Abstract
Objective
Resistance exercise (RE) stimulates growth hormone (GH) secretion in a load-dependent manner, with heavier loads producing larger GH responses. However, new research demonstrates that low-load RE performed with blood flow restriction (BFR) produces potent GH responses that are similar to or exceed those produced following high-load RE. We hypothesized that low-load RE with vascular restriction would attenuate the known age-related reduction in GH response to RE.
Design
In a randomized crossover design, ten young (28±7.8 years) and ten older (67.4±4.6 years) men performed bilateral knee extension RE with low-load [20% of one-repetition maximum (1RM)] with BFR and high-load (80% 1RM) without BFR. GH and lactate were measured every 10 minutes throughout a 150-minute testing session (30 minutes prior to and 120 minutes following completion of the exercise); IGF-I was measured at baseline and 60 minutes post-exercise.
Results
Area under the GH curve indicated that both age groups responded similarly to each exercise condition. However, young men had a significantly greater maximal GH response to low-load RE with BFR than the high-load condition without BFR. Additionally, younger men had greater maximal GH concentrations to low-load RE with BFR than older men (p=0.02). The GH responses were marginally correlated to lactate concentration (r=0.13, p=0.002) and IGF-I levels were unchanged with RE.
Conclusions
GH responses to low-load RE with vascular restriction are slightly higher than high-load RE without vascular restriction in young men. However, low-load RE with vascular restriction did not attenuate the known age-related reduction in GH response with exercise. These data suggest that while low-load RE with vascular restriction is as effective for inducing a GH response than traditionally-based high-load RE, there is a more potent response in young men.
Keywords: KAATSU, Blood flow restriction, muscle, growth hormone, vascular, hypoxia, ischemia, endocrine
INTRODUCTION
Growth hormone (GH) is a pleiotropic peptide hormone that has primary roles in the regulation of metabolism, via stimulation of lipid mobilization and oxidation [1], and in the promotion of collagen synthesis within musculotendionous tissue [2]. GH induces both direct and indirect effects on metabolism and protein synthesis, via GH receptor dimerization and/or stimulation of systemic and tissue-specific insulin-like growth factor (IGF)-I expression [1–3]. GH and IGF-I concentrations peak during adolescence and undergo a precipitous decline throughout the age span, which is thought to partially underlie the elevations in visceral fat mass and reductions in musculotendinous quality that occur in the elderly [4]. In this regard, exogenous GH administration does not promote accretion of skeletal muscle mass in adults [5], but instead reduces visceral and subcutaneous fat-mass in GH deficient populations [6] and promotes collagen synthesis in musculotendinous tissue [2]. However, the side-effects associated with GH administration [7] and its limited efficacy in promoting muscle function in older individuals [8] exclude its recommendation as an anti-aging therapy. Alternatively, exercise induces GH secretion in an intensity-dependent manner [9], which may at least partially underlie the influence of exercise on lipid oxidation [1] and/or collagen synthesis within both muscle and tendon [10]; although, this remains to be determined.
Traditional high-load resistance training performed in excess of 70% of one-repetition maximum (1RM) is the most established means of increasing both muscle and tendon cross-sectional area (CSA) and strength in young and older populations [11,12]. High-load resistance exercise (RE) results in a robust, yet transient, increase in circulating GH in young individuals, a result which is blunted in older populations [13]. Emerging evidence also indicates that low-load RE performed under conditions of local muscle blood flow restriction (BFR) produces comparable muscle hypertrophy to that observed with traditional high-load RE [14] and that low-load RE alone stimulates intramuscular collagen synthesis [15]. Interestingly, in healthy young persons, low-load RE coupled with BFR increases post-exercise GH concentrations to an equal or greater magnitude than that of high-load RE [16–22]; suggesting that low-load RE with BFR may be an effective means of attenuating metabolic and musculotendinous decrements that occur with aging. However, it remains unknown whether low-load RE with BFR is capable of increasing circulating GH in older populations who typically have low GH concentrations and who exhibit a blunted GH response to traditional high-load RE [13,23,24].
The primary purpose of this study was to compare the GH responses following low-load RE performed with BFR and high-load RE in both healthy young and older adults. Based on previous data (in young adults), we hypothesized that low-load RE with BFR would attenuate the age-related reduction in GH response to high-load RE. As secondary purposes, we examined the acute change in IGF-I, exercise volume, perceived exertion and pain as potential factors to explain the GH response.
METHODS
Subjects
Ten young men (28 ± 7.8 years) and ten older men (67.4 ± 4.6 years) were recruited for this study. To be included, subjects had to be sedentary untrained males (< 60 minutes per week exercise participation and performing no resistance exercise in the past 6 months) between 20–40 or 60–75 years of age. Subjects were excluded if they had a body mass index (BMI) > 30 kg/m2, diagnosed hypertension or use of anti-hypertensive medications, a resting blood pressure ≥ 140/90, ankle-brachial index < 0.95, an orthopedic limitation that would preclude lower extremity RE, were currently undergoing active treatment for cancer, testosterone therapy, or anticoagulant therapy, or had a history of blood clotting disorders, congestive heart failure (NYHA class II, III or IV), stroke (< 6 mo), peripheral vascular disease, coronary artery disease (myocardial infarction < 6 mo), valvular heart disease, major psychiatric disease, rheumatoid arthritis, macular degeneration, severe anemia, liver or renal disease, uncontrolled diabetes or hypertension, severe osteoarthritis, blindness or deafness, fracture in upper or lower extremity within the last 6 months, upper or lower extremity amputation, Mini-mental state exam score ≤ 24, family history or carrier of sickle cell anemia, currently smoking, alcohol or drug abuse in the past 6 months. The University of Florida Institutional Review Board approved the protocol and all subjects gave written informed consent to participate in the study.
