Abstract
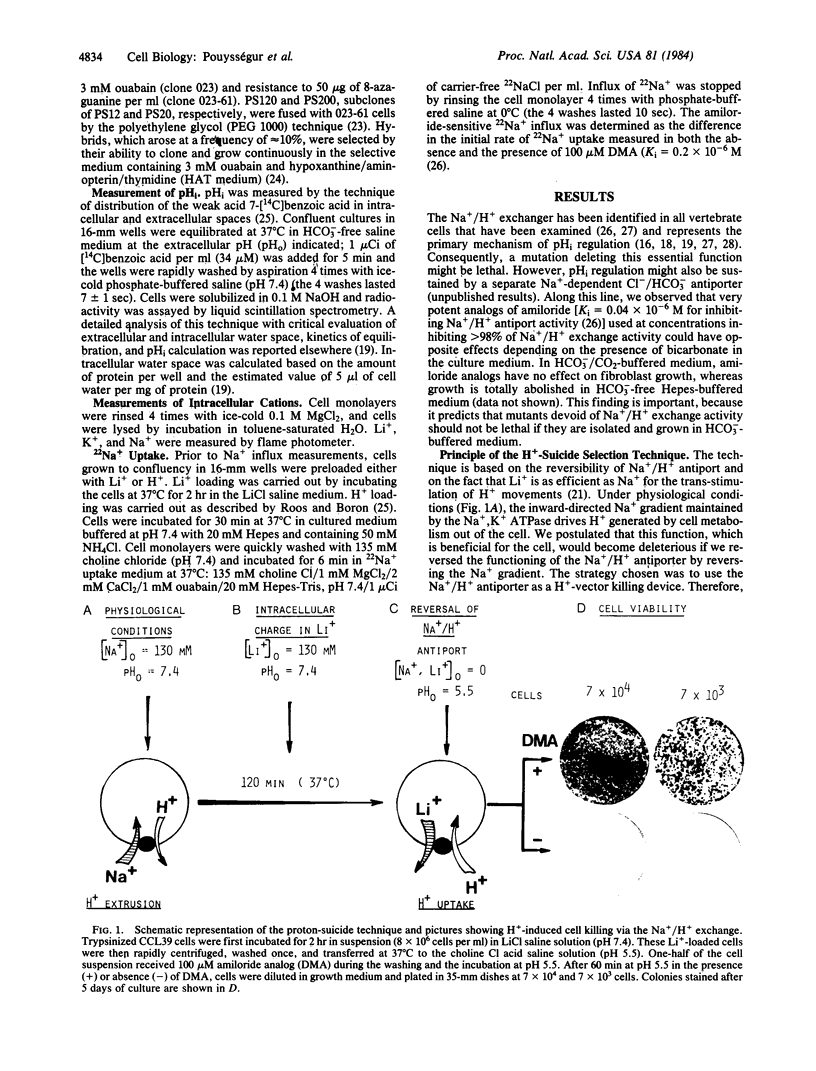
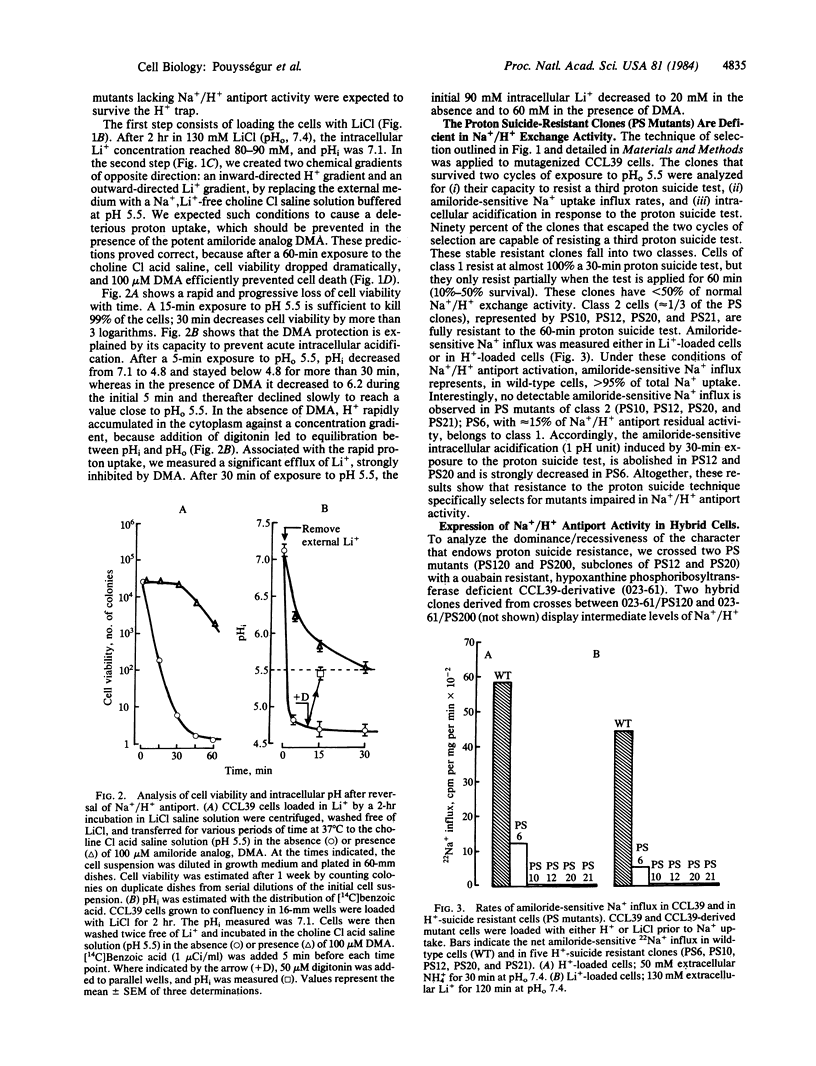
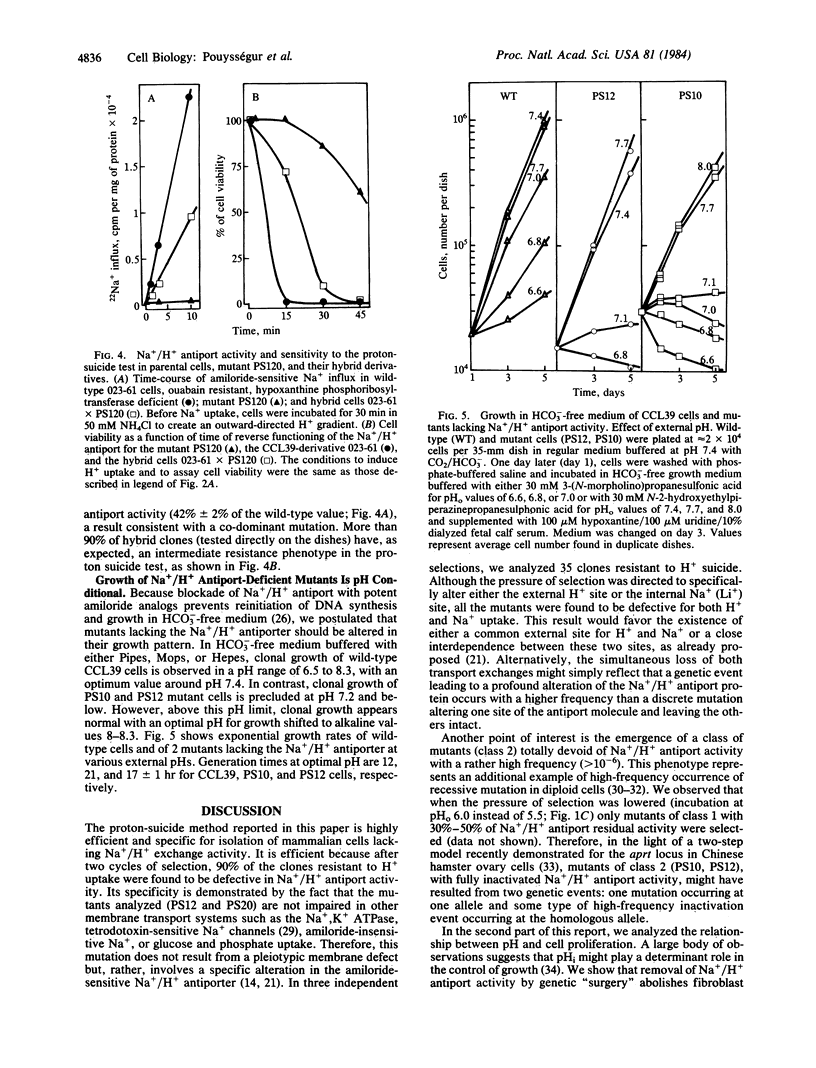
A H+-suicide technique based on the reversibility of Na+/H+ antiport was developed for the selection of mutants deficient in this membrane-bound activity. The strategy was to use the Na+/H+ antiporter as a H+-vector killing device. Chinese hamster lung fibroblasts (CCL39) were loaded with LiCl and incubated in Na+-, Li+-free choline Cl saline solution (pH 5.5). Under these conditions, intracellular pH dropped in 5 min from 7.1 to 4.8, leading to a rapid loss of cell viability (less than 0.1% survival after 30 min). Cytoplasmic acidification and cell death were prevented by treatment with 5-N,N-dimethylamiloride, a potent inhibitor of Na+/H+ antiport. Of the H+-suicide resistant clones that survived two cycles of selection, 90% were found deficient in Na+/H+ antiport activity. One class of mutants (PS10, PS12) fully resistant to the H+-suicide test, does not acidify the cell interior in response to an outward-directed Li+ gradient and has no detectable amiloride-sensitive Na+ influx measured either in Li+- or H+-loaded cells. Growth of these fibroblast clones lacking Na+/H+ antiport was found to be pH conditional in HCO3(-)-free medium. Whereas wild-type cells can grow over a wide range of external pHs (6.6-8.2), PS mutants cannot grow at neutral and acidic pHs (pH less than 7.2); their optimal growth occurs at alkaline pH values (pH 8-8.3). These findings strongly suggest that the Na+/H+ antiport activity through regulation of intracellular pH plays a crucial role in growth control.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Cassel D., Rothenberg P., Zhuang Y. X., Deuel T. F., Glaser L. Platelet-derived growth factor stimulates Na+/H+ exchange and induces cytoplasmic alkalinization in NR6 cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6224–6228. doi: 10.1073/pnas.80.20.