuracy nearly always peaked at maximum conflict (i.e. when the likelihood that a difference in preferences is found between randomly drawn pairs of group members is maximal; figure 1, solid lines). The exception was when the probability p(k) that option PLUS is the correct option was distributed unimodally across environmental situations k with a PLUS base rate above 0.5 and a small-to-medium number of decision-makers (figure 1e,f, black and dark grey solid lines). Here, a slight bias among the decision-makers in favour of PLUS-animals and a slightly lower degree of conflict (proportion of PLUS-animals = 0.75; conflict = 0.5) resulted in the highest collective decision accuracy.
Figure 1.
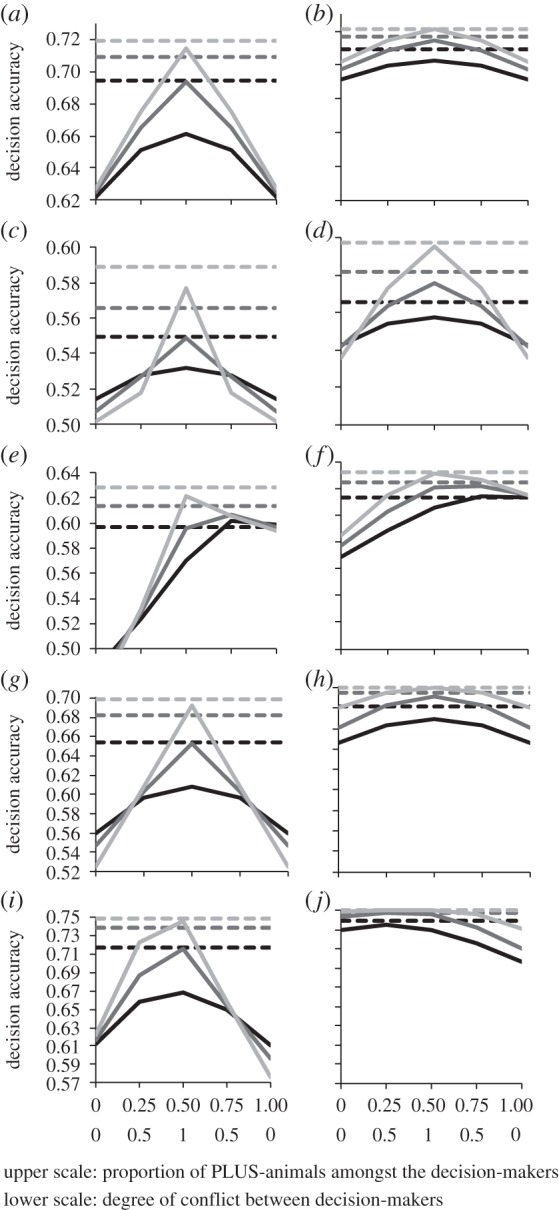
Expected decision accuracy as a function of the proportion of PLUS-animals among the decision-makers and as a function of the degree of conflict of preferences within a group. Note, that degree of conflict and the proportion of PLUS-animals are closely related to each other (both on the x-axes): the degree of conflict is maximal (conflict = 1) if the proportion of PLUS- and MINUS-animals is equal (i.e. at point 0.5 on the upper x-scales); the degree of conflict is minimal (conflict = 0) if the whole group consists either of MINUS-animals only (proportion = 0 on upper scale); or of PLUS-animals only (proportion = 1 on upper scale). The solid lines indicate decision accuracy of groups with conflict (i.e. groups consisting of PLUS- and MINUS-animals). For reasons of comparison, I also give the expected decision accuracies of Condorcet juries of similar size and expertise in the same environments as the conflict groups (dashed lines). Decision accuracies are given for the following parameter combinations of individuals' expertise, environments and number of decision-makers (i.e. group size). (i) Levels of individual expertise (low individual expertise: a, c, e, g, i, n = 3; medium individual expertise: b, d, f, h, j, n = 7; to save space, results for high individual expertise n = 25 are not shown but are qualitatively similar); (ii) environments are reflected by different distributions of the probability p(k) that option PLUS is the correct choice in the kth environmental situation (a, b: uniform distribution; c, d: symmetric unimodal distribution; e, f: asymmetric unimodal distribution; g, h: symmetric bimodal distribution; i, j: asymmetric bimodal distribution; details see table 2) and (iii) numbers of decision-makers (low number of decision-makers: black lines, m = 4; medium number of decision-makers: dark grey lines, m = 8; large number of decision-makers: light grey lines, m = 24). Upper scale: proportion of PLUS-animals among the decision-makers and lower scale: degree of conflict between decision-makers.
