Abstract
Considerable advances have occurred in the development of tissue-engineered blood vessels (TEBVs) to repair or replace injured blood vessels, or as in vitro systems for drug toxicity testing. Here we summarize approaches to produce TEBVs and review current efforts to (1) identify suitable cell sources for the endothelium and vascular smooth muscle cells, (2) design the scaffold to mimic the arterial mechanical properties and (3) regulate the functional state of the cells of the vessel wall. Initial clinical studies have established the feasibility of this approach and challenges that make TEBVs a viable alternative for vessel replacement are identified.
Keywords: engineered blood vessels, vascular endothelium, smooth muscle cells, clinical studies
INTRODUCTION
Due to limitations of existing approaches to treat obstructed coronary, carotid and peripheral arteries and arteriovenous shunts for hemodialysis patients, tissue-engineered blood vessels (TEBVs) hold the potential of providing a readily available source to treat a number of complications that arise from cardiovascular disease. While considerable progress has been made towards developing TEBVs and clinical studies have begun, the following key challenges to produce functional engineered vessels remain: (1) produce nonthrombogenic and nonimmunogenic surfaces in contact with blood; (2) develop vessels with appropriate material and mechanical properties to withstand pulsatile blood pressures without failure, permanent deformation or stenosis; and (3) enable physiological vasoconstriction and vasodilation. For clinical application, vessels should be readily available with limited processing time and at a cost competitive to existing procedures. Requirements for cell harvesting and tissue fabrication are specified by Food and Drug Administration (FDA) guidance documents (http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/default.htm). While these requirements are demanding, they also provide opportunities for innovative ways to design TEBVs.
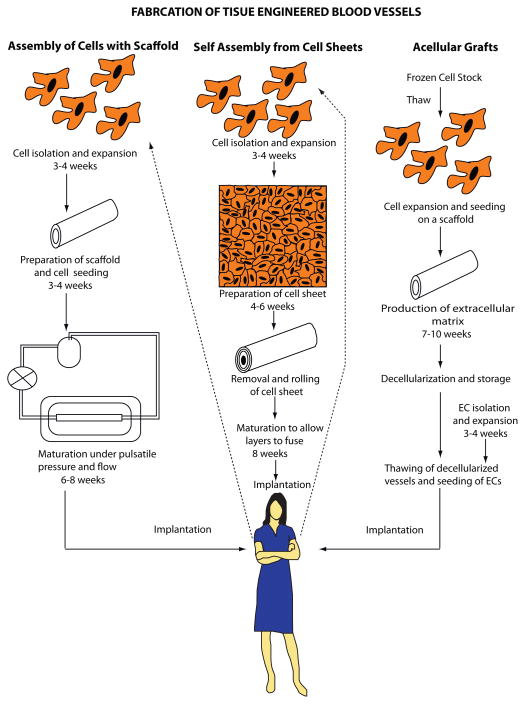
Three general approaches are used to develop TEBVs for clinical applications [1,2] (Figure 1 and Box 1): (1) in vitro assembly of vessels with cells and degradable synthetic or biological scaffolds; (2) in vitro self-assembly from cell sheets; and (3) in vivo vessel formation of implanted acellular grafts derived from decellularized blood vessels, subintestinal submucosa or cultured allogeneic smooth muscle cells (SMCs) [3].
Figure 1.
Schematic of different approaches to fabricate tissue engineered blood vessels. Advantages and challenges with each approach are summarized in Box 1.
Box 1. Fabrication of Tissue Engineered Blood Vessels Figure 1.
Method I – Cell-seeding of scaffold
Advantages
Cells in the scaffold enable TEBVs to respond to physiological stimuli. Fluid shear stress stimulates ECs to produce nitric oxide and prostacyclin, which are antithrombotic and promote vasodilation by SMCs. The SMCs produce extracellular matrix proteins and enable remodeling of TEBVs. The scaffold provides the mechanical properties necessary for functioning TEBVs in addition to attachment sites for ECs.
Challenges
Since the cells need to be autologous to avoid rejection by the recipient’s immune system, these vessels have to be produced far in advance of the planned surgery to expand cells ex vivo and enable the TEBV to develop suitable mechanical properties. The cell expansion process must satisfy stringent regulatory requirements and is costly.
Future Directions
Isolating cells at the point-of-care could eliminate the ex vivo culture period.
Method II – Self-assembly from cell sheets
Advantages
This method does not require a scaffold. The cell sheet production and rolling parameters can control the number and orientation of cell layers within the TEBV. SMCs can be utilized to enable the TEBV to respond to physiological stimuli and ECs may be incorporated to provide an antithrombotic surface.
Challenges
As with method I, the time to prepare TEBVs is long due to culture of autologous cells, preparation of cell sheets, and maturation of the vessel.