Experimental Design
This project was designed to test hypotheses comparing response differences in age (young vs. old) and exercise condition (low-load RE with BFR vs. high-load RE). Because neither BFR alone [16,25] nor exercise performed at low-loads [26] produces a GH response, we did not include these comparison conditions. Therefore, this study adopted a randomized crossover design where subjects completed a baseline screening visit and two exercise sessions which were separated by a minimum of 4 days. The average number of days between testing session was 13 days. During the baseline screening visit, patients’ lower extremities were inspected to identify risk factors for deep vein thrombosis (DVT) using the Wells criteria [27] and an ECG was performed to detect potential cardiac arrhythmias. We chose knee extension exercise because it isolates t he muscle group to specifically examine the effect of BFR on exercise-induced GH secretion without potential confounding effects of agonist muscles. Additionally, there is a large body of literature on GH responses to knee extension exercise in older adults with which to compare results. Prior to exercise and on a separate day, knee extension 1RM was performed according to a standard protocol using a selectorized knee extension machine (Paramount Fitness Corp. XL series). Test-retest coefficient of variation of older adults performing maximal strength testing is 11% as noted previously by our group [28]. Heart rate (HR), blood pressure (BP) were monitored throughout the exercise conditions to ensure the safety of the participants. Ratings of perceived exertion (RPE) and pain were assessed immediately after each set of exercise as described below.
Subjects reported to the laboratory between 0700 and 0800 hours following overnight fast (subjects were permitted to consume water ad libitum). Exercise sessions were performed in a temperature-controlled room under direct supervision of a nurse. During each exercise session, subjects performed four sets of knee extension exercise at either 80% 1RM (high-load RE) without BFR or 20% 1RM (low-load) with concurrent BFR until volitional fatigue. During low-load training, BFR was initiated 2 minutes prior to the start of exercise and the cuff remained inflated for the duration of the exercise and rest periods. Specifically, an 11 cm wide blood pressure cuff (Hokanson, Bellevue, WA) was inflated around the upper thigh according to published tourniquet safety guidelines [29]. The degree of blood flow occlusion between individuals was standardized by inflating the cuff to 1.5 times the brachial systolic blood pressure of each subject (Cuff inflation range: 135–186 mmHg) that was assessed in a sitting position prior to the start of the exercise. In addition, the GH response to RE is partially contingent on rest between sets and the volume of exercise performed [30], as such we standardized rest intervals between sets at two minutes and recorded training volume by calculating the number of repetitions performed multiplied by mass lifted in kilograms in each condition.
Subjects also self-assessed their rate of perceived exertion (RPE) and pain using two validated scales developed by Borg [31]. Each scale was administered using standardized instructions [31]. Immediately following the bout of exercise, subjects were asked to recall their RPE on a 6 to 20 scale and localized leg pain levels on a 0–10 CR10 scale at the end of the exercise bout. Average RPE, average pain, and peak pain experienced were calculated and used for data analysis.
Blood acquisition
Upon acquisition of the sample, an aliquot of whole blood was placed on a portable lactate analyzer (Accutrend © Lacate Analyzer, Sports Resource Group Inc, Minneapolis MN). The remaining samples were acquired in EDTA tubes from an antecubital forearm vein via indwelling catheter at 10 minute intervals beginning 30 minutes prior to exercise and concluding 120 minutes after the onset of exercise. The catheters used for this study did not contain heparinized tubing because heparin interferes with the analysis of clotting agents within the plasma. As such, the catheter was flushed with normal saline solution after each blood draw in order to reduce likelihood of clotting within the catheter. Despite our attempts to avoid clotting, we were unable to collect blood on 33 out of 640 (~5%) time points because of clotting that occurred in the catheter. Most were missed 100 minutes post-exercise (21 out of the 33 samples). Chilled blood samples were centrifuged at 3000 rpm for 15 min at 4°C and plasma was separated and stored frozen at −80°C prior to analysis.
Biochemical analysis
Stored samples were analyzed in duplicate and in a single run. Plasma GH was determined by a commercially available enzyme-linked immunosorbent assay (ELISA) with a sensitivity of 0.03 ng/ml and an inter-assay variance of 5.1% (Diagnostic Systems Laboratories, Inc., Webster, Texas). Plasma IGF-I was determined at baseline and 60 minutes post-exercise by a commercially available non-extraction ELISA with a sensitivity of 0.01 ng/ml and an inter-assay variance of 5.8% (Diagnostic Systems Laboratories, Inc., Webster, Texas). For safety purposes, we assessed formation of fibrin clotting products 30 minutes following the exercise. D-dimer was measured using a semi-quantitative latex agglutination assay (Fisher Scientific, Suwanee, GA). This test indicated positive when D-dimer levels exceed 50 ug/ml - a clinically meaningful level indicating a high pre-probability risk of developing a deep-vein thrombosis in a low-risk population such as those enrolled in the study [27].