Not surprisingly, individual decision accuracy was nearly always highest for the animals that maximize accuracy (i.e. for CONDORCET-animals). As a result, Condorcet juries (figure 1, dashed lines) were usually more accurate than groups in which members had preferences with respect to avoiding false negative or false positive decisions (figure 1: compare solid lines with dashed lines in all graphs). There was again an exception when the probability p(k) that option PLUS is the correct option was distributed unimodally across environmental situations k with a PLUS base rate above 0.5 and a small number of decision-makers (figure 1e,f, black lines). Here, groups with conflicting preferences achieved even higher overall decision accuracy than Condorcet juries if the decision-makers were biased in favour of PLUS-animals (proportion of PLUS-animals ≥ 0.75; conflict = 0.5), albeit the difference was very small. This advantage of groups with conflicting preferences in comparison to Condorcet juries vanished completely as the number of decision-makers increased (figure 1e,f, grey lines).
A rather surprising result was observed when the probability p(k) that option PLUS is the correct option was distributed bimodally across environmental situations k with a PLUS base rate above 0.5. Here, when the animals' expertise is medium to high, a group which is biased towards a higher proportion of MINUS-animals makes more accurate decisions than groups with a higher degree of conflict or groups with a bias towards a higher proportion of PLUS-animals, despite the fact that the base rate is higher for the PLUS than the MINUS option (figure 1j; solid lines). It follows, that it is not always best if the majority of decision-makers in a group is biased in its preferences in favour of the option with the higher overall base rate.