Future Directions
Non-immunogenic ‘universal donor cells’ could shorten the time to produce cell sheets. Allogeneic mesenchymal stem cells have already been tested in clinical trials and found to have immunosuppressive effects. However, MSCs are not antithrombotic, therefore ECs would still be needed on the inner surface of the cell sheets.
Method III – Acellular grafts
Advantages
Since the tissue is decellularized before implantation and is non-immunogenic, enabling harvested tissue or allogeneic human cells to be used. This allows for storage of decellularized vessels resulting in ‘off-the-shelf’ products.
Challenges
To ensure sufficient mechanical strength, acellular grafts may need to be reinforced with synthetic materials. In this case, the polymer resorption rate needs to be balanced with the TEBV remodeling rate to obtain the appropriate burst strength and compliance. Acellular TEBVs fail if their diameter is less than 6 mm because of thrombosis. For these smaller diameter vessels, an endothelial lining shortly after implantation is crucial.
Future Directions
Production time could be reduced with point-of-care EC isolation or novel methods to rapidly endothelialize acellular tissue grafts shortly after implantation.
In vitro methods often require extended culture periods for cells to produce and remodel the extracellular matrix (ECM) so that TEBVs have suitable mechanical strength [2], whereas acellular approaches rely upon the in vivo growth of cells from adjacent vessels into decellularized grafts to promote remodeling. Maturation of acellular grafts may be compromised in individuals with cardiovascular disease, leading to incomplete graft remodeling and reduced vasoactivity and endothelialization. Animal studies suggest that addition of cells to acellular grafts prior to implantation may improve their in vivo performance [4]. Given that endothelialization of grafts by ingrowth from adjacent vessels is limited, TEBVs with inner diameters less than 6 mm may need to be seeded with endothelial cells (ECs) to prevent thrombosis.
Addressing these challenges involves identifying suitable autologous or derived cell sources for the endothelium and vascular smooth muscle cells, designing the scaffold to mimic the arterial mechanical properties and regulating the functional state of the cells of the vessel wall. After discussing recent clinical studies, we review progress in each of these areas.
Clinical Studies
The first clinical trial of TEBVs to treat single ventricle congenital defects involved 25 patients ranging in age from 1 to 24 years [5]. Biodegradable scaffolds of woven polyglycolic acid, poly-L-lactide and ε-caprolactone (50:50) were seeded with autologous bone marrow mononuclear cells and implanted as grafts to reconfigure portions of the pulmonary circulation. Over a mean follow-up time of 5.8 years, no graft-related deaths occurred, and all vessels remained patent. The major complication of the implanted grafts was stenosis in 24% of the patients, which could be treated with balloon angioplasty. These studies demonstrated the feasibility of using TEBVs to replace low-pressure blood vessels.
L’Heureux and colleagues at Cytograft Tissue Engineering produced an entirely autologous blood vessel using cell sheet tissue engineering [6]. Human fibroblasts were extracted from skin biopsies and ECs were harvested from a superficial vein. These small-diameter TEBVs had sufficient mechanical strength and were successfully used for hemodialysis in a clinical trial involving 10 patients with a total of 68 patient-months of patency [7]. Seeding of the lumen with autologous ECs also provided grafts with the necessary antithrombotic lining. In an effort to reduce the time required to produce the completely autologous vascular graft, the L’Heureux group is examining the use of allogeneic human fibroblasts, non-endothelialized vessels, and the assembly of three-dimensional vessels from threads of cell-synthesized extracellular matrix (ECM) [8].
Investigators at Humacyte [9] generated decellularized scaffolds by first growing human SMCs on a tubular polyglycolic acid (PGA) scaffold, and then removing all cellular material with detergents to leave behind a TEBV comprised completely of ECM. These TEBVs have favorable mechanical properties, in part through the incorporation of PGA, and are nonimmunogenic since all cellular material is removed before implantation. These TEBVs (inner diameter ≥ 6 mm) were tested in an arteriovenous (AV) fistula model between the axilliary artery and the brachial vein of baboons, and exhibited >80% patency for up to 6 months. After implantation all TEBVs showed extensive medial layer remodeling and exhibited partially endothelialized regions near the anastomotic sites with native vessels. Based on promising preclinical studies (Dahl et al. Circulation. 2013; 127: 2071–2072, doi: 10.1161/CIR.0b013e318295baf5) Humacyte began a multi-center European clinical trial in December 2012, and additional patient enrollment was approved after a safety review in April 2013. In June 2013, the FDA approved U.S. clinical trials to assess safety and function of these acellular grafts in dialysis patients who are unable to undergo AV fistula formation due to prior vessel damage.
Cell Sources for Vascular Tissue Engineering
The ideal TEBV cell sources for vascular endothelial and smooth muscle cells should be autologous, capable of many cell divisions, and able to differentiate into the mature phenotype. Adult stem populations represent a promising source of autologous cells with the capacity to differentiate to vessel wall cells [10,11]. Autologous mesenchymal stem cells (MSCs) from bone marrow, adipose tissue or muscle can differentiate to form a contractile cell type similar to vascular smooth muscle [11].