Statistical analysis
Subject characteristics across age groups were compared using a one-way analysis of variance (ANOVA). Peak GH and IGF-I responses, RPE, perceived pain, and total exercise volume were evaluated in a condition (80% vs. 20% RE) by age group repeated measures ANOVA. GH and lactate time point parameters were analyzed using a mixed model regression [32]. Additionally, GH area under the curve (AUC) was calculated using cubic splines, as described elsewhere [33], and the maximal GH concentration and GH AUC were compared across age groups and conditions using the same mixed model approach. Pearson correlation coefficients were calculated to investigate potential involvement of blood lactate concentration on the GH response found during the exercise conditions. All GH data met the homogeneity of variance assumption, but were non-normally distributed. As a result, the GH data were log-transformed and subsequently met the normality assumption for parametric analyses described above. Statistical differences were decided using log-transformed data, but means ± SEM were presented in figures out of convention and comparison to the literature. An alpha of p ≤ 0.05 was considered the criterion for statistical significance and all analyses were performed in STATA 10.0.
RESULTS
Subject characteristics
The anthropometric, blood pressure, and ankle brachial index characteristics were similar between the young and old subjects. However, 1RM knee extension strength was approximately 20% lower in the older subjects (Table 1).
Table 1.
Demographics and baseline characteristics.
| Young | Old | P-value | |
|---|---|---|---|
| Age (years) | 28 ± 7.8 | 67.4 ± 4.6 | <0.001 |
| Weight (kg) | 78.1 ± 7.6 | 85.6 ± 10.5 | 0.086 |
| Height (cm) | 173.7 ± 7.3 | 177.4 ± 5.4 | 0.213 |
| Body mass index (kg/m2) | 25.9 ± 2.8 | 27.1 ± 2.5 | 0.344 |
| Systolic BP (mm Hg) | 120 ± 10.2 | 122 ± 9.1 | 0.540 |
| Diastolic BP (mm Hg) | 82.4 ± 4.7 | 84.4 ± 7.5 | 0.485 |
| Ankle Brachial Index | 1.3 ± 0.18 | 1.42 ± 0.11 | 0.346 |
| Knee extension 1RM (kg) | 115 ± 19.6 | 92.7 ± 12.7 | 0.007 |
Note: MMSE = Mini-Mental State Exam Score, RM = Repetition Maximum Values = Mean ± Standard Deviation
Subjects in both age groups performed a greater exercise volume during the 80% bout of RE compared with the 20% bout with BFR (Young: 20% RE = 2519 ± 208 vs. 80% RE = 4283 ± 312 kg; Old: 20% RE = 1722 ± 241 vs. 80% RE = 2830 ± 180 kg). (condition effect: p < 0.001). Additionally, the young subjects performed a larger training volume under both conditions (age effect: p < 0.001). There was no clinically significant evidence of D-dimer formation during both exercise conditions.
Table 2 displays information on RPE, perceived pain, repetitions achieved and exercise volume. Subjects in both age groups performed a greater exercise volume during the 80% bout of RE compared with the 20% bout with BFR (condition effect: p < 0.001). Additionally, when compared to the old, young subjects performed additional repetitions and had a larger exercise volume under both conditions. As seen with the significant age group by condition interaction, young adults also increased their exercise volume from 20% BFR RE to 80% RE to a greater extent than older adults. No differences in RPE were present between age group or exercise condition (p = 0.387; Table 2). Conversely, both average and peak pain levels were approximately 2–4 points higher during the low-load RE with BFR compared to the high-load RE in both age groups (p = 0.003, Table 2).
Table 2.
Rating of perceived exertion (RPE), pain, and total exercise volume in young and old subjects for low-load (20%) resistance exercise (RE) with blood flow restriction (BFR) and high-load (80%) RE conditions.
| Young | Old | P-Value | |||||
|---|---|---|---|---|---|---|---|
| 20% BFR BFR |
80% RE | 20% BFR BFR |
80% RE | Condition Effect |
Age Effect |
Condition by Age Interaction |
|
| RPE (6–20) | 17.9 ± 0.5 | 17.9 ± 0.5 | 17.6 ± 0.8 | 18.3 ± 0.4 | 0.299 | 0.968 | 0.387 |
| Average Pain (0–10) | 6.3 ± 0.6 | 4.3 ± 0.7 | 7.3 ± 0.7 | 3.9 ± 0.9 | 0.003 | 0.733 | 0.305 |
| Peak Pain (0–10) | 7.5 ± 0.5 | 5.6 ± 0.8 | 8.7 ± 0.6 | 4.7 ± 1.1 | 0.005 | 0.776 | 0.211 |
| Repetitions performed ‡ | 110.3 ± 8.6 | 46.1 ± 2.1 | 80.3 ± 12.5 | 37.5 ± 1.5 | < 0.001 | 0.032 | 0.146 |
| Exercise volume (rep*kg) | 2519 ± 208 | 4283 ± 312 | 1722 ± 241 | 2830 ± 180 | <0.001 | <0.001 | 0.055 |
Note: Exercise volume = total number of repetitions * mass lifted in kg; Average and peak pain calculated over the 5 sets of resistance exercise.
Represents the total number or repetitions achieved over 5 sets of knee extension exercise to volitional failure.