In all types of groups, unsurprisingly, decision accuracy increased with the degree of expertise of individual animals (i.e. with n; compare solid lines within each panel of figure 1). In Condorcet groups and in conflict groups with preference conflict, decision accuracy also increased with the number decision-makers (i.e. with m; figure 1: compare dashed lines within each panel).
3.3. False positives versus false negatives
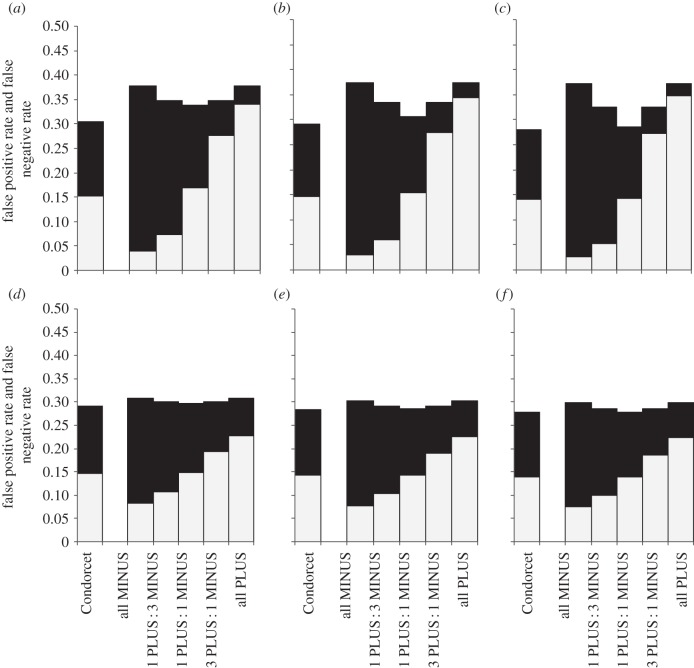
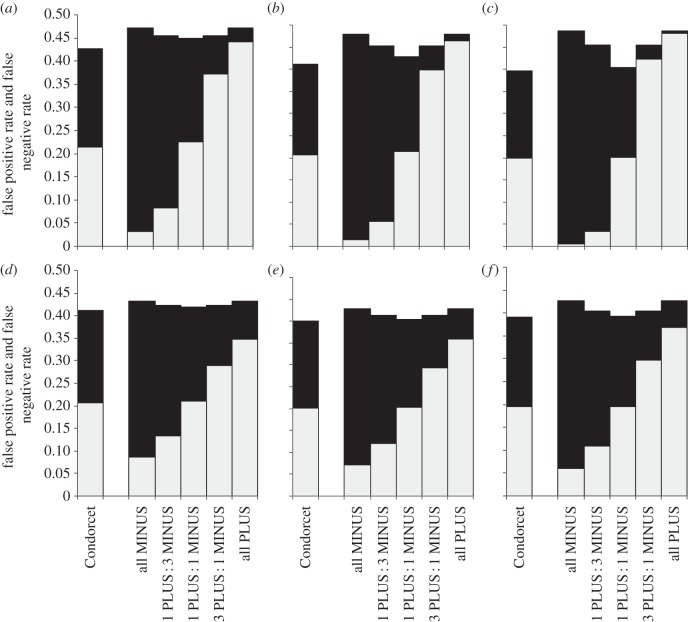
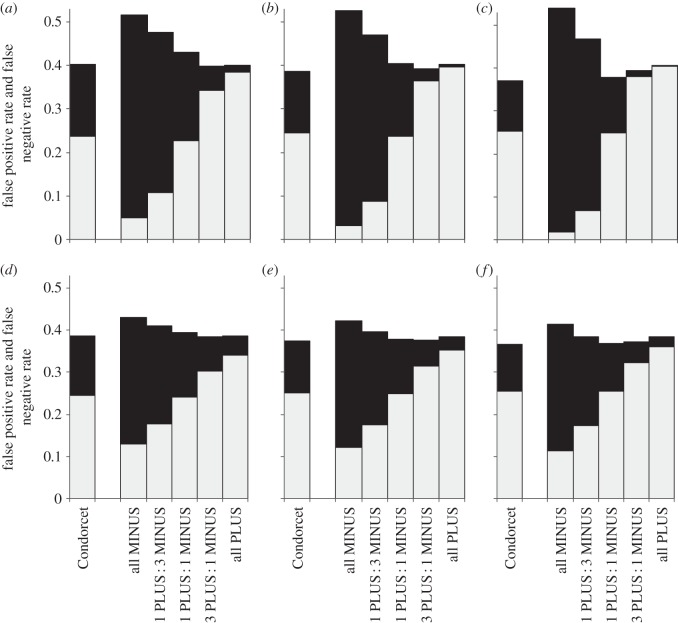
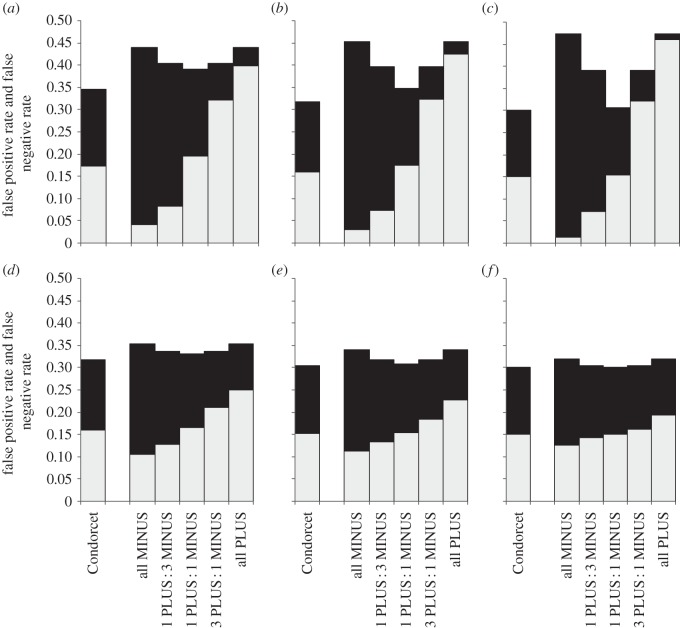
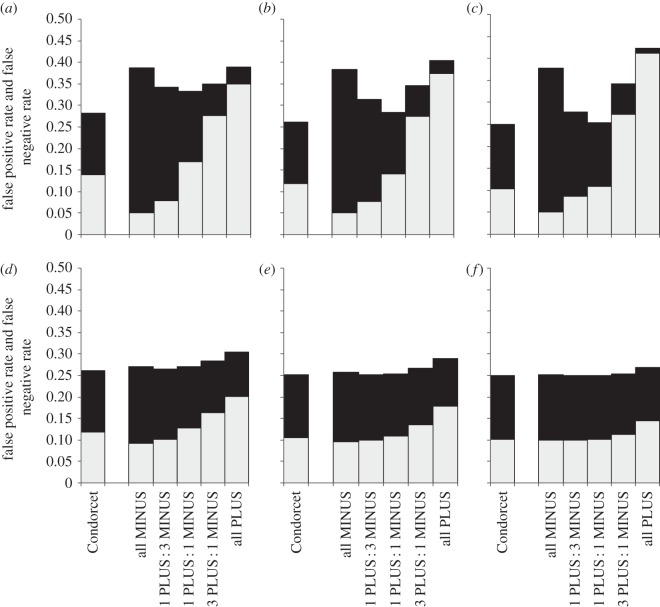
Not surprisingly, the rate of false positives increased with proportion of PLUS-animals among decision-makers and the rate of false negatives increased with the proportion of MINUS-animals (figures 2–6). Groups with the maximum degree of preference conflict had by-enlarge similar rates of false positives and false negatives to those of Condorcet juries (figures 2–6, rates of false positives: white part of bars; rates of false negatives: black parts of bars). However, already through a relatively slight skew towards more PLUS- than MINUS-animals among decision-makers, the rate of expected false negatives versus false positives usually decreased considerably, often with only a relatively slight loss of overall decision accuracy (figures 2–6: decision accuracy = 1 – rate of false negatives – rate of false positives). This was particularly so, when the animals' expertise n was low (figures 2–6a–c). An exception was when the probability p(k) that option