Source of Vascular ECs
The rapid generation of an endothelial layer is especially important for functional TEBVs with diameters less than 6 mm [2]. Autologous human microvascular ECs can be obtained from jugular or saphenous vein or liposuctioned fat, but these procedures are unattractive because of the limited number and lifespan of cells obtained, and the invasiveness of the procedures. Adipose-derived microvascular cell cultures are often contaminated with other cell types (e.g. macrophages and fibroblasts) resulting in an increased rather than decreased development of intimal hyperplasia in a dog model [12] and a decreased patency in transluminally seeded vessels in a rabbit model [13].
Promising approaches for deriving autologous ECs that do not suffer from these limitations include (1) differentiating MSCs directly to ECs [14], (2) differentiating of blood-derived late outgrowth endothelial progenitor cells (EPCs) into ECs [15], (3) dedifferentiating host stromal cells to intermediate induced pluripotent stem (iPS) cells that then differentiate to ECs [16,17], or (4) transdifferentiating harvested host stromal cells directly to ECs without inducing pluripotency [18].
Bone marrow derived cells initially generated considerable enthusiasm because large numbers of mononuclear cells (MNCs) are readily available via bone marrow aspiration from the iliac crest and could be utilized on the day of TEBV implantation. The expectation was that bone marrow derived MNCs would differentiate into ECls in vivo [19]. Instead, bone marrow MNCs evoke an inflammatory-mediated process of remodeling [5]. Addition of EC-specific growth factors such as vascular endothelial growth factor and fibroblast growth factor were not successful in producing ECs [11]. Exposure of bone marrow MSCs to fluid shear stress does induce expression of EC-specific molecules such as von Willebrand Factor (vWF), platelet-endothelial cell adhesion molecule and VE-cadherin, suggesting that ECs could be derived from MSCs [20].
Our work has focused on EPCs as a readily available source of host ECs that can be obtained from adult peripheral blood or umbilical cord blood [21]. Depending on the isolation method, two functionally distinct populations known as early-outgrowth and late-outgrowth EPCs can be obtained. Only late-outgrowth EPCs, or endothelial colony forming cells (ECFCs), exhibit conventional EC behavior and EC surface markers [22,23], as well as a high proliferative potential. Late-outgrowth EPCs isolated from healthy individuals and from patients with cardiovascular disease exhibit senescence. ECs derived from late-outgrowth EPCs elicit substantially lower alloimmune reaction than aortic ECs [24] and may be an allogeneic source. EPCs from umbilical cord blood can undergo 50–60 cell divisions before senescence [25]. Together with new protocols that permit higher yields of EPCs from small blood volumes [26], over 1010 cells could be banked from each isolation, making this an attractive cell source.
Late-outgrowth EPCs resemble mature ECs and are not derived from bone marrow [27]. ECs derived from human umbilical cord blood [28] and adult blood [25] function the same as human aortic ECs (HAECs) with regard to: expression of VE-cadherin, CD31, vWF; uptake of acetylated low-density lipoprotein; elongation and increased nitric oxide (NO) levels at physiologic shear stresses; elongation and alignment with flow direction; and up-regulation of key EC genes Krüppel-like factor 2, endothelial NO synthase (eNOS), cyclo-oxygenase 2, and thrombomodulin after exposure to flow.
Porcine late-outgrowth EPCs can spread and proliferate on titanium surfaces in vivo, protecting against thrombosis even in the low shear environment of the inferior vena cava [29]. Likewise, EPCs from blood of humans with cardiovascular disease seeded onto 1mm diameter expanded poly-tetrafluoroethylene vascular grafts had 28-day patency rates of 75–88%, although intimal hyperplasia was observed near the proximal and distal anastomoses [15].
Sources of Vascular Smooth Muscle Cells
Human vascular SMCs often have limited proliferative and synthetic capability, compromising their ability to produce mechanically strong TEBVs. Cells with SMC properties could be derived from bone marrow mononuclear cells, mesenchymal stem cells from bone marrow, adipose tissue and skeletal muscle [11]. A promising source of vascular smooth muscle is iPS cells derived from late outgrowth EPCs [30]. These EPCs can be easily reprogrammed and do no exhibit copy number variations [30], raising the possibility of deriving pure populations of autologous cells to generate TEBVs.