Growth hormone and IGF-I
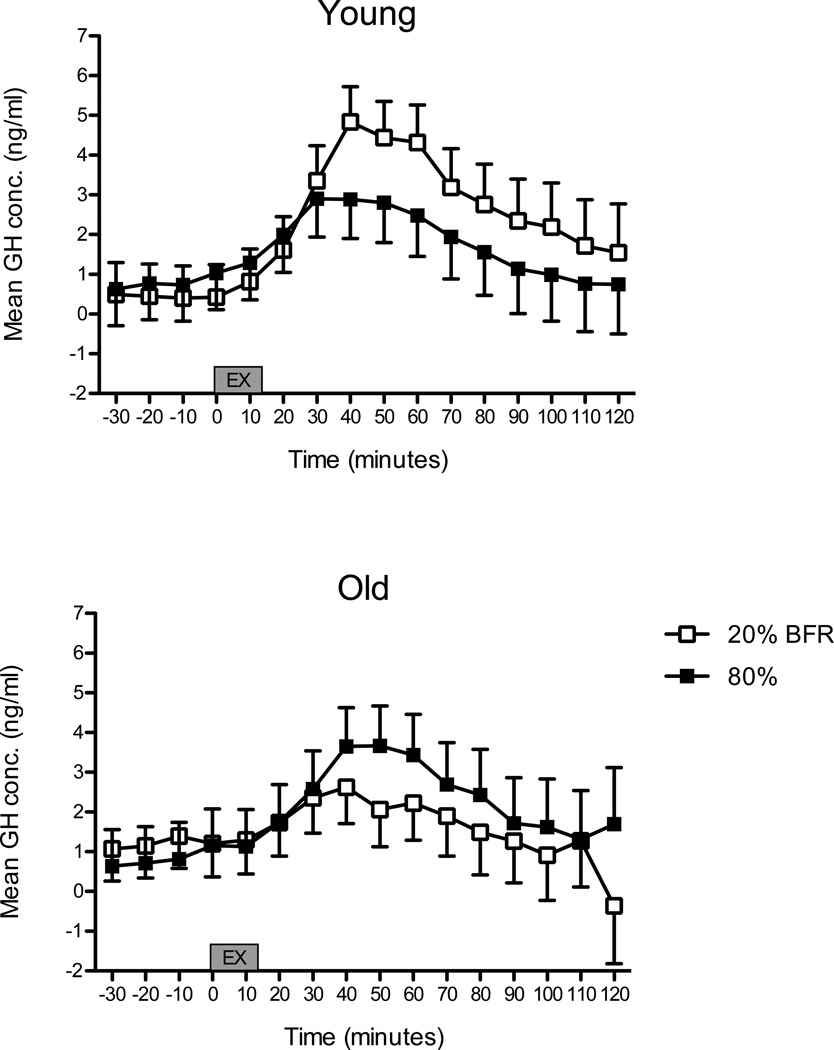
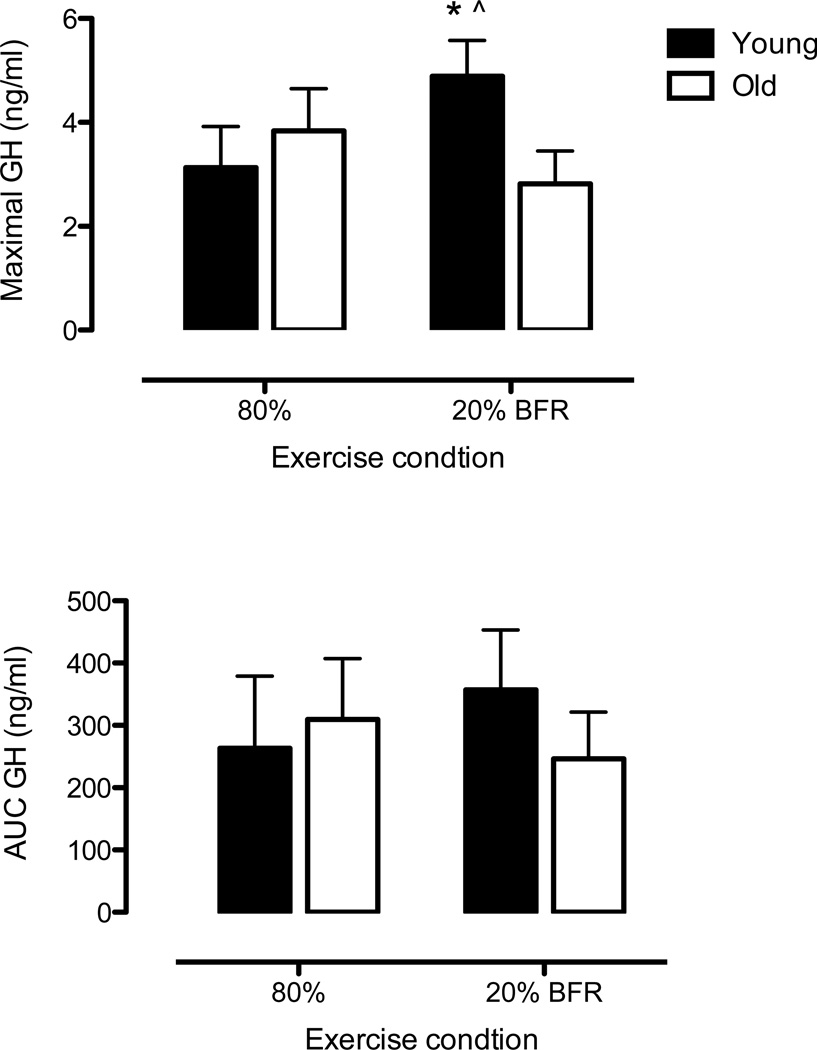
Prior to beginning exercise, baseline plasma GH concentrations were ≤ 1ng/ml in both young and older subjects. A significant three-way interaction indicated differences across age groups, exercise conditions and sampling time following the onset of exercise (p = 0.006). Figure 1 illustrates data according to differences between exercise conditions. The GH responses of young and old subjects were similar between exercise conditions. Knee extension RE at 20% 1RM with BFR had double the GH response to BFR at 40 and 50 minutes post-onset of exercise than old subjects, but these differences were not statistically different (p = 0.15 for both time points). There was no difference in the GH response between age groups in the high-load RE condition (all p-values > 0.50). Maximal concentrations of GH were higher in the young during the 20% BFR condition when compared to the old group and the 80% RE condition (Figure 2: upper panel). GH AUC was similar between age groups and conditions (Figure 2: bottom panel).