PLUS is the correct option was distributed bimodally across environmental situations k, animals had a high degree of expertise and the overall number of decision-makers was large: here, the ratio of false negatives : positives rates hardly changed with the ratio of PLUS- : MINUS-animals (figures 5 and 6f).
Figure 2.
Expected rates of false positives (light grey) and false negatives (black) for groups that consist of Condorcet juries or of various combinations of PLUS- and MINUS-animals (i.e. ratios of 0 : 1, 1 : 3, 1 : 1, 3 : 1 and 1 : 0 PLUS : MINUS-animals, respectively). The probability p(k) (that option PLUS is the correct choice in the kth environmental situation) follows a uniform distribution. Note, the degree of conflict is maximal if the proportion of PLUS- and MINUS-animals is equal (i.e. at 1 PLUS: 1 MINUS-animal on the x-axes). The rates are given for different levels of individual expertise (a–c: low expertise, n = 3; d–f: medium expertise, n = 7; to save space, results for high expertise n = 25 are not shown but are qualitatively similar); and for different numbers of decision-makers (a/d: m = 4; b/e: m = 8; c/f: m = 24).
Figure 3.
Expected rates of false positives (light grey) and false negatives (black) for groups that consist of Condorcet juries or of various combinations of PLUS- and MINUS-animals (i.e. ratios of 0 : 1, 1 : 3, 1 : 1, 3 : 1 and 1 : 0 PLUS : MINUS-animals, respectively). The probability p(k) (that option PLUS is the correct choice in the kth environmental situation) follows a symmetric unimodal distribution with low variance (σx = 0.6; to save space, results for high variance σx = 1 are not shown but are qualitatively similar). Note, same as figure 2 legend.