Extracellular Matrix Production by Medial Cells to Regulate Mechanical Properties of TEBVs
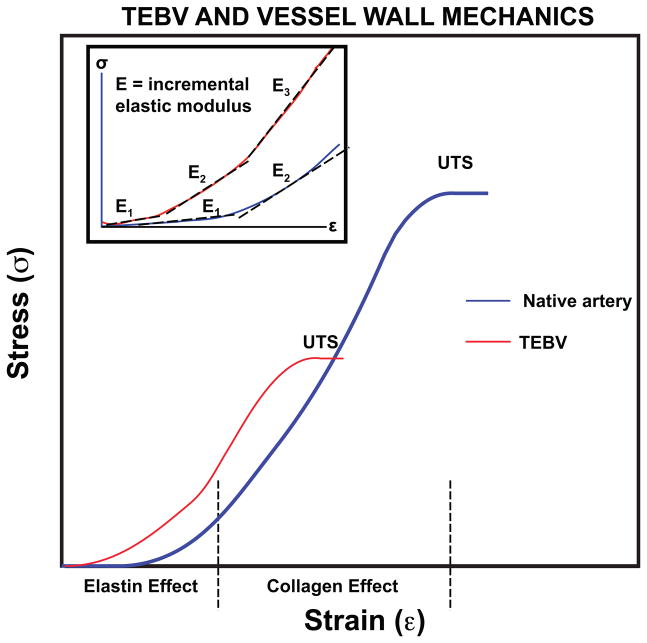
The mechanical behavior of arteries enables their expansion after ejection of blood from the left ventricle with only a modest rise in pressure, reducing the work on the heart. The media of small-diameter muscular arteries, such as coronary arteries, contains SMCs arranged in concentric layers within an ECM comprised primarily of collagen and elastin [31]. Elastin fibers are located in concentric lamellae between SMC layers to support the compliance of the vessel during pulsatile flow [32] (Figure 2 and Box 2). Collagen fibrils in native arteries are organized in circumferential, helical, and axial directions [33], providing tensional strength to the native artery at high strains. Replicating the ECM composition of native arteries in a tissue engineered vascular construct has proven to be difficult. Transmission electron microscopy indicated that collagen in tissue engineered arteries was surrounded by glycosaminoglycans (GAGs) and SMCs, while that of native arteries was surrounded by elastin or other collagen fibrils [33]. Thus, the natural ECM environment has not yet been properly recreated within TEBVs.
Figure 2.
Stress-strain behavior of native arteries and current best behavior of TEBVs. Highlighted are the incremental modulus (Einc) and ultimate tensile strength (UTS). Definitions are provided in Box 2.
Box 2. TEBV and Vessel Wall Mechanics Figure 2.
Blood vessels and TEBVs behave as nonlinear elastic materials. A constitutive relationship has not yet been developed that is valid over the wide ranges of stresses and strains to which blood vessels are normally exposed. However, key features of the mechanical properties can be defined using concepts for elastic materials.
The nonlinear mechanical behavior of blood vessels and TEBVs can be clearly shown by plots of stress versus strain (Figure 2). Such plots are generated using rectangular strips of TEBVs and stretching in circumferential direction. Alternatively, the vessel can be pressurized and the relationship between pressure and volume in the lumen or inner diameter of vessel determined.
For an elastic material, the elastic modulus is the slope of the stress versus strain, σ =Eε. For a nonlinearly elastic material, the incremental modulus is the local slope of the stress-strain curve
| (1) |
Shown in the inset to Figure 2 are several values of incremental modulus.
As an alternative to the elastic modulus, many investigators report compliance. Several different types of compliance are used. A common form is known as the volume compliance, CV.
| (2) |
The units of compliance are m3 Pa−1 or more commonly ml (mm Hg)−1. This definition is used due to the manner in which the compliance of blood vessels is measured. The volume compliance depends upon the vessel dimensions (inner radius R and length L) and incremental elastic modulus:
| (3) |
where the volume is V=πR2L. Note that while the elastic modulus is a material property, the compliance is not, since it is sensitive to geometry.
The ultimate tensile strength (UTS, Pa) is the maximum stress that can be applied before the vessel fails. This quantity is related to the pressure at which an intact vessel fails (burst pressure, Pb) by application of the law of Laplace.
| (4) |
Where t is the vessel wall thickness and Di is the initial inner diameter.
Compared to native vessels, small-diameter synthetic grafts are stiffer, stronger and less compliant, and fail due to thrombosis and neointimal hyperplasia from compliance and diameter mismatch between the graft and the native artery [34]. TEBVs are generally weaker, although the compliance more closely matches values for native vessels.
A balance between mechanical strength and chemical functionality is needed when choosing a support matrix for TEBV manufacture. PGA fiber matrices have been widely used since they degrade slowly, allowing sufficient time for the maturing vessel media to develop sufficient mechanical strength [9,35]. Fibrin matrices stimulate ECM protein production leading to stronger TEBVs [36,37]. The burst strength of fibrin vessels with human dermal fibroblasts approaches values of native vessels after pulsatile stretch at physiological pressures for 7–9 weeks [38], emphasizing the importance of biomechanical stimuli.