Figure 1. Growth hormone concentration between e xercise conditions.
20% BFR: knee extension exercise performed at 20% of maximum strength with blood flow restriction. 80%: knee extension exercise performed at 80% of maximum strength without blood flow restriction. Values are predicted means ± SEM, n = 10 per condition. *Indicates p < 0.05 for difference between exercise conditions.
Figure 2. Growth hormone concentration expressed as area under the curve (AUC) and maximal concentration.
Values are predicted means ± SEM, n = 10 per age group. *Indicates p ≤ 0.05 for differences between age groups. ^Indicates p ≤ 0.05 for differences between exercise condition.
Basal plasma IGF-I concentrations were approximately 50% lower in the old compared to young subjects prior to each exercise condition (p < 0.01). IGF-I concentrations remained unaltered in young (80% baseline: 273.2 ± 34.8; 80% post-exercise 282 ± 33.5 ng/ml; 20% BFR baseline: 269.0 ± 22.6; 20% BFR post-exercise: 255.4 ± 26.9 ng/ml) and old subjects (80% baseline: 116.9 ± 10.2; 80% post-exercise: 138.4 ± 12.5; 20% BFR baseline: 125.8 ± 10.0; 20% BFR post- exercise: 129.9 ± 14.2 ng/ml) following both exercise conditions.
Lactate
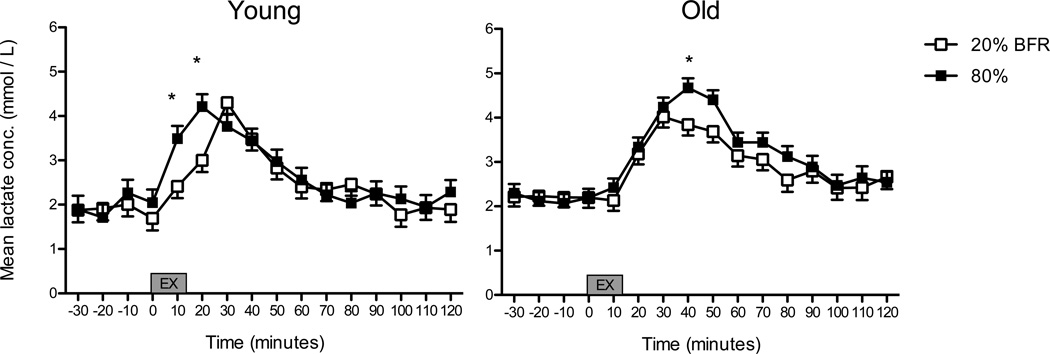
Lactate concentration increased 10 minutes following the first bout of both exercise conditions in both young and old subjects. In young subjects, the peak lactate concentration (4.4 ± 0.3 mM) occurred 20 minutes after the onset of high-load RE (p ≤ 0.05, Figure 4). A similar peak lactate concentration occurred following low-load RE with BFR (4.2 ± 0.3 mM), albeit at a slightly delayed time point (i.e., 30 minutes). In older subjects, high-load RE produced a peak lactate concentration of 4.6 ± 0.2 mM which occurred 40 minutes after the onset of exercise, while the lactate peak was lower (3.8 ± 0.2 mM) and occurred at an earlier time point (30 minutes after the onset of exercise) following low-load RE with BFR (p ≤ 0.05). No differences between age groups were noted. Of note, lactate concentration was positively correlated with the GH concentration when age groups and exercise conditions were collapsed (r = 0.13, p = 0.002). Upon further analyses, this association was stronger in older men when RE was performed at low loads with BFR (r = 0.24, p = 004) compared to RE at 80% (r = 0.03, p = 0.67). Young men showed a consistently low and non-significant association between GH and lactate concentration (20% RE: r = 0.13, p = 0.10; 80% RE: r = 0.10, p = 0.18)
Figure 4. Serum Lactate concentration in response to exercise conditions.
Values are predicted means ± SEM, n = 10 per condition. *Indicates p ≤ 0.05 for differences between exercise conditions.
DISCUSSION
To our knowledge, this is the first study comparing the effects of low-load RE with BFR and high-load RE in both young and old men. The two main findings of our study are as follows: 1) older men exhibited a blunted maximal GH response to low-load RE with BFR compared to younger men and 2) young men experienced a higher maximal GH concentration, but similar AUC GH concentrations following low-load RE with BFR, despite performing a lower total exercise volume with BFR. In addition, circulating IGF-I was substantially lower in older men and remained unaltered by either exercise protocol. Our hypothesis that low-load RE with BFR attenuates the age-related loss of GH stimulation to high-load RE was rejected.
