Abstract
Recolonization of enterococci, at a non-point source beach known to contain high background levels of bacteria, was studied after a full-scale beach renovation project. The renovation involved importation of new exogenous sand, in addition to infrastructure improvements. The study's objectives were to document changes in sand and water quality and to evaluate the relative contribution of different renovation activities towards these changes. These objectives were addressed: by measuring enterococci levels in the sand and fecal indicator bacteria levels (enterococci and fecal coliform) in the water, by documenting sediment characteristics (mineralogy and biofilm levels), and by estimating changes in observable enterococci loads. Analysis of enterococci levels on surface sand and within sediment depth cores were significantly higher prior to beach renovation (6.3 to 72 CFU/g for each sampling day) when compared to levels during and after beach renovation (0.8 CFU/g to 12 CFU/g) (p<0.01). During the renovation process, sand enterococci levels were frequently below detection limits (<0.1 CFU/g). For water, exceedances in the regulatory thresholds that would trigger a beach advisory decreased by 40% for enterococci and by 90% for fecal coliform. Factors that did not change significantly between pre- and post- renovation included the enterococci loads from animals (approx. 3 × 1011 CFU per month). Factors that were observed to change between pre- and post- renovation activities included: the composition of the beach sand (64% versus 98% quartz, and a significant decrease in biofilm levels) and loads from direct stormwater inputs (reduction of 3 × 1011 CFU per month). Overall, this study supports that beach renovation activities contributed to improved sand and water quality resulting in a 50% decrease of observable enterococci loads due to upgrades to the stormwater infrastructure. Of interest was that the change in the sand mineralogy also coincided with changes in biofilm levels. More work is needed to evaluate the relationships between beach sand mineralogy, biofilm characteristics, and the retention of fecal indicator bacteria in sand.
Keywords: beach sand, fecal indicator bacteria, enterococci, fecal coliform, recreational water quality, beach renovation
1. Introduction
Beach advisories are issued based upon microbial measures of the water at recreational beaches (U.S. EPA 1986). Beach sand quality can potentially impact a beach's corresponding water quality (Byappanahalli and Fujioka 2004; Yamahara et al. 2007; Beversdorf et al. 2007; Imamura et al. 2011; Phillips et al. 2011) and there is reason to believe that sand quality can have human health effects. Heaney et al. (2012) found an association between indicator microbe levels in sand and gastrointestinal (GI) illness and diarrhea among beach goers who dig or bury themselves in sand. An epidemiologic study conducted at Hobie Cat beach, the chosen site for this beach renovation study, showed a relationship between enterococci levels in water and skin illness, but no association with gastrointestinal illness (Fleisher et al. 2010; Sinigalliano et al. 2010; Abdelzaher et al. 2011). The primary source of the enterococci to the water column at this site is the shoreline sand, as evidenced through the numerous studies that have evaluated the spatial and temporal distribution of enterococci at this beach (Shibata et al. 2004; Wright et al. 2011; Enns et al. 2012; Feng et al. 2013). Moreover, the presence of enterococci in the sand has also correlated with pathogens (including yeast, helminthes, and pathogenic bacteria, Shah et al. 2011) further emphasizing the potential public health significance of sand fecal indicator bacteria levels.
A beach renovation project conducted at Hobie Cat Beach has provided the opportunity to evaluate the recolonization of enterococci in newly imported sand and subsequently the impact of a full-scale beach renovation on sand and water quality. Specifically the objectives of this study were to evaluate the impact of beach renovation on sand and water quality and to quantify the change in enterococci loads associated with the beach renovation activities. Although studies have described the impacts of beach renovation on shoreline animals (Steinitz et al. 1998; Grippo et al. 2007) and physical properties of the beach (Park et al. 2009; Bocamazo et al 2011), this is the first study that documents the changes in sand and water bacterial quality in conjunction with beach renovation activities.
2. Materials and Methods
2.1 Site description
Hobie Cat Beach is a 1.6 km strip of beach that consists of the coastal waters lining the Rickenbacker Causeway (Figure S-1), which connects mainland Miami to Virginia Key (8.7 km distance). Its subtropical climate is characterized by an average ambient temperature of 24.8°C, and annual average rainfall of 149 cm. Hobie Beach has relatively poor water circulation due to its shallow depth and location within a cove (Zhu et al. 2011). Hobie experiences no point sources of pollution such as sewage outfalls, failing lift stations, cross-connections of sewage with storm drains, or less obvious non-point sources such as septic tanks (Shibata et al. 2004). Primary non-point sources of contamination include humans, birds, and pets, especially dogs, with their major impact previously observed on the sand closest to the shore (Wright et al. 2009). Despite the fact that animals are allowed, dog owners are not required to pick up the dog waste. Hobie has been the site for multiple indicator bacteria studies due to its history of high enterococci levels and beach advisories (e.g., Shibata et al 2010; Fleisher et al. 2010; Sinigalliano et al. 2010; Abdelzaher et al. 2010). Although usually in compliance with regulatory monitoring criteria, the beach has exceeded the EPA Poor Water Quality Guideline of 104 colony forming units (CFU) per 100 ml approximately 7% of the time on average from 2000 to 2011 (FDOH 2012). Hobie Cat Beach has been known to have high levels of enterococci in the inter-tidal zone, below the seaweed line (20±10 CFU/g of dry sand), and in the supra-tidal zone, above the seaweed line (300±159 CFU/g of dry sand) (Wright et al. 2011). Fecal coliform has also been documented in the sand within the inter-tidal (8.4 CFU/g dry sand) and supra-tidal zones (1400 CFU/g dry sand) (Shah et al. 2011). Thus the sand at this site (ultimately receiving diffuse bacteria inputs from dogs, humans, and birds) is believed to represent the major source of fecal indicator bacteria to the water column (Wright et al. 2011). Local wind waves occurring at high tides can release a significant amount of enterococci from the sand and potentially cause water quality exceedances (Feng et al. 2013).
2.1.1 Pre-renovation
Hobie Cat Beach is located immediately adjacent to the southwest side of the Rickenbacker Causeway with a parallel local-access paved road (6 m wide) just 13 m from the mean high tide line. Before renovation, the sand above the high tide line was characterized by sparse patches of grass with an increasing gradient of grass density towards the local-access paved road. The lack of designated parking for visitors led to vehicles being parked over the sand/grass on either side of the local-access paved road, with a preference of parking towards the shore side, which lies within a few meters of the intertidal zone (Figure S-2). Prior to renovation, Hobie Cat Beach had no storm water management infrastructure causing rain runoff to flow from the paved access road directly to the beach through natural ditches that would form after storm events. Trash cans were placed on both sides of the access road with one line of trash bins as close as 5 m to the shore, allowing for rainwater coming in direct contact with the waste to flow into the natural drainage ditches.
2.1.2 Beach Renovation
On September 9, 2009, a U.S. $ 6.8 million environmental and roadway protection project was initiated for this beach. The primary motivation for this renovation was to maintain highway access as the shoreline was starting to encroach on the adjacent Rickenbacker Causeway. The improvements, in addition to shoreline stabilization and exotic vegetation removal (Figure S-3), included storm-water management and parking improvements. New exogenous sand was imported (25,000 cubic meters from Ortona quarries located about 340 kilometers north of the study site). The new sand resulted in an increase in the beach surface elevation by about 0.5 to 1.5 meters above high tide line and extended the length of the beach by 15 meters (Figure S-4).
Upon renovation, the local-access paved road was removed. A new semi-pervious paver-block (Eco-stone) system was used to replace the existing access road, and a curb was installed to separate the newly placed beach sand from the parking areas. The semi-pervious roadway and parking areas minimized direct runoff with excess runoff designed to accumulate in retention areas north of the beach site away from the shore. Garbage cans were moved as part of the renovation, from the beach sand near the shore (within 10 m) into the parking areas (20 m from shore) within the corresponding drainage system that drains toward the retention areas located to the north of the roadway away from the shore line (Figure S-5).
The renovation of the 1.6 km beach strip was conducted in two phases with the western most 0.8 km closed from March 31, 2010 through June 31, 2010; and the eastern 0.8 km closed starting July 01, 2010 through November 19, 2010 (Figure S-6). The renovation of the eastern side of the beach where sand samples were collected included the removal and grading of native sand from July 01, 2010 to August 11, 2010. New sand was spread from August 20, 2010 to October 29, 2010. More details about the renovation timeline including before and after renovation photos of the beach are provided in the supplemental text (Table S-1).
2.2 Laboratory sediment composition analysis
In order to evaluate the change in sand characteristics as a result of the renovation process, sand samples (n=20) collected from the supratidal zone were analyzed for their quartz and calcium carbonate composition and compared to the values measured earlier by Piggot et al. (2012). The quartz versus calcium carbonate composition of the sediment was analyzed gravimetrically in triplicate. Pre-weighed and dried sediment (110 °C for 24 hours) was immersed in 10% HCl to dissolve the calcium carbonate fraction. The remaining sediment was rinsed with de-ionized water three times and re-dried and re-weighed. The percentage of calcium carbonate was then computed as the weight fraction lost.