To enhance the strength of collagen gels as a support matrix for TEBVs, newly formed collagen gels may be plastically compressed to produce dense collagen scaffolds with collagen fibrillar densities comparable to those of the native ECM [39]. Dense collagen gels made from plastic compression have demonstrated good incorporation with cells, allowing for good adhesion and proliferation [40,41]. Further conditioning under pulsatile pressure increases burst strengths to 1,000 mm Hg [40].
Importance of Elastin Production in TEBVs
Elastin fiber production is currently lacking from most TEBV approaches and has not been a primary focus of TEBV production until recently. However, elastin is critical for the mechanical and signaling properties of vascular tissues. Elastic fibers comprise 30–50% of the dry weight of native vascular tissues and play important roles in the induction of actin stress fiber organization as well as the inhibition of SMC proliferation and migration [42,43]. The proliferation of arterial SMCs is modulated by the transduction of signaling pathways activated by the interaction of soluble elastin degradation products with the elastin receptor [44]. SMC proliferation produces stenoses in arteries when extracellular elastin is not present [45].
Functional extracellular elastin formation is a complex process involving elastin protein production, secretion and fibril formation. Elastin fiber production in engineered vascular tissues is largely prevented by reduced translation of tropoelastin mRNA in cells older than neonatal cells and by inefficient tropoelastin recruitment and cross-linking into the elastic matrix [46]. Elastin synthesis in the native environment is promoted by cyclic GMP, insulin-like growth factor 1, transforming growth factor β1 (TGFβ1), and fibrin degradation products[ 46].
Substrate composition and topography influence the elastin production by medial cells such as vascular SMCs or fibroblasts. Elastin-based substrates are subject to enzymatic degradation without providing biochemical and biomechanical signals promoting elastin synthesis by the medial cells [47]. Although collagen promotes the quiescent, contractile phenotype of SMCs, this can limit the synthesis of elastin precursors and assembly of elastin structures in the ECM [46,48]. 3D scaffold topography and the presence of TGF-β1 increased elastin gene expression and synthesis and expression of contractile markers by human coronary artery SMCs [49]. Interestingly, TGF-β1 did not affect elastin synthesis in 2D cultures. Furthermore, a correlation was found between the pore size of a substrate seeded with baboon and porcine SMCs and elastin and collagen production [50]. Vascular SMCs embedded in 3D collagen gels exposed to long-term cyclic distention increased production of elastin but not collagen [51]. Dense collagen gels have been combined with elastin protein polymer layers to create robust, mechanically strong TEBVs without the incorporation of medial cells [52].
TEBVs created from cell sheets of SMCs transduced with splice variant 3 of the proteoglycan versican and cultured with reduced exposure to ascorbate exhibited greater tropoelastin production, elastin crosslinks, and thicker collagen fiber bundles [53]. The presence of hyaluronan oligomers and TGF-β1 increases the elastin matrix deposition of adult rat aortic SMCs seeded within 3D collagen gels [54]. Rapamycin promote the contractile phenotype of SMCs and elastin synthesis in normal SMCs and iPS cells derived from patients with Williams-Beuren Syndrome, which involves a micro-deletion of one copy of the tropoelastin gene on chromosome 7 [55]. Since rapamycin is used clinically in drug eluting stents to inhibit SMC proliferation, this drug could be added to TEBVs after the vessel wall cells have proliferated sufficiently, thereby inducing a contractile phenotype and initiating elastin synthesis. Rapamycin addition would need to be coordinated so as not to interfere with EC adhesion and growth.
TEBVs for Drug Toxicity Testing
Endothelialized TEBVs would allow for the creation of in vitro drug testing models [56]. To date TEBVs have been studied under ideal conditions for healthy individuals. However, in the clinic, TEBVs would be implanted in patients with atherosclerosis and systemic inflammation. Therefore, evaluation of the vasoreactive response under these conditions would provide more realistic models for how the TEBV would behave after implantation. The endothelium serves as a primary target for medications for blood pressure and inflammation. An endothelialized, vasoreactive TEBV would enhance the in vitro study of new drugs to regulate cholesterol and treat hypertension, diabetes and autoimmune diseases such as lupus.
Acute inflammatory responses may be elicited through exposure to tumor necrosis factor-α (TNF-α), causing endothelium to express inflammatory markers such as vascular cell adhesion molecule – 1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), and E-selectin. Furthermore, inflammation has been shown to impair endothelial vasomotor function [2]. Statins, which are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, lower blood cholesterol levels and improves endothelium-dependent vasodilation [57]. Atorvastatin has reduces endothelial cell expression of ICAM-1 and VCAM-1 induced by TNF-α exposure in endothelial cells cultured alone [58]. Creating a vasoresponsive TEBV would open the door for more accurate in vitro models for testing endothelial response to medications.