The GH response to RE has been well characterized [34] and is traditionally known to be influenced by chronological age, amount of muscle mass recruited, type of muscle action (concentric or eccentric), exercise load, training status (an athlete versus a sedentary individual), volume of exercise, and the amount of rest given between sets of exercise. In young men, we observed that low-load RE with BFR produces a similar increase in GH to that of high-load RE, which corroborates the findings of several previous studies [16–22]. However, contrary to our hypothesis, we observed that the maximal post-exercise GH response was attenuated in old men during low-load RE with BFR. The GH response to low-load RE with BFR that we observed may seem low compared with that which others report [21]; however, it is of a slightly higher magnitude than that reported by Fry et al., using the same knee exercise protocol in older adults (mean peak response of ~1 ng/ml) [25]. Interestingly, neither low-load RE alone nor BFR alone increase circulating GH concentrations, while the combination of low-load RE with BFR produces a robust increase in GH [22,26]. This suggests that the coupling of these two modalities produces an adjunct elevation in circulating GH that appears to be reliant on both local muscle ischemia and physical exertion.
The accumulation of metabolic byproducts produced by exercising muscle is involved in the hypothalamic-stimulated release of GH [35,36]. Specifically, metabolic acidosis in the form of lactate accumulation is involved in GH release, as evidenced by Luger and colleagues who infused lactate to produce the same serum concentrations observed during an exercise bout and observed roughly half the GH response resulting from exercise [35]. In our study, while we observed that lactate concentration was correlated with GH concentrations, we found no clear differences between the conditions or age groups that could explain the elevated peak GH response. Alternatively, exercise volume is known to influence the GH response to exercise. However, we find it unlikely that the observed differences in exercise volume influenced the GH response in older individuals because the amount of mechanical work performed was not related to the GH response in our study. As such, exercise volume and lactate concentration may play a lesser role in GH secretion following low-load RE with BFR than others have suggested [21,22].
In agreement with the findings of several previous studies [13,24,30], we report that basal GH concentrations were not different between younger and older men. However, our results appear to at least partially conflict with a number of studies, which have reported that both the maximal and integrated AUC GH responses to high-load RE are blunted in old individuals [13,23,24]. In fact, we observed no difference in the GH AUC between young and old individuals in our study and the maximal GH responses to high-load RE were also similar between ages. The older men enrolled in our study were thoroughly screened and apparently healthy for their respective age (i.e., no subjects were hypertensive – a common condition in older adults). Thus, our recruitment of a healthy sample of older adults likely skewed comparisons to other studies where screening was less stringent or where subjects were of an even older age [13,30]. Conversely, in older men, we observed that the maximal GH response to low-load RE with BFR was blunted compared with that following high-load RE which may have resulted from a reduced maximal force production due to BFR, which we [26] and others [37] have previously reported. In particular, maximal EMG is reduced both during [37] and following [38] RE with BFR, a result that is amplified by the severity of blood flow occlusion [38]. It remains unknown whether these deficits result from central or peripheral mechanisms; however, both metaboreceptor activation [39] and central command influence GH secretion [40,41]. Additionally, the relative importance of the maximal GH response to stimuli versus that of the total GH response (typically measured as AUC) remains somewhat controversial. The fact that GH is typically secreted in a pulsatile manner [1], as opposed to continuously, suggests that the maximal or peak GH response may be of some importance when evaluating the physiological effects of this hormone; although, this remains to be determined.
BFR RE resulted in similar reported exertion levels, but higher perceived localized pain during exercise. These results suggest that pain receptors are activated to a greater extent during BFR RE than high-load RE. While the mechanism is not completely clear, its plausible that decreased venous outflow during BRF reduces clearance of metabolic acidosis resulting in activation of proton-activated nociceptors [42]. Importantly, acute pain is known to regulate GH secretion through stimulation of opioid receptors [43]. For example, Greisen and coworkers found a significant increase in GH secretion following electrical stimulation to the abdominal skin [44]. Additionally, Jubeau et al. reported a higher GH secretion with electrical stimulated versus voluntary maximal muscle contractions [45]. The researchers speculated that high acute pain perceived during the stimulated contractions contributed to the elevated GH response. It would appear that the higher levels of pain induced by low-load BFR contributed to the GH release compared to the relatively painless high-load RE bout. From a more applied perspective, elevated pain levels seen during BFR RE reduce its potential utility as a viable modality for the public. A modification of the BFR exercise protocol that minimizes pain levels by releasing cuff pressure between sets of exercise might improve tolerability, but it is unknown how this will impact the physiological responses. Additional research is needed to ensure low-load BFR RE is tolerable while providing the physiological signals that are associated with muscle adaptation.
GH circulates in multiple molecular forms, only some of which are biologically active [46]. In men, the bulk of GH secretion occurs in a pulsatile manner at night, while daytime levels are comparatively low [1]; however, several stimuli including exercise [9] and amino acid administration [47] induce GH secretion. For this study, we measured the intact, immuno-reactive GH (irGH) molecule, which is the most prevalent within the circulation and which consists of several GH subfractions, including immunofunctional GH (ifGH), GH bound to GH binding protein, and GH fragments [48]. irGH expresses two separate receptor-binding domains, both of which are required to dimerize the GH receptor and initiate signal transduction [48]. Conversely, ifGH, which represents approximately 50% of irGH [47] is considered biologically active because ifGH expresses both binding domains [48]. As such, GH may exert direct systemic effects via receptor dimerization or indirect effects via the stimulation of systemic or local IGF-I expression [1]; although, we did not observe changes in circulating IGF-I following either exercise protocol. Importantly, it is no longer thought that GH stimulates accretion of skeletal muscle mass in adults [5], considering that GH does not induce myofibrillar protein synthesis [2] and that exogenous GH administration does not improve muscle performance in healthy young individuals, GH-deficient individuals [49], or older men [8]. In humans, the primary roles of GH are as a key regulator of fuel metabolism and as an initiator of protein synthesis with connective tissue [50]. For example, GH induces rapid dose-dependent increases in lipid mobilization and oxidation, a result which is reversed with pharmacologic GH receptor blockade [1], and moderately reduces fat mass in both obese [51] and GH deficient individuals [6] following administration. In addition, GH influences musculotendinous tissue via stimulation of collagen protein synthesis either directly or indirectly, through effects on IGF-I [2,3]. Considering the clear roles of GH in metabolic and musculotendinous health, future research examining the effects of low-load RE with BFR on circulating ifGH and tissue-specific (i.e., musculotendinous collagen or adipose tissue) IGF-I expression appears warranted.