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Franchi A., Le Cam A., Pouysségur J. Growth factor-stimulated protein phosphorylation in G0/G1-arrested fibroblasts. Two distinct classes of growth factors with potentiating effects. J Biol Chem. 1983 Feb 10;258(3):1706–1713. [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. The amiloride-sensitive Na+/H+ antiport in 3T3 fibroblasts. J Biol Chem. 1983 May 25;258(10):6272–6276. [PubMed] [Google Scholar]
- Glenn K., Bowen-Pope D. F., Ross R. Platelet-derived growth factor. III. Identification of a platelet-derived growth factor receptor by affinity labeling. J Biol Chem. 1982 May 10;257(9):5172–5176. [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical characterization of mutants of CHO cells resistant to the protein synthesis inhibitor trichodermin. Somatic Cell Genet. 1978 May;4(3):355–374. doi: 10.1007/BF01542848. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Mancuso M. G. Chemicals which promote cell hybridization. Somatic Cell Genet. 1981 Jul;7(4):473–488. doi: 10.1007/BF01542991. [DOI] [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Moss M., Wiley H. S., Fenton J. W., 2nd, Cunningham D. D. Photoaffinity labeling of specific alpha-thrombin binding sites on Chinese hamster lung cells. J Biol Chem. 1983 Mar 25;258(6):3996–4002. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Biochemical characterization of the amiloride-sensitive Na+/H+ antiport in Chinese hamster lung fibroblasts. J Biol Chem. 1983 Mar 25;258(6):3503–3508. [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Salomon J. C., Silvestre P. Isolation of a Chinese hamster fibroblast mutant defective in hexose transport and aerobic glycolysis: its use to dissect the malignant phenotype. Proc Natl Acad Sci U S A. 1980 May;77(5):2698–2701. doi: 10.1073/pnas.77.