CONCLUSIONS AND RECOMMENDATIONS
Development of mechanically strong and vasoactive TEBVs is critical for clinical advancement in small-diameter bypass grafts and vascularization of tissues. Acellular TEBVs easily manufactured and show great potential for off-the-shelf accessibility. These vessels can be used for large vessel replacement or high flow situations, such as hemodialysis access shuts. For bypass procedures, both mural cells and endothelium are required to create a vasoactive small diameter TEBV capable of integration with the native vasculature. A confluent endothelium provides a non-thrombogenic surface and releases nitric oxide for flow-mediated vasodilation, while a differentiated mural layer populated with SMCs, MSCs, or fibroblasts exhibits contractility in response to pulsatile flow or drug agonists. Creating a small-diameter TEBV that closely replicates the native vascular environment would enable more robust in vitro models for drug toxicity testing. Future efforts in this field should focus on finding an easily expandable cell source and more closely replicating the native ECM environment and mechanical properties while maintaining short production times.
Highlights.
Initial clinical results with tissue engineered blood vessels are promising
Acellular grafts can be rapidly fabricated for applications in high flow
Cell-based engineered vessels are needed to reproduce full function of arteries
A confluent endothelium still appears to be needed to replace small diameter vessels
New approaches to produce vessels should address clinical challenges
Acknowledgments
The work was supported by UH2TR000505 and the NIH Common Fund for the Microphysiological Systems Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• Of special interest
•• Of outstanding interest
- •1.Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. Vascular tissue engineering: the next generation. Trends in Molecular Medicine. 2012;18:394–404. doi: 10.1016/j.molmed.2012.04.013. This review nicely summarizes clinical studies using vascaulr grafts and identifies barriers to the development and translation of TEBV to clinical application. [DOI] [PubMed] [Google Scholar]
- 2.Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nat Rev Cardiol. 2013;10:410–421. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- ••3.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. Readily Available Tissue-Engineered Vascular Grafts. Science Translational Medicine. 2011;3:68ra69. doi: 10.1126/scitranslmed.3001426. Vascular grafts were produced from the extracellular matrix produced by cultured human smooth muscle cells and shown to have suitable mechanical strength and patency for up to 6 months in a baboon arteriovenous graft model. This represents a promising approach to provide an access vessel for hemodialysis patients. [DOI] [PubMed] [Google Scholar]
- 4.Yazdani SK, Watts B, Machingal M, Jarajapu YPR, Van Dyke ME, Christ GJ. Smooth Muscle Cell Seeding of Decellularized Scaffolds: The Importance of Bioreactor Preconditioning to Development of a More Native Architecture for Tissue-Engineered Blood Vessels. Tissue Eng Part A. 2009;15:827–840. doi: 10.1089/ten.tea.2008.0092. [DOI] [PubMed] [Google Scholar]
- 5.Hibino N, Villalona G, Pietris N, Duncan DR, Schoffner A, Roh JD, Yi T, Dobrucki LW, Mejias D, Sawh-Martinez R, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25:2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. New Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- ••7.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. The authors report on the clinical results of ten patients who received completely biological TEBVs with autologous cells. This constitutes a landmark study because it is the only known clinical trial to date with patients who received grafts that were entirely produced from their own cells using sheet-based tissue engineering. [DOI] [PubMed] [Google Scholar]
- 8.Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N. The Evolution of Vascular Tissue Engineering and Current State of the Art. Cells Tissues Organs. 2012;195:144–158. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra69. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 10.Bajpai VK, Andreadis ST. Stem Cell Sources for Vascular Tissue Engineering and Regeneration. Tissue Eng Part B Rev. 2012;18:405–425. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: A review. Biomaterials. 2012;33:3388–3400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Arts CH, Hedeman Joosten PP, Blankensteijn JD, Staal FJ, Ng PY, Heijnen-Snyder GJ, Sixma JJ, Verhagen HJ, de Groot PG, Eikelboom BC. Contaminants from the transplant contribute to intimal hyperplasia associated with microvascular endothelial cell seeding. Eur J Vasc Endovasc Surg. 2002;23:29–38. doi: 10.1053/ejvs.2001.1532. [DOI] [PubMed] [Google Scholar]
- 13.Arts CH, De Groot PG, Attevelt N, Heijnen-Snyder GJ, Verhagen HJ, Eikelboom BC, Blankensteijn JD. In vivo transluminal microvascular endothelial cell seeding on balloon injured rabbit arteries. J Cardiovasc Surg. 2004;45:129–137. [PubMed] [Google Scholar]