We report here that low-load RE with BFR stimulates GH secretion in an amount that is comparable to that produced by high-load RE without BFR in young men. However, the post-exercise GH response to low-load RE with BFR is lower in older men when compared with that seen in young men and to some extent compared to traditional high-load RE.
ACKNOWELDGEMENTS
Todd Manini’s work on this project was supported in part by Award Number P30AG028740 from the NIA Claude D. Pepper Center from the National Institute on Aging. Stephen Borst’s work on this project supported by a Veteran’s Administration Merit Award.
CITATIONS
- 1.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 2.Doessing S, Heinemeier KM, Holm L, et al. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010;588:341–351. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doessing S, Holm L, Heinemeier KM, et al. GH and IGF1 levels are positively associated with musculotendinous collagen expression: experiments in acromegalic and GH deficiency patients. Eur J Endocrinol. 2010;163:853–862. doi: 10.1530/EJE-10-0818. [DOI] [PubMed] [Google Scholar]
- 4.Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C. Modulation of GH/IGF-1 axis: potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev. 2008;129:593–601. doi: 10.1016/j.mad.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Bravata DM, Olkin I, et al. Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med. 2008;148:747–758. doi: 10.7326/0003-4819-148-10-200805200-00215. [DOI] [PubMed] [Google Scholar]
- 6.Egger A, Buehler T, Boesch C, Diem P, Stettler C, Christ ER. The effect of GH replacement therapy on different fat compartments: a whole-body magnetic resonance imaging study. Eur J Endocrinol. 2011;164:23–29. doi: 10.1530/EJE-10-0702. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 8.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age Ageing. 2004;33:548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 9.Gibney J, Healy ML, Sonksen PH. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr Rev. 2007;28:603–624. doi: 10.1210/er.2006-0052. [DOI] [PubMed] [Google Scholar]
- 10.Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol. 2006;91:483–498. doi: 10.1113/expphysiol.2005.032896. [DOI] [PubMed] [Google Scholar]
- 13.Pyka G, Wiswell RA, Marcus R. Age-dependent effect of resistance exercise on growth hormone secretion in people. J Clin Endocrinol Metab. 1992;75:404–407. doi: 10.1210/jcem.75.2.1639942. [DOI] [PubMed] [Google Scholar]
- 14.Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev. 2009;37:78–85. doi: 10.1097/JES.0b013e31819c2e5c. [DOI] [PubMed] [Google Scholar]
- 15.Holm L, van Hall G, Rose AJ, et al. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E257–E269. doi: 10.1152/ajpendo.00609.2009. [DOI] [PubMed] [Google Scholar]
- 16.Fujita S, Abe T, Drummond MJ, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903–910. doi: 10.1152/japplphysiol.00195.2007. [DOI] [PubMed] [Google Scholar]
- 17.Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc. 2008;40:258–263. doi: 10.1249/mss.0b013e31815c6d7e. [DOI] [PubMed] [Google Scholar]
- 18.Madarame H, Sasaki K, Ishii N. Endocrine responses to upper- and lower-limb resistance exercises with blood flow restriction. Acta Physiol Hung. 2010;97:192–200. doi: 10.1556/APhysiol.97.2010.2.5. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda T, Fujita S, Ogasawara R, Sato Y, Abe T. Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: a pilot study. Clin Physiol Funct Imaging. 2010;30:338–343. doi: 10.1111/j.1475-097X.2010.00949.x. [DOI] [PubMed] [Google Scholar]
- 20.Reeves GV, Kraemer RR, Hollander DB, et al. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J Appl Physiol. 2006;101:1616–1622. doi: 10.1152/japplphysiol.00440.2006. [DOI] [PubMed] [Google Scholar]
- 21.Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000;88:61–65. doi: 10.1152/jappl.2000.88.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol. 2005;95:65–73. doi: 10.1007/s00421-005-1389-1. [DOI] [PubMed] [Google Scholar]
- 23.Craig BW, Brown R, Everhart J. Effects of progressive resistance training on growth hormone and testosterone levels in young and elderly subjects. Mech Ageing Dev. 1989;49:159–169. doi: 10.1016/0047-6374(89)90099-7. [DOI] [PubMed] [Google Scholar]
- 24.Marcell TJ, Wiswell RA, Hawkins SA, Tarpenning KM. Age-related blunting of growth hormone secretion during exercise may not be soley due to increased somatostatin tone. Metabolism. 1999;48:665–670. doi: 10.1016/s0026-0495(99)90069-0. [DOI] [PubMed] [Google Scholar]
- 25.Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199–1209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierce JR, Clark BC, Ploutz-Snyder LL, Kanaley JA. Growth hormone and muscle function responses to skeletal muscle ischemia. J Appl Physiol. 2006;101:1588–1595. doi: 10.1152/japplphysiol.00585.2006. [DOI] [PubMed] [Google Scholar]