Figure 4.
Expected rates of false positives (light grey) and false negatives (black) for groups that consist of Condorcet juries or of various combinations of PLUS- and MINUS-animals (i.e. ratios of 0 : 1, 1 : 3, 1 : 1, 3 : 1 and 1 : 0 PLUS : MINUS-animals, respectively). The probability p(k) (that option PLUS is the correct choice in the kth environmental situation) follows an asymmetric unimodal distribution with low variance (σx = 0.6) and a base rate of B = 0.595 (to save space, results for high variance σx = 1 are not shown but are qualitatively similar). Note, same as figure 2 legend.
Figure 5.
Expected rates of false positives (light grey) and false negatives (black) for groups that consist of Condorcet juries or of various combinations of PLUS- and MINUS-animals (i.e. ratios of 0 : 1, 1 : 3, 1 : 1, 3 : 1 and 1 : 0 PLUS : MINUS-animals, respectively). The probability p(k) (that option PLUS is the correct choice in the kth environmental situation) follows a symmetric bimodal distribution with high variance (σx = 1; to save space, results for low variance σx = 0.6 are not shown but are qualitatively similar). Note, same as figures 2 legend.
Figure 6.
Expected rates of false positives (light grey) and false negatives (black) for groups that consist of Condorcet juries or of various combinations of PLUS- and MINUS-animals (i.e. ratios of 0 : 1, 1 : 3, 1 : 1, 3 : 1 and 1 : 0 PLUS : MINUS-animals, respectively). The probability p(k) (that option PLUS is the correct choice in the kth environmental situation) follows a asymmetric bimodal distribution with a high variance and a base rate of B = 0.55 (to save space, results for low variance are not shown but are qualitatively similar). Note, same as figure 2 legend.
4. Discussion
As expected, under most circumstances, the personal choice of an animal was most accurate, if the animal chose option PLUS when, in its past experience, option PLUS had been the correct choice in a majority of cases in the given environmental situation, and vice versa for option MINUS [41]. When option PLUS was a priori more likely to be correct (averaged across all environmental situations), then a slightly lower decision threshold (i.e. a more lenient threshold in favour of option PLUS) could lead to more accurate choices than a threshold which required that option PLUS had been correct in a majority of experienced cases in the past. However, this was not invariably the case.
In particular, when in most environmental situations the probability that option PLUS is the correct choice is either high or low (i.e. a bimodal distribution of p(k)), then the most accurate decision threshold can be higher (and more stringent) than a majority of experienced cases. Perversely, in such a scenario, the most accurate decision threshold for choosing option PLUS can increase as the PLUS base rate (and the a priori probability that option PLUS is the correct option) increases. The underlying reason is, that in such a case, a raised decision threshold discriminates better between environmental situations with a high and with a low probability that option PLUS is the correct choice. This is best illustrated by an example.
Consider the extreme case that in half of environmental situations, it is p(khigh) = 1 and in the other half of environmental situations, it is p(klow) = 0.4. Here, the PLUS base rate is high with B = 0.7 and the a priori probability that option PLUS is the correct option is more than 2.3 times as high as the a priori probability that option MINUS is the correct choice. However, the best decision threshold here is, to choose option PLUS if, and only if, in the past experience option PLUS was the correct choice in every single case. Only then can the animal be in an environmental situation in which it is p(khigh) = 1. In any other environmental situation, it should favour option MINUS. I conclude that knowing the base rate of the success of an option, without knowing the underlying distribution of p(k), can be very misleading, and that animals should ignore base rates when setting decision thresholds, unless detailed further information is available (that is, they should instead use a simple majority threshold in each environmental situation, [41]). Base rate neglect is a known and widely discussed phenomenon in humans [42]. In wild animals, sufficiently detailed data are urgently required to test whether animals ignore base rates in an adaptive manner.