- 14.Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. STEM CELLS. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 15.Stroncek JD, Ren LC, Klitzman B, Reichert WM. Patient-derived endothelial progenitor cells improve vascular graft patency in a rodent model. Acta Biomaterialia. 2012;8:201–208. doi: 10.1016/j.actbio.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, et al. Endothelial Cells Derived From Human iPSCs Increase Capillary Density and Improve Perfusion in a Mouse Model of Peripheral Arterial Disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel R, Daheron L, Liao S, Vardam T, Kamoun WS, Batista A, Buecker C, Schäfer R, Han X, Au P, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1310675110. published ahead of print July 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Huang NF, Zou J, Laurent TJ, Lee JC, Okogbaa J, Cooke JP, Ding S. Conversion of Human Fibroblasts to Functional Endothelial Cells by Defined Factors. Arterioscler Thromb Vasc Biol. 2013;33:1366–1375. doi: 10.1161/ATVBAHA.112.301167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura G, Miyagawa-Tomita S, Shin’oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 20.Yuan L, Sakamoto N, Song G, Sato M. High-level Shear Stress Stimulates Endothelial Differentiation and VEGF Secretion by Human Mesenchymal Stem Cells. Cellular and Molecular Bioengineering. 2013;6:220–229. [Google Scholar]
- 21.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis Thrombosis and Vascular Biology. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 23.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133(+) cells or CD45(+) hematopoietic precursors. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 24.Ladhoff J, Fleischer B, Hara Y, Volk HD, Seifert M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovascular Research. 2010;88:121–129. doi: 10.1093/cvr/cvq109. [DOI] [PubMed] [Google Scholar]
- 25.Stroncek JD, Grant BS, Brown MA, Povsic TJ, Truskey GA, Reichert WM. Comparison of endothelial cell phenotypic markers of late outgrowth EPCs isolated from coronary artery disease patients and healthy volunteers. Tissue Eng. 2009;35:3473–3486. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang SD, Carlon TA, Jantzen AE, Lin FH, Ley MM, Allen JD, Stabler TV, Haley NR, Truskey GA, Achneck HE. Isolation of Functional Human Endothelial Cells from Small Volumes of Umbilical Cord Blood. Ann Biomed Eng. 2013 doi: 10.1007/s10439-013-0807-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tura O, Skinner EM, Barclay GR, Samuel K, Gallagher RC, Brittan M, Hadoke PW, Newby DE, Turner ML, Mills NL. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. STEM CELLS. 2013;31:338–348. doi: 10.1002/stem.1280. [DOI] [PubMed] [Google Scholar]
- 28.Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of Umbilical Cord Blood Derived Late Outgrowth Endothelial Progenitor Cells Exposed to Laminar Shear Stress. Tissue Eng Part A. 2009;15:3575–3587. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Jantzen AE, Lane WO, Gage SM, Jamiolkowski RM, Haseltine JM, Galinat LJ, Lin FH, Lawson JH, Truskey GA, Achneck HE. Use of autologous blood-derived endothelial progenitor cells at point-of-care to protect against implant thrombosis in a large animal model. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.07.066. This study illustrates that it is feasible to rapidly seed bare metal tubes with late-outgrowth endothelial cells at the point-of-care, which suggests that it is also possible to seed TEBVs (or acellular grafts) within minutes of implantation and achieve a functional EC layer that protects the graft against thrombosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •30.Geti I, Ormiston ML, Rouhani F, Toshner M, Movassagh M, Nichols J, Mansfield W, Southwood M, Bradley A, Rana AA, et al. A Practical and Efficient Cellular Substrate for the Generation of Induced Pluripotent Stem Cells from Adults: Blood-Derived Endothelial Progenitor Cells. Stem Cells Translational Medicine. 2012;1:855–865. doi: 10.5966/sctm.2012-0093. The iPS cells generated from late out outgrowth EPCs do not suffer from limitations that other iPS cells exhibit: they have normal karyotypes, can be reprogrammed rapidly and with high efficiently, and have high growth rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH, Simon A. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. American Journal of Physiology. 1991;260:H1870–1877. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- 32.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl SL, Vaughn ME, Niklason LE. An ultrastructural analysis of collagen in tissue engineered arteries. Annals of Biomedical Engineering. 2007;35:1749–1755. doi: 10.1007/s10439-007-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu S, Kambic H. On matching compliance between canine carotid arteries and polyurethane grafts. Artif Organs. 1997;21:1247–1254. doi: 10.1111/j.1525-1594.1997.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoerstrup SP, Zund G, Sodian R, Schnell AM, Grunenfelder J, Turina MI. Tissue engineering of small caliber vascular grafts. Eur J Cardiothorac Surg. 2001;20:164–169. doi: 10.1016/s1010-7940(01)00706-0. [DOI] [PubMed] [Google Scholar]