2.2.1 Quantification of Biofilm within Beach Sediments
Biofilm, or extracellular polymeric substance, (EPS), consisting of primarily polysaccharides excreted by microorganisms, was extracted using a modified protocol previously described (Piggot et al. 2012). Briefly, approximately 1 gram of sample was allowed to stand in 0.5 mM EDTA for 15 min at 40°C with gentle shaking every 5 minutes for three consecutive treatments. After each treatment, samples were centrifuged at 8000 × g and the supernatant was pooled together. The supernatant was mixed with cold (4°C) ethanol (final concentration of 70%) for 8 hours to precipitate extracted EPS. Precipitate of extracted material was collected by centrifugation, dissolved in 1 ml of de-ionized water and used for the quantification of EPS by the phenol-sulfuric acid method. Each 1 ml sample of dissolved EPS was incubated with 3.2 ml of sulfuric acid for 1 minute, cooled to room temperature in a water bath and 50 μl of 90% phenol was added. The sample was incubated at room temperature for 1 hour and the absorbance was measured spectrophotometrically at 490 nm. The amount of carbohydrate present was determined by comparison with a calibration curve using D-glucose. The sediments from the samples were washed with de-ionized water to remove salts and were dried for the determination of dry weight to calculate the measure of μg EPS per g of dry sand.
2.3 Evaluation of Enterococci Source Loads
Loads (in units of CFU per month) from two major sources of enterococci were quantified: the loads from animals (humans, dogs, and birds) and loads from stormwater. The animal loads were based upon the method of Wang et al. (2010). In brief this method utilizes camera images of the beach taken at regular time intervals to determine the number of people and animal visitors. The developed method translates raw image counts into daily and monthly visitation rates. Enterococci source functions were computed from the observed number of unique individuals for average days of each month of the year, and from average load contributions for humans and for animals (Wright et al. 2009; Elmir et al. 2007). We estimate that this analysis provides an order-of-magnitude level of accuracy with respect to enterococci loads from animal sources. Camera images (location of camera shown in Figure S-6) were available for the period from November 12, 2009 through October 31, 2011. Human, dog (specifically big dogs), and seagull counts were obtained from images taken closest to 12 noon on Tuesdays, Thursdays, Saturdays, and Sundays during this time period. Missing images during this time (representing less than 8% of the total number of images evaluated) were interpolated from the nearest week days or weekend days for which data were available.
Loads from stormwater were estimated from the method developed by Feng et al. (2013) which is based upon the standard stormwater estimation method known as the “rational formula.” The rational formula requires rainfall intensity as an input. Daily intensities were available from a station operated by the S. Florida Water Management District (http://www.sfwmd.gov/dbhydroplsql/show_dbkey_info.main_menu, Station ID: Miami2). This station was located along the coast within 4 km of the study site. The estimated stormwater volumes were multiplied by the average level of enterococci measured in stormwater (1.5 × 108 CFU m-3) to estimate the enterococci load in units of CFU for each storm event. In order to further evaluate the influence of stormwater, we evaluated the enterococci and fecal coliform record in time series and compared it to the rainfall record. Additional comparisons were performed to evaluate whether there were statistical differences in hydrometeorologic and hydrologic conditions before versus after renovation. The hydrometeorologic measures included rainfall rate, wind magnitude and direction, and solar insolation while hydrologic measures included tide and wave heights. Sources of data and details of the analysis procedures are included in the supplement.
2.4 Sand Sampling Strategy for Enterococci Measures
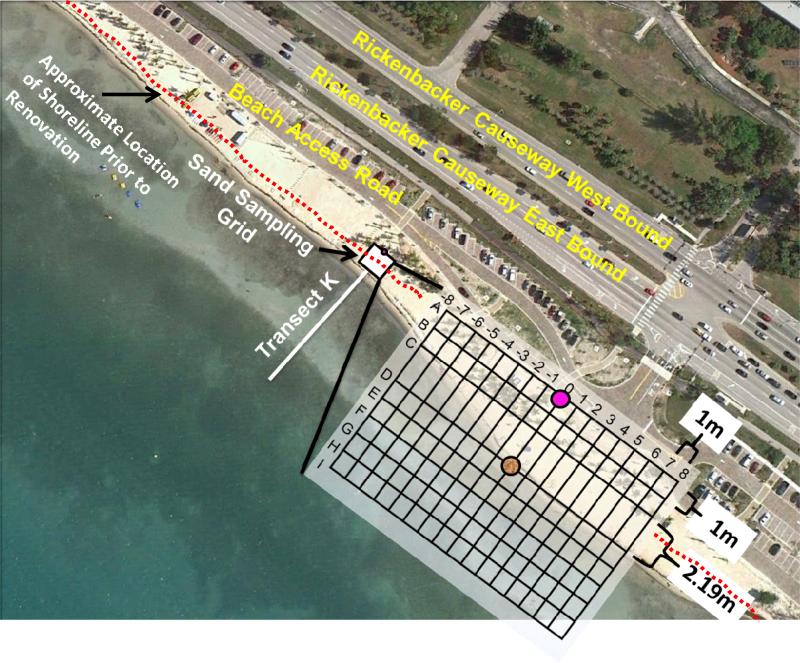
Sand samples were collected on the eastern side of the beach at a predefined transect as marked by an offshore buoy and identified in prior studies as transect K (Figure 1). A sampling grid was established referenced to this transect. Sand samples were collected regularly utilizing two different methods: a large diameter core (depth core) was used for evaluating enterococci levels with sand depth, and shallow, small diameter cores (surface cores) were used for analysis of enterococci levels in surface sediment. One special sample-set we term the “excavation sample-set” was collected in an effort to document the distribution of enterococci over a larger depth after the addition of newly imported sand.
Figure 1.
Beach configuration and surface core sampling grid. The offshore buoy marks transect K, which corresponds to column “0” of the grid. The highlighted circle in row A corresponds to the on-land stationary reference point used to establish the northern extent of the grid. Row A is farthest from the water. Rows D through E were in the intertidal zone prior to renovation. Rows F through I were added after addition of new sand to gather data from the “new” intertidal zone. The brown shaded circle along row D and column “0” designates the location where the depth cores were collected.
Sediment samples were collected starting August 2009 to October 2011 approximately every two to three weeks. This time period corresponded to 26 months, 11 months before, 4 months during, and 11 months after the renovation of the eastern portion of the beach. For comparative purposes, sand data were separated into before, during, and after renovation. The excavation sample set was collected on October 4, 2010 during the peak of the renovation process, immediately following placement of the new sand over the sampling area.
2.4.1 Depth Cores
Depth cores were collected at column “0”, row D of the sampling grid (see Figure 1) using a sterile large core 20 cm in diameter and 40 cm in length. The top 13 cm were sampled for measuring enterococci levels. Upon collection, the core was transported back to the lab where it was sectioned into horizontal segments 0.5 cm in depth, and placed into separate Whirl-pak™ bags, homogenized, and then subjected to both enterococci (10 g sand) and moisture content analyses (20 g sand).
2.4.2 Excavation Sample Set
Upon renovation of the beach, the new sand resulted in a higher surface elevation of the beach due to new sand being placed on the surface of the original sand. Of interest was to determine whether bacteria levels remained elevated in the original beach sand now buried. The excavation sample set went much deeper than the depth cores, to a depth of 66 cm. This sample set was collected by first digging a hole to the groundwater table. At every 5 cm depth, a sample was taken by scraping the side of the hole sideways with a new sterile spoon to expose new sand. Another new sterile spoon was used to collect the sample into a sterile Whirlpak™ bag. This excavation sample set was collected along the same row (row D) as the original depth core at a distance of 9 meters, so as not to interfere with the sampling grid used to collect surface cores.
2.4.3 Surface Cores
A grid-like strategy was used to analyze the presence of enterococci on the surface of the sediment in the supra-tidal and inter-tidal zones as represented in Figure 1. Small sterile cores (4 cm deep and 2.54 cm in diameter) were dug into the sediment for collection and then emptied into sterile Whirl-pak™ bags. The samples were then taken immediately back to the lab, homogenized, and analyzed for enterococci. The grid (16 m by 10 m) was distributed around a set location known to have high levels of enterococci prior to beach renovation (Figure 1). The grid consisted of 17 columns spaced 1 meter apart. These columns were marked perpendicular to the shoreline with the center marked as 0 (Transect K) and the ends marked as +8 and −8. Columns sampled were ±1, ±6, and ±8. Rows of the grid were marked in 1 meter increments from A through I, with the exception of a 2.19 m distance between C and D, allowing row D and column 0 (Transect K) to align with the sampling location of the depth core. Based on the pre-renovation beach layout, the sampling grid was designed to include the supra-tidal zone (rows A-C) and the inter-tidal zone (rows D-E). Upon placement of the new sand, the beach extended farther southwest allowing for the establishment of rows A through I, with F through I located within the new inter-tidal zone. The location of transects A through E remained unchanged before, during, and after the renovation periods, with the exception of the surface elevation due to the placement of new sand.