Individuals within a group might differ with respect to their preferences for avoiding false negatives versus avoiding false positives [1,8]. An important example are decisions about predator avoidance [2,12,13,23,35]. When a group of foraging animals decides whether to flee from an expected predator attack, a false negative could cost the life of one or more group members. However, a false positive (i.e. a false alarm) can also have considerable costs: all group members forgo foraging opportunities and waste energy on an unnecessary flight [40]. Here, for a small, vulnerable animal the costs of a false negative are likely to be particularly high; for a hungry or non-satiated animal, the costs of a false positive are relatively high [23,43]. This can lead to inter-individual differences in preferences. In foraging decisions, non-satiated and satiated individuals are likely to differ with respect to their preferences for false negatives and false positives when deciding whether to stay in a partially depleted foraging patch or to move on and search for a potentially better patch [15,17,19,20]. A further example are mating and breeding decisions, in which the relative costs of false positives and false negatives (e.g. with respect to correct species recognition) can differ for animals of opposite sex.
In this study, for groups with preference conflict, decision accuracy nearly always peaked at maximum conflict. This is in good agreement with previous suggestions that another form of conflict, namely a conflict with respect to principal goals, might enhance decision accuracy [30,34]. However, groups with no preferences (Condorcet juries) were nearly always more accurate than groups in which group members did have preferences with respect to avoiding false positives versus avoiding false negatives. Thus, groups with preferences cannot achieve decision accuracy as high as that of groups without such preferences. Moreover, for the most accurate groups with preference conflict, the ratio of false positives versus false negatives was very similar to that ratio in decisions by Condorcet groups. Thus, the loss of accuracy in groups with preferences does not even result in a shift between the danger of false negatives versus false positives.
Nevertheless, such a shift was possible at the expense of a slightly further loss in decision accuracy: usually, a relatively slight skew towards more PLUS- than MINUS-animals among decision-makers decreased the rate of expected false negatives versus false positives considerable, while resulting in a relatively slight loss of overall decision accuracy.
I conclude that in ecological situations in which decision accuracy is extremely crucial for fitness and survival, animals cannot ‘afford’ preferences with respect to avoiding false positives versus avoiding false negatives. However, when decision accuracy is slightly less crucial, then animals might have such preferences. In such a case, it is in the interest of all stakeholders, that the decision-makers are composed of both, animals with a preference of avoiding false negatives over avoiding false positives and animals with a preference of avoiding false positives over avoiding false negatives. Only if animals with different preferences are relatively balanced among decision-makers can a reasonable overall decision accuracy be achieved.
However, there remains the conflict itself. While overall a relatively balanced group of decision-makers is desirable for every stakeholder, ‘PLUS-animals’ will prefer a slight bias towards ‘PLUS-animals’ among the decision-makers, and ‘MINUS-animals’ a slight bias towards ‘MINUS-animals’, since this strongly shifts the ratio of false negatives versus false positives. How this conflict resolves will depend on the specific situation, and in particular on the different pay-offs that are connected with avoiding false positives and avoiding false negatives for all the individual stakeholders [13,16,18–20,28,37]. In any case, the group is likely to avoid that type of error more strongly that the majority of group members prefer to avoid.
Unfortunately, empirical data in the biological literature on collective decision-making are still scarce and not very detailed [30]. One purpose of this model is to encourage (i) the collection of relevant quantitative data by field ecologist (i.e. the relative costs of false negatives/positives for different group members; the personal choice thresholds for different individuals; the collective decision outcomes in relation to group composition) and (ii) the design of adequate experiments to test predictions about collective decision-making.
Acknowledgement
I would like to thank Andrey Morozow for the organization of an interesting and inspiring conference.