- 36.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 37.Ahmann KA, Weinbaum JS, Johnson SL, Tranquillo RT. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng Part A. 2010;16:3261–3270. doi: 10.1089/ten.tea.2009.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32:714–722. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghezzi CE, Marelli B, Muja N, Nazhat SN. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomaterialia. 2012;8:1813–1825. doi: 10.1016/j.actbio.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Ghezzi CE, Muja N, Marelli B, Nazhat SN. Real time responses of fibroblasts to plastically compressed fibrillar collagen hydrogels. Biomaterials. 2011;32:4761–4772. doi: 10.1016/j.biomaterials.2011.03.043. [DOI] [PubMed] [Google Scholar]
- •41.Ghezzi CE, Risse PA, Marelli B, Muja N, Barralet JE, Martin JG, Nazhat SN. An airway smooth muscle cell niche under physiological pulsatile flow culture using a tubular dense collagen construct. Biomaterials. 2013;34:1954–1966. doi: 10.1016/j.biomaterials.2012.11.025. Dense collagen gels were seeded with human pulmonary SMCs and cultured under pulsatile flow to create immediately perfusable TEBVs with burst pressures of up to 1000 mm Hg after one week of culture. This methodology enables collagen to be used as a scaffold for TEBV. [DOI] [PubMed] [Google Scholar]
- 42.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 43.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40–49. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. Journal of Biological Chemistry. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- 45.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 46.Bashur CA, Venkataraman L, Ramamurthi A. Tissue engineering and regenerative strategies to replicate biocomplexity of vascular elastic matrix assembly. Tissue Eng Part B Rev. 2012;18:203–217. doi: 10.1089/ten.teb.2011.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovascular Research. 2006;71:40–49. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Thie M, Schlumberger W, Semich R, Rauterberg J, Robenek H. Aortic smooth muscle cells in collagen lattice culture: effects on ultrastructure, proliferation and collagen synthesis. European Journal of Cell Biology. 1991;55:295–304. [PubMed] [Google Scholar]
- •49.Lin S, Sandig M, Mequanint K. Three-dimensional topography of synthetic scaffolds induces elastin synthesis by human coronary artery smooth muscle cells. Tissue Eng Part A. 2011;17:1561–1571. doi: 10.1089/ten.TEA.2010.0593. Human coronary artery SMCs seeded on 3D polyurethane scaffolds increased elastin gene expression two-fold compared to 2D scaffolds. This paper indicates that substrate topography influences elastin production in SMCs. [DOI] [PubMed] [Google Scholar]
- 50.Lee KW, Stolz DB, Wang Y. Substantial expression of mature elastin in arterial constructs. Proc Natl Acad Sci U S A. 2011;108:2705–2710. doi: 10.1073/pnas.1017834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937–949. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 52.Kumar VA, Caves JM, Haller CA, Dai E, Liu L, Grainger S, Chaikof EL. Acellular vascular grafts generated from collagen and elastin analogs. Acta Biomaterialia. 2013;9:8067–8074. doi: 10.1016/j.actbio.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keire PA, L’Heureux N, Vernon RB, Merrilees MJ, Starcher B, Okon E, Dusserre N, McAllister TN, Wight TN. Expression of Versican Isoform V3 in the Absence of Ascorbate Improves Elastogenesis in Engineered Vascular Constructs. Tissue Engineering Part A. 2010;16:501–512. doi: 10.1089/ten.tea.2009.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkataraman L, Ramamurthi A. Induced elastic matrix deposition within three-dimensional collagen scaffolds. Tissue Eng Part A. 2011;17:2879–2889. doi: 10.1089/ten.tea.2010.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kinnear C, Chang WY, Khattak S, Hinek A, Thompson T, de Carvalho Rodrigues D, Kennedy K, Mahmut N, Pasceri P, Stanford WL, et al. Modeling and Rescue of the Vascular Phenotype of Williams-Beuren Syndrome in Patient Induced Pluripotent Stem Cells. Stem Cells Translational Medicine. 2013;2:2–15. doi: 10.5966/sctm.2012-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Truskey GA, Achneck HE, Bursac N, Chan HF, Cheng CS, Fernandez C, Hong S, Jung Y, Koves T, Kraus WE, Leong K, et al. Design Considerations for an Integrated Microphysiological Muscle Tissue for Drug and Tissue Toxicity. Stem Cell Research & Therapy. 2013 doi: 10.1186/scrtX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schindler T, Nitzsche EU, Olschewski M, Magosaki N, Mix M, Prior JO, Facta AD, Solzbach U, Just H, Schelbert HR. Chronic Inflammation and Impaired Coronary Vasoreactivity in Patients with Coronary Risk Factors. Circulation. 2004;110:1069–1075. doi: 10.1161/01.CIR.0000140264.56496.76. [DOI] [PubMed] [Google Scholar]
- 58.Wu K, Tian S, Zhou H, Wu Y. Statins protect human endothelial cells from TNF-induced inflammation via ERK5 activation. Biochemical Pharmacology. 2013;85:1753–1760. doi: 10.1016/j.bcp.2013.04.009. [DOI] [PubMed] [Google Scholar]