2.5 Laboratory sediment sample analysis for enterococci
Processing of sediment samples required extraction of enterococci from sediment into water by adding a measured amount of sediment (approximately 10 g) into a sterile plastic bottle with 100 mL of sterile phosphate buffered dilution water. These samples were shaken vigorously for 2 minutes to promote the transfer of bacteria into the water (Boehm et al., 2009). Sediment was allowed to settle for 30 seconds, and then 5 and 50 mL of the supernatants were analyzed by standard membrane filtration method which is based upon plating on mEI agar (Method 1600, U.S. EPA 2002). Units are reported in CFU per gram of dry sand.
2.6 Water Quality Analyses
Weekly water quality monitoring data (enterococci and fecal coliform) were obtained from Miami-Dade Department of Health to evaluate the levels of fecal indicator levels prior to and after beach renovation. The water sampling location for regulatory monitoring is located on the west side of the beach (Figure S-6). Samples are collected by wading to a depth of about 1 meter, mid-way between knee to waist deep water. The water samples were analyzed by membrane filtration methods using standard protocols (U.S. EPA, 2000). The time period evaluated included a 40-month period prior to beach closure for the west side of the beach (June 2006 to October 2009, n = 178). Similarly a 39-month period of water quality data was evaluated when the west side of the beach was reopened (June 2010 to September 2013, n = 167). No water samples were collected while the beach was closed. Time periods evaluated corresponded to the same months of the year to control for seasonal variability. Only data corresponding to weekly routine monitoring were included in the evaluation. Exploratory samples collected after exceedances were not included in the analysis to avoid biasing the data.
The water quality data were compared to Florida Department of Health regulatory thresholds for marine waters. These thresholds are based upon enterococci measures that are used to establish beach “advisories” and fecal coliform measures that are used to issue beach “warnings.” For enterococci, the threshold levels are 35 colony-forming units per 100 ml (CFU/100 ml) for establishing “moderate” water quality and 104 CFU/100 ml for issuing a beach advisory (FDOH 2012). For fecal coliform, the threshold levels are 200 CFU/100 ml for “moderate” water quality and 400 CFU/100 ml for issuing a beach warning (FDOH 2012).
2.7 Statistical Analyses
Comparisons between top versus bottom of depth core, and between other groups were made by using an unpaired t-test with Welch's correction, not assuming equal variances. Statistical significance was taken as a two-sided P value <0.05. Correlations were assessed using Pearson r statistic. Correlation analysis was conducted to compare enterococci levels in sand versus moisture content and to also compare bacteria levels in water against prior same day, prior two days, and prior week rainfall records. Ordinary one-way ANOVA was used to compare data from before, during, and after renovation phases of the study. P <0.05 is considered significant. Results are expressed as mean ± the standard error of the mean (SEM). The statistical package employed was GraphPad Prism, Version 4 (La Jolla, USA).
3. Results
3.1 Sediment composition analysis
Composition analysis of sand showed that the new sand imported onto Hobie Cat Beach was almost entirely and uniformly composed of quartz (98 ± 2% quartz). The pre-renovation sediment composition was a mixture of quartz and calcium carbonate, with an average composition of 64% in the supra-tidal zone (n=40 for each location, Piggot et al. 2012).
3.1.1 Sediment EPS Quantification
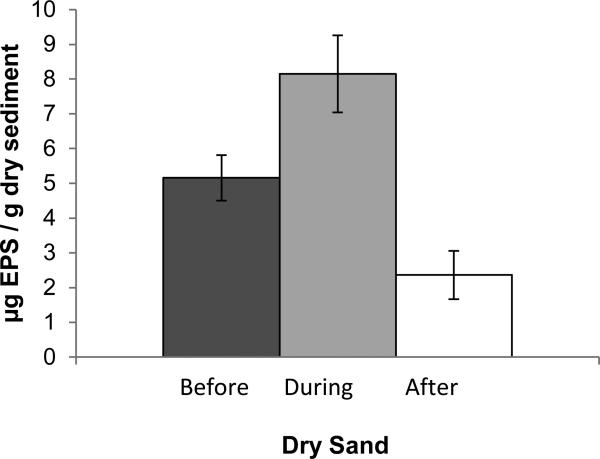
EPS levels within the supratidal sands varied, on average, from 5.2 μg EPS per g dry sand before renovation, to 8.1 μg/g during renovation, to 2.4 μg/g after renovation (Figure 2). These means were statistically different between before and after renovation (p = 0.02) and between during and after renovation (p = 0.02), but were not statistically different between before and during renovation (p=0.08). Piggot et al. (2012) observed the highest levels of enterococci when biofilm levels were in the 4 to 9 μg/g range. Prior to renovation, the biofilm levels appeared to be optimum for enterococci retention, whereas after renovation, biofilm levels generally fell below the optimum range.
Figure 2.
Sediment Biofilm (EPS) quantification in supra-tidal zone before, during, and after beach renovation. Error bars correspond to the standard error of mean. The means before and after renovation (p = 0.02) and between during and after renovation (p = 0.02) were statistically different (p=0.02 for both). The means were not statistically different between before and during renovation (p=0.08).
3.2 Enterococci Source Loads
Enterococci loads from animals ranged from 8 × 108 per month to 7 × 1011 per month. During the 4.5 months of beach renovation, these loads were estimated at 4 × 1010 CFU per month, on average. The average monthly loads before and after beach renovation were 3 × 1011 CFU per month, on average. The loads during renovation were statistically less than loads before (p=0.03) and after (p<0.01). The loads before and after renovation were not statistically different (p=0.47). The dominant source among dogs, humans, and birds, given the procedure of Wang et al. (2010), was dog fecal inputs (Figure S-7D). Although the beach was closed during the July to November 2010 period, dogs were still observed at the beach site (4 and 16 during September and October 2010, respectively) (Figure S-7A). Data indicates the peak number of dogs observed prior to renovation was 356 for the month of May 2010; the maximum number of dogs observed after renovation was 305 for the month of February 2011. The inputs from humans were on the order of 107 CFU per month while the beach was closed during the active renovation process (14 and 10 humans during September and October 2010, respectively) and on the order of 1010 CFU per month both before and after the renovation process. Peak numbers of humans were observed during June 2010 (n = 1676), June 2011 (n=1657) and July 2011 (n=2062). Low numbers of humans were observed when the beach was closed during July 2010 (n=42) (Figure S-7B). Seagull inputs were on the order of 104 to 107 CFU per month. The highest numbers of seagulls were observed during the winter months (n= 1099 January 2010, n=755 March 2011) and during times when the beach was closed (n=1272 during October 2010 and n=832 during November 2010) (Figure S-7C). Loads estimated for dogs, humans, and seagulls were similar both before and after beach renovation with values consistent with those reported by Wang et al. (2010) for this beach. Other than during the active beach renovation process, the enterococci loads at this beach appeared to have maintained relatively consistent seasonal patterns in comparison to measurements made by Wang et al. (2010).
Contributions from storm water for the 12-month period preceding the infrastructure upgrades were estimated at 3.2 × 1011 per month, almost the same as the average value observed for animal inputs. Apparently stormwater and animal inputs appear to contribute equally to the enterococci load at the beach. Average rainfall the same day, two days prior, and one week prior to water fecal indicator sampling dates, had no correlation with enterococci levels in the water (R2 = 0.003, R2 = 0.001, R2 = 0.015, respectively) or fecal coliform levels (R2 = 0.001, R2 < 0.001, R2 = 0.008, respectively). Measures before and after renovation for rainfall, solar insolation, wind magnitude including alongshore and cross-shore were not statistically different (Table S-2). However, mean daily tidal height was statistically different between before (3.5 cm) and after (5.7 cm) renovation conditions. The majority of the increase occurred from September 2012 to August 2013 almost 2 years after the beach was reopened. This difference in tidal height did not significantly impact the hydrodynamics of the study site.