References
- 1.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 2.List C. 2004. Democracy in animal groups: a political science perspective. Trends Ecol. Evol. 19, 168–169. ( 10.1016/j.tree.2004.02.004) [DOI] [PubMed] [Google Scholar]
- 3.Couzin ID, Ioannou CC, Demirel G, Gross T, Torney CJ, Hartnett A, Conradt L, Levin SA, Leonard NE. 2011. Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580. ( 10.1126/science.1210280) [DOI] [PubMed] [Google Scholar]
- 4.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 5.Codling EA, Pitchford JW, Simpson SD. 2007. Group navigation and the ‘many-wrongs principle’ in models of animal movement. Ecology 88, 1864–1870. ( 10.1890/06-0854.1) [DOI] [PubMed] [Google Scholar]
- 6.List C, Elsholtz C, Seeley TD. 2009. Independence and interdependence in collective decision making: an agent-based model of nest-site choice by honeybees. Phil. Trans. R. Soc. B 364, 755–762. ( 10.1098/rstb.2008.0277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moussaid M, Garnier S, Theraulaz G, Helbing D. 2009. Collective information processing and pattern formation in swarms, flocks, and crowds. Top. Cogn. Sci. 1, 469–497. ( 10.1111/j.1756-8765.2009.01028.x) [DOI] [PubMed] [Google Scholar]
- 8.Lusseau D, Conradt L. 2009. The emergence of unshared consensus decisions in bottlenose dolphins. Behav. Ecol. Sociobiol. 63, 1067–1077. ( 10.1007/s00265-009-0740-7) [DOI] [Google Scholar]
- 9.Sumpter DJ, Pratt S. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753. ( 10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause J, Ruxton GD, Krause S. 2010. Swarm intelligence in animals and humans. Trends Ecol. Evol. 25, 28–34. ( 10.1016/j.tree.2009.06.016) [DOI] [PubMed] [Google Scholar]
- 11.Katsikopoulos KV, King AJ. 2010. Swarm intelligence in animal groups: when can a collective out-perform an expert? PLoS ONE 5, e15505 ( 10.1371/journal.pone.0015505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward AJW, Herbert-Read JE, Sumpter DJT, Krause J. 2011. Fast and accurate decisions through collective vigilance in fish shoals. Proc. Natl Acad. Sci. USA 108, 2312–2315. ( 10.1073/pnas.1007102108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause J, Bumann D, Todt D. 1992. Relationship between the position preference and nutritional state of individuals in schools of juvenile roach (Rutilus rutilus). Behav. Ecol. Sociobiol. 30, 177–180. ( 10.1007/BF00166700) [DOI] [Google Scholar]
- 14.Conradt L, Roper T. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 15.Clutton-Brock TH, Guiness FE, Albon SD. 1982. Red deer: behaviour and ecology of two sexes. Chicago, IL: University of Chicago Press. [Google Scholar]
- 16.Conradt L, Roper TJ. 2007. Democracy in animals: the evolution of shared group decisions. Proc. R. Soc. B 274, 2317–2326. ( 10.1098/rspb.2007.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruckstuhl KE, Neuhaus P. 2002. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 77, 77–96. ( 10.1017/S1464793101005814) [DOI] [PubMed] [Google Scholar]
- 18.Conradt L, Krause J, Couzin ID, Roper TJ. 2009. ‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304–312. ( 10.1086/596532) [DOI] [PubMed] [Google Scholar]
- 19.Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. 2003. Spontaneous emergence of leaders and followers in foraging pairs. Nature 423, 432–434. ( 10.1038/nature01630) [DOI] [PubMed] [Google Scholar]
- 20.King AJ, Douglas CMS, Huchard E, Isaac NJB, Cowlishaw G. 2008. Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838. ( 10.1016/j.cub.2008.10.048) [DOI] [PubMed] [Google Scholar]