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Silvestre P. Relationship between increased aerobic glycolysis and DNA synthesis initiation studied using glycolytic mutant fibroblasts. Nature. 1980 Oct 2;287(5781):445–447. doi: 10.1038/287445a0. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Jacques Y., Lazdunski M. Identification of a tetrodotoxin-sensitive Na+ channel in a variety in fibroblast lines. Nature. 1980 Jul 10;286(5769):162–164. doi: 10.1038/286162a0. [DOI] [PubMed] [Google Scholar]
- Rabin M. S., Gottesman M. M. High frequency of mutation to tubercidin resistance in CHO cells. Somatic Cell Genet. 1979 Sep;5(5):571–583. doi: 10.1007/BF01542695. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rothenberg P., Glaser L., Schlesinger P., Cassel D. Activation of Na+/H+ exchange by epidermal growth factor elevates intracellular pH in A431 cells. J Biol Chem. 1983 Oct 25;258(20):12644–12653. [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of Na influx, Na-K pump activity and DNA synthesis in quiescent cultured cells. Adv Enzyme Regul. 1980;19:61–85. doi: 10.1016/0065-2571(81)90009-1. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W. High-frequency mutation at the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells due to deletion of the gene. Proc Natl Acad Sci U S A. 1983 Feb;80(3):810–814. doi: 10.1073/pnas.80.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villereal M. L. Sodium fluxes in human fibroblasts: effect of serum, Ca+2, and amiloride. J Cell Physiol. 1981 Jun;107(3):359–369. doi: 10.1002/jcp.1041070307. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]
- Zilberstein D., Padan E., Schuldiner S. A single locus in Escherichia coli governs growth in alkaline pH and on carbon sources whose transport is sodium dependent. FEBS Lett. 1980 Jul 28;116(2):177–180. doi: 10.1016/0014-5793(80)80637-5. [DOI] [PubMed] [Google Scholar]