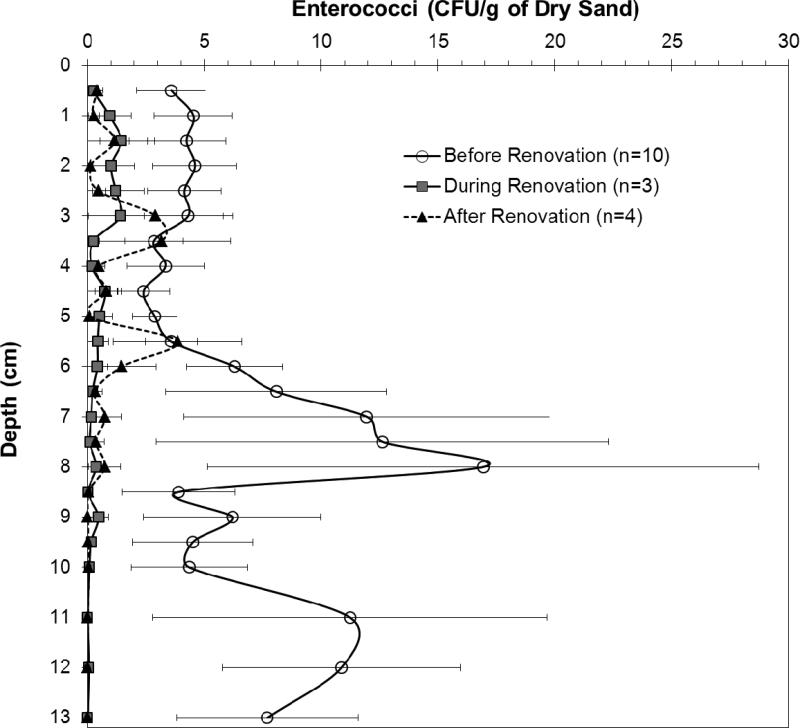
3.3 Depth Cores
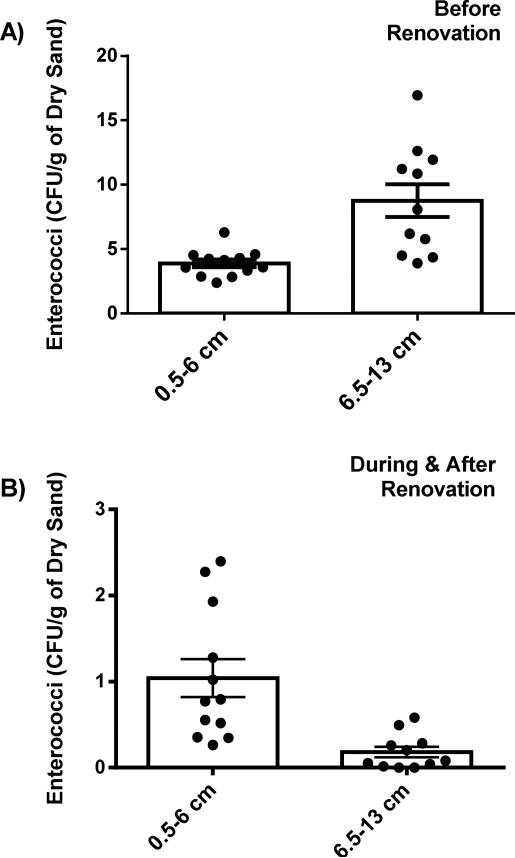
Beach renovation resulted in decreased average levels of enterococci throughout the depth cores (see Figure 3). Prior to renovation the average enterococci level was 6.3 CFU/g, while during renovation, the average level was 0.45 CFU/g. Finally, 11 months after the beach was opened, data shows that enterococci levels were still low with a value of 0.76 CFU/g, on average. These averages were statistically different (P <0.01). Comparing the top portion of the depth core (0.5 cm-6 cm) to the bottom portion (6.5 cm-13 cm) for the pre-renovation period demonstrated that average enterococci levels were significantly higher (P <0.01) in the bottom portion of the core (8.9 CFU/g) as opposed to the top (3.9 CFU/g) as shown in Figure 4A. The opposite was observed for cores collected during and after renovation (Figure 4B), where average levels of enterococci were significantly higher in the top portion of the core (P <0.01; 0.7 CFU/g for the top portion collected during renovation and 1.3 CFU/g for top portion collected after renovation, as compared to 0.15 CFU/g and 0.21 CFU/g for the bottom portions, respectively).
Figure 3.
Averaged levels of enterococci recorded with depth before, during, and after beach renovation. Analyses were conducted every 0.5 cm. Pre-renovation data consisted of 10 depth cores starting August 28, 2009 and ending July 8, 2010. Data collected during renovation corresponded to 3 depth cores starting September 6, 2010 and ending October 13, 2010. Data collected after renovation corresponded to 4 depth cores starting January 7, 2011 and ending October 8, 2011. Data is expressed as mean ± SEM.
Figure 4.
Comparison of top portion (0.5-6 cm) versus bottom portion (6.5-13 cm) of depth core. A) Averaged levels of enterococci before beach renovation, consisting of 10 depth cores starting August 28, 2009 and ending July 8, 2010. Top is significantly lower than bottom, P <0.05. B) Averaged levels of enterococci during and after beach renovation, corresponding to 7 depth cores starting September 6, 2010 and ending October 8, 2011. Top is significantly higher than bottom, P <0.05. Data is expressed as mean ± SEM.
Similar patterns were observed for the SEM of the depth core measurements. The SEM of the enterococci levels was higher before renovation as compared to during and after renovation, with P <0.01. The SEM variation with depth also followed the pattern of average enterococci levels with higher standard errors in the lower half of the core during pre-renovation conditions as opposed to larger standard errors observed for the upper half of the cores collected both during and after renovation. In summary, sand characterized by higher average enterococci levels is also characterized by higher variability.
Upon closure of the beach, the enterococci levels in the depth core remained relatively elevated (3.5 CFU/g on July 8, 2010 and 1.4 CFU/g on September 6, 2010). Immediately after placement of all of the new sand, enterococci levels dropped with the core collected on September 20, 2010 showing all samples below the 0.1 CFU/g detection limit. A depth core collected on October 13, 2010 was characterized by 18 layers testing below 0.1 CFU/g and the remaining 4 layers (observed in both top and bottom layers) measuring at the detection limit of 0.1 CFU/g. Once the beach reopened to the public in November 2010, the enterococci levels started to increase with the last core collected one year later (October 8, 2011) measuring at an average of 2 CFU/g. The average enterococci levels after beach reopening, however, did not rise to the average levels observed prior to beach renovation of 6.3 CFU/g. The enterococci levels in the last core collected almost one year after the beach was reopened was statistically lower than the enterococci levels observed in the cores collected prior to beach renovation (P <0.01).
Moisture data indicates that the original sand composition held more moisture than the new sand since sand moisture content was 8.4% prior to renovation, compared to 2.6% during and 2.1% after renovation (P < 0.01). However, when enterococci levels and moisture content were compared on a sample-to-sample basis no significant correlation was found for depth core or surface core data (R2=0.04, R2=0.01, respectively).
3.4 Excavation Sample Set
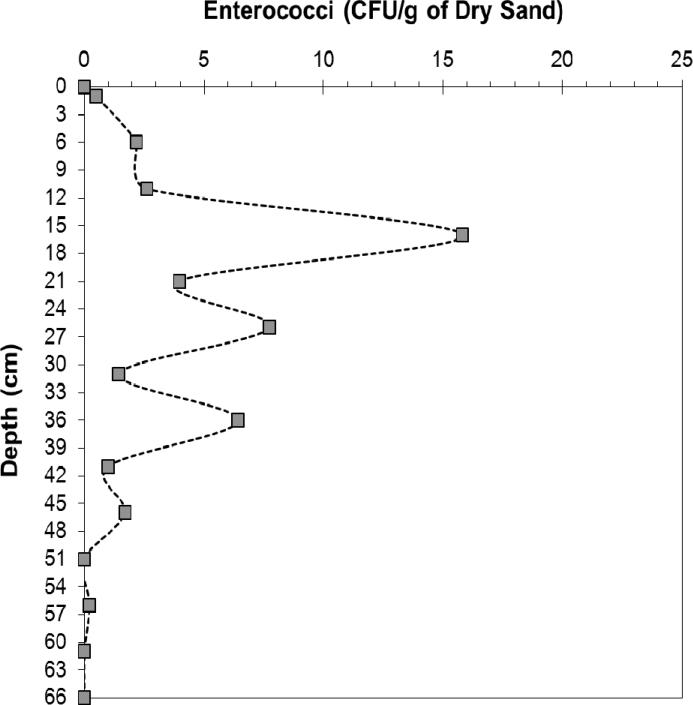
The results from the excavation sample set (Figure 5) were consistent with that of the depth cores. The enterococci population was indeed low (< 3 CFU/g) for the depths that coincide with the normal sampling depth of 0 cm to 13 cm for the depth cores. Enterococci levels were elevated between a depth of 15 to 36 cm. This data supports the idea that the original sand, which was buried, maintained elevated levels of enterococci. The 15 to 36 cm depth is consistent with the elevation of the pre-renovation sand as observed from the changes in sand elevation at the sampling point (Figure S-4).