- 21.Kerth G, Ebert C, Schmidtke C. 2006. Group decision making in fission–fusion societies: evidence from two-field experiments in Bechstein's bats. Proc. R. Soc. B 273, 2785–2790. ( 10.1098/rspb.2006.3647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austen-Smith D, Feddersen TJ. 2009. Information aggregation and communication in committees. Phil. Trans. R. Soc. B 364, 763–769. ( 10.1098/rstb.2008.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingle S, Feldman A, Boyce MS, Wilson WF. 2008. Prey behavior, age-dependent vulnerability, and predation rates. Am. Nat. 172, 712–725. ( 10.1086/591675) [DOI] [PubMed] [Google Scholar]
- 24.Hix S, Noury A, Roland G. 2009. Voting patterns and alliance formation in the European Parliament. Phil. Trans. R. Soc. B 364, 821–831. ( 10.1098/rstb.2008.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sueur C, et al. 2011. Collective decision-making and fission-fusion dynamics: a conceptual framework. Oikos 120, 1608–1617. ( 10.1111/j.1600-0706.2011.19685.x) [DOI] [Google Scholar]
- 26.Petit O, Gautrais J, Leca JB, Theraulaz G, Deneubourg JL. 2009. Collective decision-making in white-faced capuchin monkeys. Proc. R. Soc. B 276, 3495–3503. ( 10.1098/rspb.2009.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bousquet CAH, Manser MB. 2011. Resolution of experimentally induced symmetrical conflicts of interest in meerkats. Anim. Behav. 81, 1101–1107. ( 10.1016/j.anbehav.2011.02.030) [DOI] [Google Scholar]
- 28.Conradt L, Roper TJ. 2009. Conflicts of interest and the evolution of decision sharing. Phil. Trans. R. Soc. B 364, 807–819. ( 10.1098/rstb.2008.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeley TD, Buhrman SC. 1999. Group decision making in swarms of honey bees. Behav. Ecol. Sociobiol. 45, 19–31. ( 10.1007/s002650050536) [DOI] [Google Scholar]
- 30.Conradt L. 2012. Models in animal collective decision-making: information uncertainty and conflicting preferences. Interface Focus 2, 226–240. ( 10.1098/rsfs.2011.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biro D, Sumpter DJT, Meade J, Guilford T. 2006. From compromise to leadership in pigeon homing. Curr. Biol. 16, 2123–2128. ( 10.1016/j.cub.2006.08.087) [DOI] [PubMed] [Google Scholar]
- 32.Conradt L, List C. 2009. Group decisions in humans and animals: a survey. Phil. Trans. R. Soc. B 364, 719–742. ( 10.1098/rstb.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastie R, Kameda T. 2005. The robust beauty of majority rules in group decisions. Psychol. Rev. 112, 494–508. ( 10.1037/0033-295X.112.2.494) [DOI] [PubMed] [Google Scholar]
- 34.Conradt L, List C, Roper TJ. In press Swarm intelligence: When uncertainty meets conflict. Am. Nat. [DOI] [PubMed] [Google Scholar]
- 35.Wolf M, Kurvers RHJM, Ward AJW, Krause S, Krause J. 2013. Accurate decisions in an uncertain world: collective cognition increases true positives while decreasing false positives. Proc. R. Soc. B 280, 20122777 ( 10.1098/rspb.2012.2777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulte E. 2010. Information aggregation and preference heterogeneity in committees. Theory Decis. 69, 97–118. ( 10.1007/s11238-008-9118-y) [DOI] [Google Scholar]
- 37.Nagy M, Akos Z, Biro D, Vicsek T. 2011. Hierarchical group dynamics in pigeon flocks. Nature 464, U890–U899. ( 10.1038/nature08891) [DOI] [PubMed] [Google Scholar]
- 38.Swets JA. 1988. Measuring the accuracy of diagnostic systems. Science 240, 1285–1293. ( 10.1126/science.3287615) [DOI] [PubMed] [Google Scholar]
- 39.Kuncel NR, Hezlett SA. 2007. Assessment: standardized tests predict graduate students’ success. Science 315, 1080–1081. ( 10.1126/science.1136618) [DOI] [PubMed] [Google Scholar]
- 40.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman JH. 1997. On bias, variance, 0/1—loss, and the curse-of-dimensionality. Data Mining Knowl. Disc. 1, 55–77. ( 10.1023/A:1009778005914) [DOI] [Google Scholar]
- 42.Gigerenzer G, Hoffrage U. 1995. How to improve Bayesian reasoning without instruction—frequency formats. Psychol. Rev. 102, 684–704. ( 10.1037/0033-295X.102.4.684) [DOI] [Google Scholar]
- 43.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]