- 27.Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 28.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. Journal of the American Geriatrics Society. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 29.Klenerman L. The Tourniquet Manual: Principles and Practice. London: Springer; 2003. [Google Scholar]
- 30.Weltman A, Weltman JY, Roy CP, et al. Growth hormone response to graded exercise intensities is attenuated and the gender difference abolished in older adults. J Appl Physiol. 2006;100:1623–1629. doi: 10.1152/japplphysiol.01312.2005. [DOI] [PubMed] [Google Scholar]
- 31.Borg G. Perceived exertion and pain scales. Champaign IL: Human Kinetics; 1988. [Google Scholar]
- 32.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 33.Chow SC, Liu JP. Design and Analysis of Bioavailability and Bioequivalence Studies. Boca Raton, FL: Chapman & Hall; 2009. [Google Scholar]
- 34.Kraemer WJ, Nindle BC, Gordon SE. Resistance exercise: Acute and Chronic Changes in Growth Hormone Concentrations. In: Kraemer WJ, Rogol AD, editors. The Endocrine System in Sports and Exercise. Malden, Mass: Blackwell Publishing, Inc; 2005. [Google Scholar]
- 35.Luger A, Watschinger B, Deuster P, Svoboda T, Clodi M, Chrousos GP. Plasma growth hormone and prolactin responses to graded levels of acute exercise and to a lactate infusion. Neuroendocrinology. 1992;56:112–117. doi: 10.1159/000126912. [DOI] [PubMed] [Google Scholar]
- 36.Marx JO, Gordon SE, Vos NH, et al. Effect of alkalosis on plasma epinephrine responses to high intensity cycle exercise in humans. Eur J Appl Physiol. 2002;87:72–77. doi: 10.1007/s00421-002-0591-7. [DOI] [PubMed] [Google Scholar]
- 37.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda T, Brechue WF, Fujita T, Shirakawa J, Sato Y, Abe T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J Sports Sci. 2009;27:479–489. doi: 10.1080/02640410802626567. [DOI] [PubMed] [Google Scholar]
- 39.Viru M, Jansson E, Viru A, Sundberg CJ. Effect of restricted blood flow on exercise-induced hormone changes in healthy men. Eur J Appl Physiol Occup Physiol. 1998;77:517–522. doi: 10.1007/s004210050369. [DOI] [PubMed] [Google Scholar]
- 40.Kjaer M, Secher NH, Bach FW, Galbo H. Role of motor center activity for hormonal changes and substrate mobilization in humans. Am J Physiol. 1987;253:R687–R695. doi: 10.1152/ajpregu.1987.253.5.R687. [DOI] [PubMed] [Google Scholar]
- 41.Kjaer M, Secher NH, Bach FW, Sheikh S, Galbo H. Hormonal and metabolic responses to exercise in humans: effect of sensory nervous blockade. Am J Physiol. 1989;257:E95–E101. doi: 10.1152/ajpendo.1989.257.1.E95. [DOI] [PubMed] [Google Scholar]
- 42.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomasi PA, Fanciulli G, Palermo M, Pala A, Demontis MA, Delitala G. Opioid-receptor blockade blunts growth hormone (GH) secretion induced by GH-releasing hormone in the human male. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1998;30:34–36. doi: 10.1055/s-2007-978827. [DOI] [PubMed] [Google Scholar]
- 44.Greisen J, Juhl CB, Grofte T, Vilstrup H, Jensen TS, Schmitz O. Acute pain induces insulin resistance in humans. Anesthesiology. 2001;95:578–584. doi: 10.1097/00000542-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Jubeau M, Sartorio A, Marinone PG, et al. Comparison between voluntary and stimulated contractions of the quadriceps femoris for growth hormone response and muscle damage. Journal of Applied Physiology. 2008;104:75–81. doi: 10.1152/japplphysiol.00335.2007. [DOI] [PubMed] [Google Scholar]
- 46.Nindl BC, Kraemer WJ, Marx JO, Tuckow AP, Hymer WC. Growth hormone molecular heterogeneity and exercise. Exerc Sport Sci Rev. 2003;31:161–166. doi: 10.1097/00003677-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Powers ME, Yarrow JF, McCoy SC, Borst SE. Growth hormone isoform responses to GABA ingestion at rest and after exercise. Med Sci Sports Exerc. 2008;40:104–110. doi: 10.1249/mss.0b013e318158b518. [DOI] [PubMed] [Google Scholar]
- 48.Nindl BC. Exercise modulation of growth hormone isoforms: current knowledge and future directions for the exercise endocrinologist. Br J Sports Med. 2007;41:346–348. doi: 10.1136/bjsm.2006.028951. discussion 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Widdowson WM, Gibney J. The effect of growth hormone (GH) replacement on muscle strength in patients with GH-deficiency: a meta-analysis. Clin Endocrinol (Oxf) 2010;72:787–792. doi: 10.1111/j.1365-2265.2009.03716.x. [DOI] [PubMed] [Google Scholar]
- 50.Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mekala KC, Tritos NA. Effects of recombinant human growth hormone therapy in obesity in adults: a meta analysis. J Clin Endocrinol Metab. 2009;94:130–137. doi: 10.1210/jc.2008-1357. [DOI] [PubMed] [Google Scholar]