Figure 5.
Results from excavation sample set collected on October 4, 2010 after completion of new sand placement during the active renovation process. Enterococci levels were measured for a depth of 66 cm from the surface at a site 9 meters away along the row of original depth core.
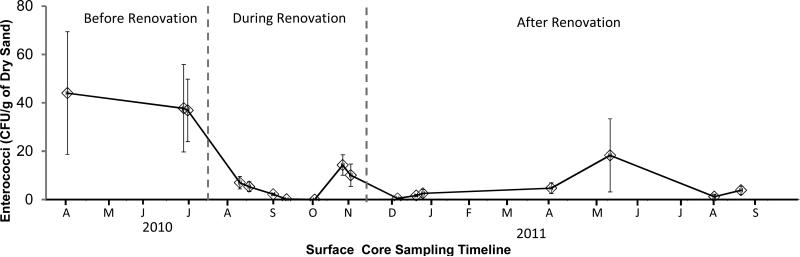
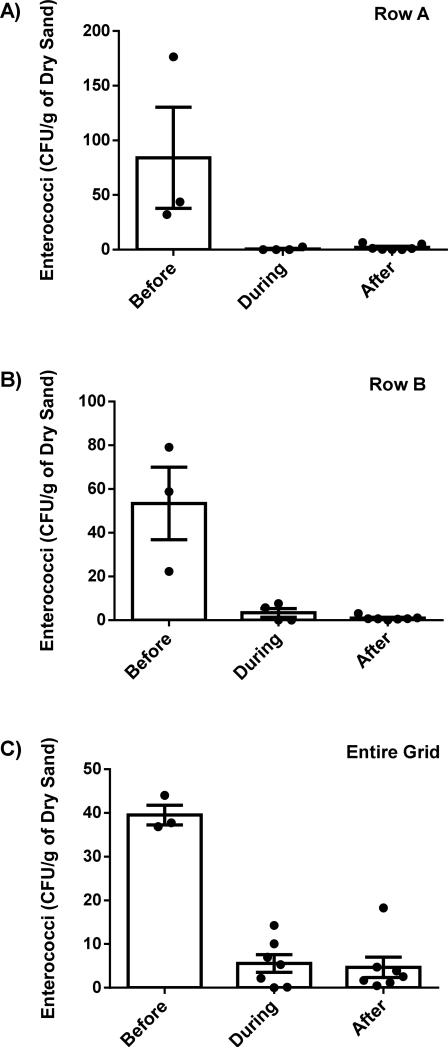
3.5 Surface Cores
Average levels of enterococci decreased significantly among surface core sediment samples as time progressed throughout the renovation process (Figure 6). Overall the differences in enterococci levels during and after the beach renovation were significant (P <0.01; Figure 7C). This trend was especially apparent in the supra-tidal zone with significant decreases in average enterococci for rows A (P <0.01) and B (P <0.01) as demonstrated in Figures 7A and 7B respectively. The average levels for row A changed from 84 CFU/g before renovation, 0.66 CFU/g during renovation, and 2.1 CFU/g after renovation. While the average levels for row B decreased from 53 CFU/g before renovation, 3.3 CFU/g during renovation, and 0.94 after renovation. The differences in pre and post renovation enterococci levels were not significant for the rows located closer to or within the inter-tidal zone (rows C, D, and E). Renovation also changed a trend that was seen in the surface grid samples. Prior to renovation, average surface sediment enterococci levels decreased as they approached the shoreline. Table 1 shows this trend starting with an average enterococci level of 84 CFU/g at row A, while decreasing continuously to 8 CFU/g at row E. Once the new sand was installed, this trend was no longer observed. In fact, as shown in Table 1, the data indicates the new inter-tidal zones (rows F, G, H, and I) showed higher enterococci levels than in the new supra-tidal zone (rows A through E). Statistically, this difference was significant at 90% confidence but not at 95% (P =0.08).
Figure 6.
Time Series of Surface Core Sampling Illustrating Enterococci Levels Before, During and After Renovation.
Figure 7.
Summary data of surface sand sampling before, during, and after beach renovation. Pre-renovation data consisted of 3 surface core grid samplings starting April 16, 2010 and ending July 15, 2010. Data collected during renovation corresponded to 7 surface core grid samplings starting August 23, 2010 and ending November 14, 2010. Data collected after renovation corresponded to 7 surface core grid samplings starting December 19, 2010 and ending September 2, 2011. A) & B) Averaged levels of enterococci for row A and row B respectively, on surface sand sample grid, per sample day. C) Averaged levels of enterococci for entire surface sand sample grid (rows A-E), per day; before, during, and after renovation. During and after are significantly lower than before, P <0.05, for figures A, B and C. Data is expressed as mean ± SEM.
Table 1.
Summary of enterococci levels for surface sand grid sampling rows before, during, and after renovation. Levels reported correspond to the mean value measured for each row and the corresponding standard error of mean. Cells filled with gray correspond to locations located within the inter-tidal zone. P values computed using 1-way ANOVA.
| Enterococci Levels for Surface Sand Sample Grid Rows (CFU/ g of Dry Sand) | ||||
|---|---|---|---|---|
| Row | Before | During | After | P value |
| A | 84 ± 46 | 0.7 ± 0.6 | 2.1 ± 1 | 0.011 |
| B | 53 ± 16 | 3.3 ± 2 | 0.9 ± 0.4 | 0.0002 |
| C | 40 ± 16 | 3.9 ± 2.8 | 12 ± 11 | 0.17 |
| D | 9.7 ± 2.9 | 6.7 ± 2.5 | 2.9 ± 0.9 | 0.14 |
| E | 8.0 ± 6.5 | 5.2 ± 1.8 | 5.9 ± 1.9 | 0.82 |
| F | 32 ± 14 | |||
| G | 17 ± 6.3 | |||
| H | 69 ± 66 | |||
| I | 2.1 ± 0.6 | |||
3.6 Water Quality Analysis
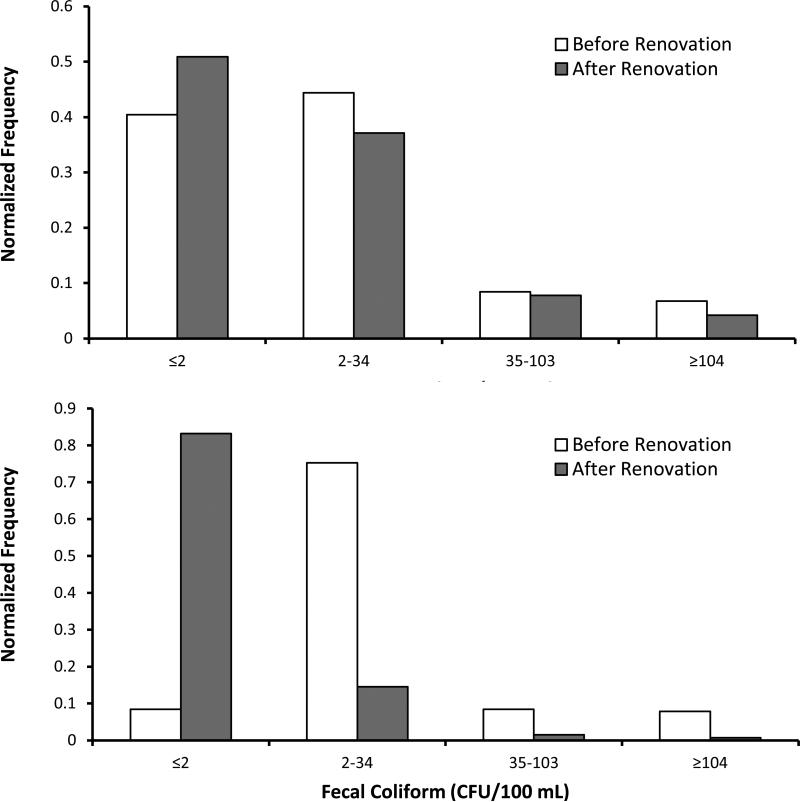
Water quality improved, on average, at the location used for regulatory monitoring. Enterococci levels decreased from 37 CFU/100 mL to 19 CFU/100 mL. Fecal coliform levels decreased from 176 CFU/100 ml to 22 CFU/100 mL. This decrease was statistically significant at 95% confidence limits for both enterococci (P =0.02) and fecal coliform (P <0.001). Histogram plots of the water quality data further illustrate the shift of fecal indicator levels towards lower levels (Figure 8). Of particular significance is that the extremely high levels that would trigger regulatory thresholds decreased by 40% for enterococci (from 6.7% to 4.2% of the samples) and by 90% for fecal coliform (from 7.9% to 0.76% of the samples).
Figure 8.
Histograms demonstrating the distribution of enterococci and fecal coliform in the water at regulatory threshold values before and after renovation. The bin range in CFU/100 mL is shown on the x-axis, while the normalized frequency of that level is shown on the y-axis. Data represents a cumulative of weekly monitoring results 40 months before and 40 months after renovation.
4. Discussion
The purpose of this study was to examine how beach renovation affected the recolonization of enterococci within beach sand and its ultimate impacts on water quality at a non-point source subtropical beach. For sand, results show that enterococci levels significantly decreased during the beach renovation. Furthermore, results show that enterococci levels in sand remained significantly lower even 287 days after the beach was reopened to the public. Of interest was that the levels of sand enterococci remained significantly high after the beach was closed to the public on July 1, 2010. It was only after all of the new sand was added (roughly 80 days later) that the average levels of the new surface sand dropped to near zero values. Camera data showed that average enterococci source loads from animals decreased by an order of magnitude from before to during renovation and yet the enterococci levels remained elevated in the buried original sand, as illustrated by the excavation sample set. We hypothesize that the elevated levels of enterococci at depth are likely due to replication of the enterococci present in the original sand as the transport pathway is not obvious from sources at depth, especially since this zone was above the groundwater table.
The results of this study support the literature that documents the potential for long-term persistence of fecal indicator bacteria in beach sand (Alm et al. 2003; Yamahara et al. 2009). This study contributes to this literature by documenting persistence under the unique conditions of a full-scale beach renovation project. Essentially this study supports that levels of enterococci observed in the sand is likely the summation of baseline environmental enterococci (from persistence and possibly regrowth) plus the contributions of additional inputs from the diffuse sources to the beach. These diffuse sources may ultimately contribute to the baseline but their consistent introduction is not required to maintain sand levels elevated.
Upon closure of the beach a considerable amount of time was taken to remove prior vegetation and re-grade the area. The process of re-grading involved scrapping the original sand and layering this sand with new sand. Of interest was the lack of carry-over of enterococci due to the use of large scale moving equipment for both the grading of the original sand and the new sand. Amazingly the new sand, upon completion of the installation, tested consistently at below detection limit values (for both the depth core on September 20, 2010 and the surface cores on September 17, 2010) even though the grading was done with equipment that was not intentionally washed.
After the placement of the new sand, the pristine sand conditions slowly changed. As observed from the 13 cm depth core data, higher levels of enterococci were observed near the surface of the sand (Figure 3 and 4B). This pattern is the opposite of what was observed prior to beach renovation where the highest enterococci levels were observed in the deeper sand (beyond 5 cm), suggesting that enterococci was persistent below the 5 cm layer. Essentially these observations may indicate that the source of “new” enterococci to the pristine sand is entering from the surface where dogs, humans and birds deposit bacteria. As the levels of enterococci did not return to the higher levels observed prior to the addition of new sand to the beach, these diffuse surface sources are likely small relative to prior beach conditions, which were characterized by uncontrolled storm water discharge that included inferior solid waste management facilities.
In addition to the depth distribution, the surface distribution of enterococci in the pristine sand also changed. Grid sampling suggests that prior to renovation enterococci levels decreased from the supra-tidal zone to the inter-tidal zone as seen in Table 1, for rows A through E. As Table 1 also shows, after renovation this trend was no longer seen. In fact, during renovation, average levels of enterococci in the inter-tidal zone were nearly an order of magnitude higher than those recorded in the supra-tidal zone. Intertidal zone rows F, G and H averaged values of 32 CFU/g, 17 CFU/g, and 69 CFU/g, while supra-tidal zone rows averaged 0.66 CFU/g to 6.7 CFU/g. This observation suggests there was a source of enterococci in the tidal area during renovation. It is unclear how this trend is reversed, but one possibility is that the original sand is mixed with the new sand in the inter-tidal zone, as opposed to less mixing of the sand in the drier supra-tidal zone. We hypothesize that the waves could cause resuspension and mixing of both layers as a prior study at this beach showed sediment resuspension and higher turbidity patterns in the presence of offshore waves (Kinzelman et al. 2009, Feng et al. 2013). Other causes responsible for this new trend could be the incorporation of bacteria in the water from dog and human bathers (Elmir et al. 2007) located upstream from the grid-sampling site. Yet, another possible source would be from the birds, which have a tendency to congregate in the intertidal zone. Camera image analysis showed that birds tend to congregate in the intertidal zone, especially during low tide and during early morning hours. Thus diffuse sources of microbes to the water (from shoreline birds, dogs, and human bathing) coupled with some carryover from the prior original sand, provides one possible explanation for the elevated levels of enterococci within the inter-tidal zone after renovation.
Decreases were also observed in water fecal indicator levels and these decreases paralleled the decrease recorded in the sand. Not only were decreases observed in the average fecal indicator levels in the water, decreases were also observed in the proportion of samples that exceeded regulatory thresholds. Before renovation enterococci exceeded the single-sample maximum value of 104 CFU/100 mL 6.7% of the time. This was reduced to 4.2% of the samples after renovation. Similarly, the single-sample maximum value of 400 CFU/100 mL for fecal coliform was exceeded 7.9% of the time prior to beach renovation and 0.76% of the afterwards. These reductions at the high end of the water quality spectrum are especially important from a beach advisory/warning point-of-view, as it would result in a decrease in the number of advisories based upon enterococci by 40% and warnings based upon fecal coliform by 90%. When looking at overall average fecal indicator bacteria levels, the decline was significant at 95% confidence for both fecal indicator bacteria.
Enterococci loads estimated from camera image analysis suggest that loads from diffuse sources (dogs, humans, and birds) were on the order of 1011 per month prior to and after beach renovation with loads at 1010 per month during the active renovation process. Stormwater inputs were also estimated at 1011 prior to beach renovation and non-existent thereafter due to diversion of stormwater away from the shoreline. Thus infrastructure improvements appear to have decreased the observable beach bacterial loads from the diffuse sources by about 50%. This reduction contributed towards the more than 50% reduction in sand bacteria levels observed within the supratidal sands (from 6.3 CFU/g to 0.76 CFU/g from pre- to post-renovation for the depth cores).
Physical changes of the beach were significant and included the redirection of storm-water, cars, and solid waste disposal facilities away from the shoreline with drainage also directed away from the shoreline. Infiltration of this drainage now occurs within the pervious parking lot and in sections adjacent to the parking lot away from the shoreline. Apparently this diversion of storm-water and sources of contamination (e.g. car tires and solid waste and their leachates) has contributed to the improvements in: sand quality at least for the 11-month post renovation-monitoring period and water quality for the 40-month post renovation period. Such observations are consistent with findings that implicate beach management practices as contributing towards improvements in beach water quality (Kinzelman et al. 2003; Skalbeck et al. 2010). For example, Kinzelman and McLellan (2009) documented a significant decrease in beach advisories within the Great Lakes Region after the redesign of a major storm water outlet and improvements in beach grooming practices. For the case of Hobie Cat Beach, stormwater was routed for infiltration away from the shoreline. The infiltration of storm-water near the beach area could potentially impact water quality through groundwater flow of this infiltrated water ultimately towards the shoreline as observed for beaches in California (Boehm et al. 2004; Russell et al. 2012) and North Carolina (Price et al. 2013). The study by Kinzelman and McLellan (2009) also found improved beach elevation and slope to play an important role in the reduction of indicator organisms in the water column. The increased elevation and depth in Hobie Beach's topography could have also contributed to the improvement in water quality.
In addition to the changes in the physical infrastructure and beach topography, changes in sediment characteristics represent another very relevant change. The new sand was composed almost entirely of quartz and was characterized by lower moisture retention. Prior to renovation the original sediment was composed of a combination of quartz and calcium carbonate and was characterized by higher moisture retention. The overall average moisture content of the new sand was lower than that of the original sand and this lower bulk moisture level can impact the survival of fecal indicators in the sand environment (Solo-Gabriele et al. 2000; Desmarais et al. 2002; Mika et al. 2009; Yamahara et al. 2009). However, our data did not show a correlation between enterococci level and moisture content. This suggests that although moisture may play a role in the growth and survival of enterococci, it was not the leading factor in measuring its presence in dry surface sand or within a short depth of 13 cm from the surface. It maybe instead that other contributions, like mineral composition, were much more important in retaining enterococci, as the change in the mineralogical composition (quartz versus calcium carbonate) was significant. The new sand was 98% quartz while the original sand was 64% quartz.
The change in the sand mineral composition may have influenced the ability of the beach to retain enterococci. Of interest was that the EPS levels in the sand prior to renovation were at optimum levels for enterococci retention, whereas during renovation the EPS increased, and after renovation it decreased to sub-optimum levels for enterococci retention (Piggot et al. 2012). We can hypothesize that the sudden increase in EPS levels during renovation may have been a by-product of earth moving activities which results in mixing of sand and releasing (freeing up/making available) nutrients that encourages microbial communities, through changes in environment, to produce excess EPS. Once the earth-moving activities were completed, the biofilm levels returned to lower equilibrium levels, presumably due to the new sand mineralogy. According to Piggot et al. (2012), the distribution of EPS is different between sand grains made of calcium carbonate versus that made of quartz. Quartz grains were observed to have a smoother surface with EPS accumulated only in sparse cracks and crevices. Calcium carbonate grains are a softer mineral, easier to abrade than quartz, and are derived from broken skeletal material giving the grains a much higher surface rugosity than quartz grains. The surfaces of calcium carbonate grains appeared to have a more homogeneous coating of EPS, which may be the result of a more uniform distribution of pits, crevices and other depressions occupied by microbial biofilms. These observations suggest that biofilms may more easily form in the original sand because of its higher calcium carbonate composition, while the new sand being composed of 98% quartz possibly prevents biofilms due to the limited crevices that allow for biofilm accumulation. These differences can possibly influence the retention and persistence of enterococci among quartz as compared to calcium carbonate sand. This observation by Piggot et al. (2012) is consistent with the overall decrease in biofilm levels observed pre- and post- renovation. It is possible that this change in sand mineralogy and biofilm, resulted in even greater benefits by further reducing the enterococci levels in the sand because of a less conducive environment for enterococci retention. The change in sand mineralogy would extend the benefits of the renovation beyond what was possible through improvements to the stormwater infrastructure alone. The reduction in sand bacteria levels by a factor of greater than 50% (greater than what was afforded by the infrastructure improvements) could have been due to the replacement of the old sand which was a mixture of calcium carbonate and quartz, with a sand that was almost entirely composed of quartz.
Rainfall, solar insolation, wind intensity, and wave height were statistically not different during the sampling period corresponding to before and after renovation. Tidal height was statistically different although the differences were small (3.5 cm versus 5.7 cm). One may expect higher levels of bacteria with higher tides, because of the greater amount of intertidal sand in contact with the water. The possibility of increased bacterial releases with higher tides was more than compensated by the beach renovation. Moreover the time period corresponding to the highest tides (September 2012 to August 2013) was almost two years after the beach was reopened. We thus believe that, for the most part, hydrometeorologic and hydrologic parameters did not make significant contributions to the differences observed in sand and water quality.
5. Conclusion
This study has indicated that the beach renovation had an added value beyond the preservation of a highway. The renovation described here resulted in a positive impact on the quality of sand and water at the beach for a time period of 287 days after reopening. This was facilitated by infrastructure improvements that include improved stormwater management and improvements to parking and solid waste disposal facilities. We estimate that these improvements resulted in a reduction of the enterococci load by 3 × 1011 CFU per month, representing a 2-fold decrease in observable enterococci loads to the beach. However, a 5-fold decrease in enterococci levels were observed between pre- and post-beach renovation which is more than would be expected from the infrastructure upgrades alone (assuming that the enterococci levels in sand respond linearly to the input loads). As a result, other factors likely contributed towards the decrease in sand enterococci levels. We hypothesize that additional decreases are associated with the mineralogy of the new sand. The type of sand added to the beach also likely had an impact on microorganisms’ ability to recolonize within the sand possibly through its influence on moisture retention and biofilm growth. Overall, this study supports that infrastructure upgrades through the diversion of stormwater resulted in considerable improvements in beach sand and water quality by decreasing enterococci loads by a factor of 2.
We recommend additional studies that quantify the impact of beach sand mineralogy in controlling the persistence of bacterial indicators in beach sand. We also recommend longer term measurements of beach microbe levels in sand along with longer term measures of biofilm to confirm whether or not enterococci return back to pre-renovation levels. We speculate that enterococci levels will remain low because of the significant reduction in enterococci from stormwater runoff and due to the intrinsic changes in sand characteristics. We also recommend a factorial analysis of the several beach renovation components to further tease out the impacts of the different renovation activities. Of interest would be to also evaluate persisting layers of enterococci to determine if there is evidence of cloning within these populations.
Supplementary Material
Impact of renovation on fecal indicator levels was examined at a nonpoint source beach.
Significant decrease in sand enterococci levels observed up to 1 year after renovation.
Regulatory exceedances for water quality decreased by 40 to 90%
Improvements to stormwater infrastructure decreased the observable enterococci load by a factor of 2.
Additional decreases were associated with the mineralogy of the new sand.
ACKNOWLEDGMENTS
Funding for this project was received through the National Science Foundation (NSF) and the National Institute of Environmental Health Sciences (NIEHS) Oceans and Human Health Center at the University of Miami Rosenstiel School [NSF 0CE0432368/0911373/1127813] and [NIEHS P50 ES12736]. Supplemental support was provided by the NSF-REU program and NSF grant OCE1127813. We would like to thank all of the students from the Oceans and Human Health Laboratory at the University of Miami for all of their contributions to this project. We also thank Dr. John Wang for provision of spreadsheets needed for computation of enterococci loads from camera image analysis. We also acknowledge Lincong and Jiangang Luo for their painstaking analyses of camera images. We also greatly appreciate the water quality monitoring data provided by the Miami-Dade Department of Health and we are thankful to Redland Company Construction and Jimmy Martincak, beach manager, for provision of information about the beach renovation process and for access to the beach site during renovation activities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelzaher AM, et al. Presence of Pathogens and Indicator Microbes at a Non-Point Source Subtropical Recreational Marine Beach. App. and Env. Micro. 2010;76:724–732. doi: 10.1128/AEM.02127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelzaher AM, Wright ME, Ortega C, Hasan AR, Shibata T, Solo-Gabriele HM, Kish J, Withum K, He G, Elmir SM, Bonilla JA, Bonilla TD, Palmer CJ, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Plano LRW, Garza AC, Zhu X, Stewart JR, Dickerson JW, Jr., Yampara-Iquise H, Carson C, Fleisher JM, Fleming LE. Daily Measures of Microbes and Human Health at a Non-point Source Marine Beach. Journal of Water and Health. 2011;9(3):443–457. doi: 10.2166/wh.2011.146. [DOI] [PubMed] [Google Scholar]
- Alm E, Burke J, Spain A. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 2003;37:3978–3982. doi: 10.1016/S0043-1354(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Beversdorf LJ, Bornstein-Forst SM, McLellan SL. The potential for beach sand to serve as a reservoir for Escherichia coli and the physical influences on cell die-off. J Appl Microbiol. 2007;102(5):1372–81. doi: 10.1111/j.1365-2672.2006.03177.x. [DOI] [PubMed] [Google Scholar]
- Bocamazo LM, Grosskope WG, Buonuiato FS. Beach Nourishment, Shoreline Change, and Dune Growth at Westhampton Beach, New York, 1996-2009. Journal of Coastal Research. 2011;59:181–191. [Google Scholar]
- Boehm AB, et al. Faecal Indicator Bacteria Enumeration in Beach Sand: A Comparison Study of Extraction Methods in Medium to Coarse Sands. Journal of Applied Micro. 2009;107:1740–1750. doi: 10.1111/j.1365-2672.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm AB, Shellenbarger G, Paytan A. Groundwater Discharge: Potential Association with Fecal Indicator Bacteria in the Surf Zone. Environ. Sci. Technol. 2004;38:3558–3566. doi: 10.1021/es035385a. [DOI] [PubMed] [Google Scholar]
- Byappanahalli M, Fujioka R. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. Water Sci Technol. 2004;50(1):27–32. [PubMed] [Google Scholar]
- Desmarais TR, Solo-Gabriele HM, Palmer CJ. Influence of Soil on Fecal Indicator Organisms in a Tidally Influenced Subtropical Environment. Applied and Environmental Microbiology. 2002;68(3):1165–1172. doi: 10.1128/AEM.68.3.1165-1172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmir S, Wright M, Abdelzaher A, Solo-Gabriele H, Fleming L, Miller G, Rybolowik M, Shih P, Pillai S, Cooper J, Quaye E. Quantitative evaluation of bacteria released by bathers in marine water. Water Res. 2007;41:3–10. doi: 10.1016/j.watres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns AA, Vogel LJ, Abdelzaher AM, Solo-Gabriele HM, Plano LRW, Gidley ML, Phillips MC, Klaus JS, Piggot AM, Feng Z, Reniers AJHM, Haus BK, Elmir SM, Zhang Y, Jimenez NH, Abdel-Mottaleb N, Schoor ME, Brown A, Khan SQ, Dameron AS, Salazar NC, Fleming LE. Spatial and Temporal Variation in Indicator Microbe Sampling is Influential in Beach Management Decisions. Water Research. 2012;46:2237–2246. doi: 10.1016/j.watres.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Reniers A, Haus BK, Solo-Gabriele HM. Modeling sediment-related enterococci loading, transport and inactivation at an embayed non-point source subtropical beach. Water Resources Research. 2013 doi:10.1029/2012WR012432, in press. [Google Scholar]
- Fleisher JM, Fleming LE, Solo-Gabriele HM, Kish JK, Sinigalliano CD, Plano LRW, Elmir SM, Wang JD, Withum K, Shibata T, Gidley ML, Abdelzaher A, He G, Ortega C, Zhu X, Wright M, Hollenbeck J, Backer LC. The BEACHES study: health effects and exposures from non-point source microbial contaminants in subtropical recreational marine waters. International Journal of Epidemiology. 2010;39(5):1291–1298. doi: 10.1093/ije/dyq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florida Department of Health Florida Healthy Beaches Program. 2012 http://esetappsdoh.doh.state.fl.us/irm00beachwater/default.aspx.
- Grippo MA, Cooper S, Massey AG. Effect of Beach Replenishment Projects on Waterbird and Shorebird Communities. Journal of Coastal Research. 2007;235:1088–1096. [Google Scholar]
- Heaney D;, Sams E, Dufour AP, Brenner KP, Haugland RA, Chern E, Wing S, Marshall S, Love DC, Serre M, Noble R, Wade TJ. Fecal Indicators in Sand, Sand Contact, and Risk of Enteric Illness Among Beachgoers. Epidemiology. 2012;23(1):95–106. doi: 10.1097/EDE.0b013e31823b504c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura GJ, Thompson RS, Boehm AB, Jay JA. Indigenous soil bacteria and low moisture may limit but allow faecal bacteria to multiply and become a minor population in tropical soils. FEMS Microbiol Ecol. 2011;77(1):40–9. DOI: 10.1111/j.1574-6941.2011.01082.x. [Google Scholar]
- Kinzelman JL, Whitman RL, Byappanahalli M, Jackson E, Bagley RC. Evaluation of beach grooming techniques on Escherichia coli density in foreshore sand. Lake and Reservoir Management. 2003;19(4):349–354. [Google Scholar]
- Kinzelman J, McLellan S. Success of science-based management practices in reducing swimming bans – a case study from Racine, WI. USA. Aquat. Ecosyst. Health Manag. 2009;12:187–196. [Google Scholar]
- Mika KB, Imamura G, Chang C, Conway V, Fernandez G, Griffith JG, Kampalath RA, Lee CM, Lin C-C, Moreno R, Thompson S, Whitman RL, Jay JA. Pilot-and bench-scale testing of faecal indicator bacteria survival in marine beach sand near point sources. Journal of Applied Microbiology. 2009;107:72–84. doi: 10.1111/j.1365-2672.2009.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-Y, Gayes PT, Wells JT. Monitoring beach renourishment along the sediment-starved shoreline of Grand Strand, South Carolina. Journal of Coastal Research. 2009;25(2):336–349. [Google Scholar]
- Piggot AM, Klaus J, Johnson S, Phillips MC, Solo-Gabriele HM. Enterococci Levels are Related to Sediment Biofilms at Recreational Beaches in South Florida. Applied and Environmental Microbiology. 2012;78(17):5973–5982. doi: 10.1128/AEM.00603-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MC, Solo-Gabriele HM, Piggot AM, Klaus JS, Zhang Y. Relationship between sand and water quality at recreational beaches. Water Research. 2011;45:6763–6769. doi: 10.1016/j.watres.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price WD, Burchell II MR, Hunta WF, Chescheira GM. Long-term study of dune infiltration systems to treat coastal stormwater runoff for fecal bacteria. Ecological Engineering. 2013;52:1–11. [Google Scholar]
- Russell TL, Yamahara KM, Boehm AB. Mobilization and Transport of Naturally Occurring Enterococci in Beach Sands Subject to Transient Infiltration of Seawater. Environ. Sci. Technol. 2012;46:5988–5996. doi: 10.1021/es300408z. [DOI] [PubMed] [Google Scholar]
- Shah AH, Abdelzher AM, Phillips M, Hernandez R, Solo-Gabriele HM, Kish J, Scorzetti G, Fell JW, Diaz MR, Scott TM, Lukasik J, Harwood VJ, McQuaig S, Sinigalliano CD, Gidley ML, Wanless D, Ager A, Lui J, Stewart JR, Plano LRW, Fleming LE. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. Journal of Applied Microbiology. 2011;110(6):1571–1583. doi: 10.1111/j.1365-2672.2011.05013.x. [DOI] [PubMed] [Google Scholar]
- Shibata T, Solo-Gabriele HM, Sinigalliano CD, Gidley ML, Plano LRW, Fleisher JM, Wang JD, Elmir SM, He G, Wright ME, Abdelzaher AM, Ortega C, Wanless D, Garza AC, Kish J, Scott T, Hollenbeck J, Backer LC, Fleming LE. Evaluation of conventional and alternative monitoring methods for a recreational marine beach with non-point source of fecal contamination. Environmental Science & Technology. 2010;44:8175–8181. doi: 10.1021/es100884w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Solo-Gabriele HM, Fleming LE, Elmir S. Monitoring Marine Recreational Water Quality using Multiple Microbial Indicators in an Urban Tropical Environment. Water Research. 2004;38:3119–3131. doi: 10.1016/j.watres.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigalliano CD, Fleisher JM, Gidley ML, Solo-Gabriele HM, Shibata T, Plano LRW, Elmir SM, Wanless D, Bartkowiak J, Boiteau R, Withum K, Abdelzaher AM, He G, Ortega C, Zhu X, Wright ME, Kish J, Hollenbeck J, Scott T, Backer LC, Fleming LE. Traditional and molecular analyses for fecal indicator bacteria in non-point source subtropical recreational marine waters. Water Research. 2010;44(13):3763–3772. doi: 10.1016/j.watres.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalbeck JD, Kinzelman JL, Mayer GC. Fecal indicator organism density in beach sands: Impact of sediment grain size, uniformity, and hydrologic factors on surface water loading. Journal of Great Lakes Research. 2010;36:707–714. [Google Scholar]
- Solo-Gabriele H, Wolfert M, Desmarais T, Palmer C. Sources of E.coli to a Sub-Tropical Coastal Environment. Applied and Environmental Microbiology. 2000;66(1):230–237. doi: 10.1128/aem.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz MJ, Salmon M, Wyneken J. Beach Renourishment and Loggerhead Turtle Reproduction: A Seven Year Study at Jupiter Island, Florida. Journal of Coastal Research. 1998;14(3):1000–1013. [Google Scholar]
- U.S. Environmental Protection Agency . Method 1600: Enterococci in Water by Membrane Filtration Using membrane-Enterococcu Indoxyl-β-D-Glucoside Agar (mEI) US EPA Office of Water; Washington DC: 2002. EPA-821-R-02-022. [Google Scholar]
- U.S. Environmental Protection Agency . Improved enumeration methods for the recreational water quality indicators: enterococci and Escherichia coli. Washington D.C: 2000. [Google Scholar]
- U.S. Environmental Protection Agency . Ambient Water Quality Criteria for Bacteria. U.S. EPA; Washington, DC: 1986. EPA 440/5-84-002. [Google Scholar]
- Wang JD, Solo-Gabriele HM, Abdelzaher AM, Fleming LE. Estimation of enterococci input from bathers and animals on a recreational beach using camera images. Marine Pollution Bulletin. 2010;60:1270–1278. doi: 10.1016/j.marpolbul.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Abdelzaher AM, Solo-Gabriele HM, Elmir S, Fleming LE. The Inter-tidal Zone is the Pathway of Input of Enterococci to a Subtropical Recreational Marine Beach. Water Science & Technology. 2011;63.3:542–549. doi: 10.2166/wst.2011.255. [DOI] [PubMed] [Google Scholar]
- Wright ME, Solo-Gabriele HM, Elmir S, Fleming LE. Microbial load from animal feces at a recreational beach. Mar. Pollut. Bull. 2009;58:1649–1656. doi: 10.1016/j.marpolbul.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara KM, Walters SP, Boehm AB. Growth of enterococci in unaltered, unseeded sands subjected to tidal wetting. Applied and Environmental Microbiology. 2009;75:1517–1524. doi: 10.1128/AEM.02278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara KM, Layton BA, Santoro AE, Boehm AB. Beach sands along the California coast are diffuse sources of fecal bacteria to coastal waters. Environ Sci Technol. 2007;41(13):4515–4521. doi: 10.1021/es062822n. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang JD, Solo-Gabriele HM, Fleming LE, Elmir S. A microbial water quality model for recreational marine beaches. Water Research. 2011;45:2985–2995. doi: 10.1016/j.watres.2011.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.